Impact of Lactobacillus acidophilus—La5 on Composition and Metabolism of the Intestinal Microbiota of Type 2 Diabetics (T2D) and Healthy Individuals Using a Microbiome Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Samples Collection
2.3. Experimental Protocol
2.4. Metabolic Activity: Ammonium () Analysis and Short-Chain Fatty Acids (SCFAs)
2.5. Survival of Lactobacillus acidophilus—La5 under Simulated Conditions of the Stomach and Duodenum in the SHIME®
2.6. Microbiological Analysis Employing 16S rRNA Gene Sequencing
2.7. Statistical Analysis
3. Results and Discussion
3.1. Survival of L. acidophilus—La5 under Simulated GI Condition
3.2. Metabolic Activity
3.3. Richness and Diversity of the Gut Microbiota
3.4. Influence of Lactobacillus acidophilus—La5 on Gut Microbiota Composition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IDF Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org/en/resources/ (accessed on 10 May 2023).
- Control CfD, Prevention. National Diabetes Statistics Report, 2020; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2020.
- Sekirov, I.; Russell, S.L.; Antunes, L.C.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, A.; Zeng, X.; Hou, C.; Liu, H.; Qiao, S. Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015, 15, 32. [Google Scholar] [CrossRef]
- Di Luccia, B.; Manzo, N.; Baccigalupi, L.; Calabrò, V.; Crescenzi, E.; Ricca, E.; Pollice, A. Lactobacillus gasseri SF1183 affects intestinal epithelial cell survival and growth. PLoS ONE 2013, 8, e69102. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Nagata, S.; Asahara, T.; Yuki, N.; Matsuda, K.; Tsuji, H.; Takahashi, T.; Nomoto, K.; Yamashiro, Y. Intestinal Microbiota Profiles of Healthy Pre-School and School-Age Children and Effects of Probiotic Supplementation. Ann. Nutr. Metab. 2015, 67, 257–266. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Yamazaki, R.; Hashimoto, S.; Yokokura, T. Antidiabetic effects of an oral administration of Lactobacillus casei in a non-insulin-dependent diabetes mellitus (NIDDM) model using KK-Ay mice. Endocr. J. 1997, 44, 357–365. [Google Scholar] [CrossRef]
- Sato, J.; Kanazawa, A.; Ikeda, F.; Yoshihara, T.; Goto, H.; Abe, H.; Komiya, K.; Kawaguchi, M.; Shimizu, T.; Ogihara, T.; et al. Gut dysbiosis and detection of “live gut bacteria” in blood of Japanese patients with type 2 diabetes. Diabetes Care 2014, 37, 2343–2350. [Google Scholar] [CrossRef]
- Matsumoto, K.; Takada, T.; Shimizu, K.; Moriyama, K.; Kawakami, K.; Hirano, K.; Kajimoto, O.; Nomoto, K. Effects of a probiotic fermented milk beverage containing Lactobacillus casei strain Shirota on defecation frequency, intestinal microbiota, and the intestinal environment of healthy individuals with soft stools. J. Biosci. Bioeng. 2010, 110, 547–552. [Google Scholar] [CrossRef]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef]
- Cordeiro, B.F.; Alves, J.L.; Belo, G.A.; Oliveira, E.R.; Braga, M.P.; da Silva, S.H.; Lemos, L.; Guimarães, J.T.; Silva, R.; Rocha, R.S.; et al. Therapeutic Effects of Probiotic Minas Frescal Cheese on the Attenuation of Ulcerative Colitis in a Murine Model. Front. Microbiol. 2021, 12, 623920. [Google Scholar] [CrossRef]
- Moayyedi, P.; Ford, A.C.; Talley, N.J.; Cremonini, F.; Foxx-Orenstein, A.E.; Brandt, L.J.; Quigley, E.M. The efficacy of probiotics in the treatment of irritable bowel syndrome: A systematic review. Gut 2010, 59, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Ondee, T.; Pongpirul, K.; Visitchanakun, P.; Saisorn, W.; Kanacharoen, S.; Wongsaroj, L.; Kullapanich, C.; Ngamwongsatit, N.; Settachaimongkon, S.; Somboonna, N.; et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 2021, 11, 6367. [Google Scholar] [CrossRef]
- Brasili, E.; Mengheri, E.; Tomassini, A.; Capuani, G.; Roselli, M.; Finamore, A.; Sciubba, F.; Marini, F.; Miccheli, A. Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 induce different age-related metabolic profiles revealed by 1H-NMR spectroscopy in urine and feces of mice. J. Nutr. 2013, 143, 1549–1557. [Google Scholar] [CrossRef]
- Zarrati, M.; Shidfar, F.; Nourijelyani, K.; Mofid, V.; Hossein zadeh-Attar, M.J.; Bidad, K.; Najafi, F.; Gheflati, Z.; Chamari, M.; Salehi, E. Lactobacillus acidophilus La5, Bifidobacterium BB12, and Lactobacillus casei DN001 modulate gene expression of subset specific transcription factors and cytokines in peripheral blood mononuclear cells of obese and overweight people. Biofactors 2013, 39, 633–643. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Siddiqui, W.J. Cholesterol Levels; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Tonucci, L.B.; Olbrich, K.M.; Oliveira, L.L.; Ribeiro, S.M.R.; Martino, H.S.D. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V.; Akbarian-Moghari, A. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J. Dairy Sci. 2011, 94, 3288–3294. [Google Scholar] [CrossRef]
- Pham, V.T.; Mohajeri, M.H. The application of in vitro human intestinal models on the screening and development of pre- and probiotics. Benef. Microbes 2018, 9, 725–742. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Larsen, N.; de Mello Tieghi, T.; Adorno, M.A.T.; Kot, W.; Saad, S.M.I.; Jespersen, L.; Sivieri, K. Modulation of gut microbiota from obese individuals by in vitro fermentation of citrus pectin in combination with Bifidobacterium longum BB-46. Appl. Microbiol. Biotechnol. 2018, 102, 8827–8840. [Google Scholar] [CrossRef]
- Bianchi, F.; Rossi, E.A.; Sakamoto, I.K.; Adorno, M.A.T.; Van de Wiele, T.; Sivieri, K. Beneficial effects of fermented vegetal beverages on human gastrointestinal microbial ecosystem in a simulator. Food Res. Int. 2014, 64, 43–52. [Google Scholar] [CrossRef]
- Dostal, A.; Baumgartner, J.; Riesen, N.; Chassard, C.; Smuts, C.M.; Zimmermann, M.B.; Lacroix, C. Effects of iron supplementation on dominant bacterial groups on the gut, faecal SCFA and gut inflammation: Arandomised, placebo-controlled intervention trial in South African children. Br. J. Nutr. 2014, 112, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, C.G.; Prosello, W.; Ghiberto, T.D.; Reinheimer, J.A. Viability of probiotic (Bifidobacterium, Lactobacillus acidophilus and Lactobacillus casei) and nonprobiotic microflora in Argentinian Fresco cheese. JDS 2000, 83, 1905–1911. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, P.Y. Conservative Fragments in Bacterial 16S rRNA Genes and Primer Design for 16S Ribosomal DNA Amplicons in Metagenomic Studies. PLoS ONE 2009, 4, e7401. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Christoff, A.P.; Sereia, A.F.R.; Boberg, D.R. Bacterial Identification through Accurate Library Preparation and High-Throughput Sequencing; White Paper: Bacterial NGS Sequencing; Neoprospecta Microbiome Technologies: Florianopolis, Brazil, 2017. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Compunting: Vienna, Austria, 2020; Available online: https://www.r-project.org (accessed on 17 January 2023).
- McMurdie, P.J.; Holmes, S.; Watson, M. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Zhou, N.; McDonald, D.; Costello, E.K.; Knight, R. Forensic identification using skin bacterial communities. Proc. Natl. Acad. Sci. USA 2010, 107, 6477–6481. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics on Food; World Health Organization: Geneva, Switzerland; Food and Agriculture Organization of the United Nations: London, ON, Canada, 2002.
- Park, M.K.; Lee, S.; Kim, Y.S. Effects of pH and Osmotic Changes on the Metabolic Expressions of Bacillus subtilis Strain 168 in Metabolite Pathways including Leucine Metabolism. Metabolites 2022, 12, 112. [Google Scholar] [CrossRef]
- Priya, A.J.; Vijayalakshmi, S.P.; Raichur, A.M. Enhanced survival of probiotic Lactobacillus acidophilus by encapsulation with nanostructured polyelectrolyte layers through layer-by-layer approach. J. Agric. Food Chem. 2011, 59, 11838–11845. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Tang, Z.; Chen, H.; Ren, Z.; Ding, Q.; Liang, K.; Sun, Z. Mutual interaction between gut microbiota and protein/amino acid metabolism for host mucosal immunity and health. Anim. Nutr. 2021, 7, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Duff, C.; Baruteau, J. Modelling urea cycle disorders using iPSCs. npj Regen. Med. 2022, 7, 56. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Davila, A.M.; Blachier, F.; Gotteland, M.; Andriamihaja, M.; Benetti, P.H.; Sanz, Y.; Tomé, D. Intestinal luminal nitrogen metabolism: Role of the gut microbiota and consequences for the host. Pharmacol. Res. 2013, 68, 95–107. [Google Scholar] [CrossRef]
- Hughes, R.; Kurth, M.J.; McGilligan, V.; McGlynn, H.; Rowland, I. Effect of colonic bacterial metabolites on Caco-2 cell paracellular permeability in vitro. Nutr. Cancer 2008, 60, 259–266. [Google Scholar] [CrossRef]
- He, X.; Parenti, M.; Grip, T.; Lönnerdal, B.; Timby, N.; Domellöf, M.; Hernell, O.; Slupsky, C.M. Fecal microbiome and metabolome of infants fed bovine MFGM supplemented formula or standard formula with breast-fed infants as reference: A randomized controlled trial. Sci. Rep. 2019, 9, 11589. [Google Scholar] [CrossRef]
- Mohiuddin, S.S.; Khattar, D. Biochemistry, Ammonia; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kalaitzakis, E.; Olsson, R.; Henfridsson, P.; Hugosson, I.; Bengtsson, M.; Jalan, R.; Björnsson, E. Malnutrition and diabetes mellitus are related to hepatic encephalopathy in patients with liver cirrhosis. Liver Int. 2007, 27, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.H.; Zuo, D.M. Oxidative deamination of methylamine by semicarbazide-sensitive amino oxidase leads to cytotoxic damage in endothelial cells. Diabetes 1993, 42, 594–603. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Lolla, L. Proteomic analysis of red blood cells and the potential for the clinic: What have we learned so far? Expert Rev. Proteom. 2017, 14, 243–252. [Google Scholar] [CrossRef]
- Li, J.J.; Voisin, D.; Quiquerez, A.L.; Bouras, C. Differential expression of advanced glycosylation end-products in neurons of different species. Brain Res. 1994, 641, 285–288. [Google Scholar] [CrossRef]
- Low, S.Y.; Taylor, P.M.; Hundal, H.S.; Pogson, C.I.; Rennie, M.J. Transport of L-glutamine and L-glutamate across sinusoidal membranes of rat liver. Biochem. J. 1992, 284 Pt 2, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ruppin, H.; Bar-Meir, S.; Soergel, K.H.; Wood, C.M.; Schmitt, M.G., Jr. Absorption of short-chain fatty acids by the colon. Gastroenterology 1980, 78, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-Chain Fatty Acids and Human Colonic Function: Roles of Resistant Starch and Nonstarch Polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Miller, S.J. Cellular and physiological effects of short-chain fatty acids. Mini. Rev. Med. Chem. 2004, 4, 839–845. [Google Scholar] [CrossRef]
- Wang, L.; Cen, S.; Wang, G.; Lee, Y.K.; Zhao, J.; Zhang, H.; Chen, W. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of constipation. J. Funct. Foods 2020, 69, 103953. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Bäckhed, F. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar]
- McNeil, N.I. The contribution of the large intestine to energy supplies in man. Am. J. Clin. Nutr. 1984, 39, 338–342. [Google Scholar]
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Fellows, R.; Denizot, J.; Stellato, C.; Cuomo, A.; Jain, P.; Stoyanova, E.; Balázsi, S.; Hajnády, Z.; Liebert, A.; Kazakevych, J.; et al. Microbiota derived short chain fatty acids promote histone crotonylation in the colon through histone deacetylases. Nat. Commun. 2018, 9, 105. [Google Scholar] [CrossRef]
- Huang, W.; Zhou, L.; Guo, H.; Xu, Y.; Xu, Y. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism 2017, 68, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-Z.; Tao, S.-B.; Ma, L.; Fu, P. Roles of short-chain fatty acids in kidney diseases. Chin. Med. J. 2019, 132, 1228–1232. [Google Scholar] [CrossRef]
- Lin, H.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and Propionate Protect against Diet-Induced Obesity and Regulate Gut Hormones via Free Fatty Acid Receptor 3-Independent Mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z. Research progress in intestinal microbiota and type 2 diabetes mellitus. Chin. J. Microecol. 2019, 31, 866–868. [Google Scholar]
- Scheithauer, T.P.M.; Rampanelli, E.; Nieuwdorp, M.; Vallance, B.A.; Verchere, C.B.; van Raalte, D.H.; Herrema, H. Gut Microbiota as a Trigger for Metabolic Inflammation in Obesity and Type 2 Diabetes. Front. Immunol. 2020, 16, 571731. [Google Scholar] [CrossRef]
- Knip, M. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol 2016, 12, 54–67. [Google Scholar] [CrossRef]
- Leite, A.Z.; Rodrigues, N.C.; Gonzaga, M.I.; Paiolo, J.C.C.; de Souza, C.A.; Stefanutto, N.A.V.; Omori, W.P.; Pinheiro, D.G.; Brisotti, J.L.; Matheucci Junior, E.; et al. Detection of Increased Plasma Interleukin-6 Levels and Prevalence of Prevotella copri and Bacteroides vulgatus in the Feces of Type 2 Diabetes Patients. Front. Immunol. 2017, 15, 1107. [Google Scholar] [CrossRef]
- Plassais, J.; Gbikpi-Benissan, G.; Figarol, M.; Scheperjans, F.; Gorochov, G.; Derkinderen, P.; Cervino, A.C.L. Gut microbiome alpha-diversity is not a marker of Parkinson’s disease and multiple sclerosis. Brain Commun. 2021, 3, fcab113. [Google Scholar] [CrossRef]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic gut microbiota dysbiosis as an inflammaging and immunosenescence condition that fosters progression of retinopathy and nephropathy. Biochim. Biophys. Acta Mol. Basis. Dis. 2019, 1865, 1876–1897. [Google Scholar] [CrossRef]
- Lee, C.B.; Chae, S.U.; Jo, S.J.; Jerng, U.M.; Bae, S.K. The Relationship between the Gut Microbiome and Metformin as a Key for Treating Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2021, 22, 3566. [Google Scholar] [CrossRef]
- Sabatino, A.; Regolisti, G.; Cosola, C.; Gesualdo, L.; Fiaccadori, E. Intestinal Microbiota in Type 2 Diabetes and Chronic Kidney Disease. Curr. Diabetes Rep. 2017, 17, 16. [Google Scholar] [CrossRef]
- Lippert, K.; Kedenko, L.; Antonielli, L.; Kedenko, I.; Gemeier, C.; Leitner, M.; Kautzky-Willer, A.; Paulweber, B.; Hackl, E. Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults. Benef. Microbes 2017, 8, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Plummer, E.; Bulach, D.; Carter, G.; Albert, M.J. Gut microbiome of native Arab Kuwaitis. Gut Pathog. 2020, 26, 10. [Google Scholar] [CrossRef]
- Shetty, S.A.; Marathe, N.P.; Lanjekar, V.; Ranade, D.; Shouche, Y.S. Comparative genome analysis of Megasphaera sp. reveals niche specialization and its potential role in the human gut. PLoS ONE. 2013, 18, e79353. [Google Scholar] [CrossRef]
- Shivaji, S. A systematic review of gut microbiome and ocular inflammatory diseases: Are they associated? Indian J. Ophthalmol. 2021, 69, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, M.; Wang, Y.; Dorfman, R.G.; Liu, H.; Yu, T.; Chen, X.; Tang, D.; Xu, L.; Yin, Y.; et al. Faecalibacterium prausnitzii Produces Butyrate to Maintain Th17/Treg Balance and to Ameliorate Colorectal Colitis by Inhibiting Histone Deacetylase 1. Inflamm. Bowel Dis. 2018, 24, 1926–1940. [Google Scholar] [CrossRef] [PubMed]
- De Andrés, J.; Manzano, S.; García, C.; Rodríguez, J.M.; Espinosa-Martos, I.; Jiménez, E. Modulatory effect of three probiotic strains on infants’ gut microbial composition and immunological parameters on a placebo-controlled, double-blind, randomised study. Benef. Microbes 2018, 9, 573–584. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Belzer, C.; Goossens, M.; Kleerebezem, M.; De Vos, W.M.; Thas, O.; De Weirdt, R.; Kerckhof, F.M.; Van de Wiele, T. Butyrate-producing Clostridium cluster XIVa species specifically colonize mucins in an in vitro gut model. ISME J. 2013, 7, 949–961. [Google Scholar] [CrossRef]
- Blatchford, P.; Stoklosinski, H.; Eady, S.; Wallace, A.; Butts, C.; Gearry, R.; Gibson, G.; Ansell, J. Consumption of kiwifruit capsules increases Faecalibacterium prausnitzii abundance in functionally constipated individuals: A randomised controlled human trial. J. Nutr. Sci. 2017, 6, e52. [Google Scholar] [CrossRef]
- Sokol, H.; Seksik, P.; Furet, J.P.; Firmesse, O.; Nion-Larmurier, I.; Beaugerie, L.; Cosnes, J.; Corthier, G.; Marteau, P.; Doré, J. Low counts of Faecalibacterium prausnitzii in colitis microbiota. Inflamm. Bowel Dis. 2009, 15, 1183–1189. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Forslund, K.; Hildebrand, F.; Nielsen, T.; Falony, G.; Le Chatelier, E.; Sunagawa, S.; Prifti, E.; Vieira-Silva, S.; Gudmundsdottir, V.; Pedersen, H.K.; et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 2015, 528, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fang, Z.; Zhang, C.; Xia, H.; Jie, Z.; Han, X.; Chen, Y.; Ji, L. Effects of Acarbose on the Gut Microbiota of Prediabetic Patients: A Randomized, Double-blind, Controlled Crossover Trial. Diabetes Ther. 2017, 8, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Stärkel, P.; Windey, K.; Tremaroli, V.; Bäckhed, F.; Verbeke, K.; et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef]
- Abusleme, L.; Dupuy, A.K.; Dutzan, N.; Silva, N.; Burleson, J.A.; Strausbaugh, L.D.; Gamonal, J.; Diaz, P.I. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013, 7, 1016–1025. [Google Scholar] [CrossRef]
- Shi, T.T.; Xin, Z.; Hua, L.; Wang, H.; Zhao, R.X.; Yang, Y.L.; Xie, R.R.; Liu, H.Y.; Yang, J.K. Comparative assessment of gut microbial composition and function in patients with Graves’ disease and Graves’ orbitopathy. J. Endocrinol. Investig. 2021, 44, 297–310. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2021, 14, 166. [Google Scholar] [CrossRef]
- Kunasegaran, T.; Balasubramaniam, V.R.M.T.; Arasoo, V.J.T.; Palanisamy, U.D.; Ramadas, A. The Modulation of Gut Microbiota Composition in the Pathophysiology of Gestational Diabetes Mellitus: A Systematic Review. Biology 2021, 10, 1027. [Google Scholar] [CrossRef] [PubMed]
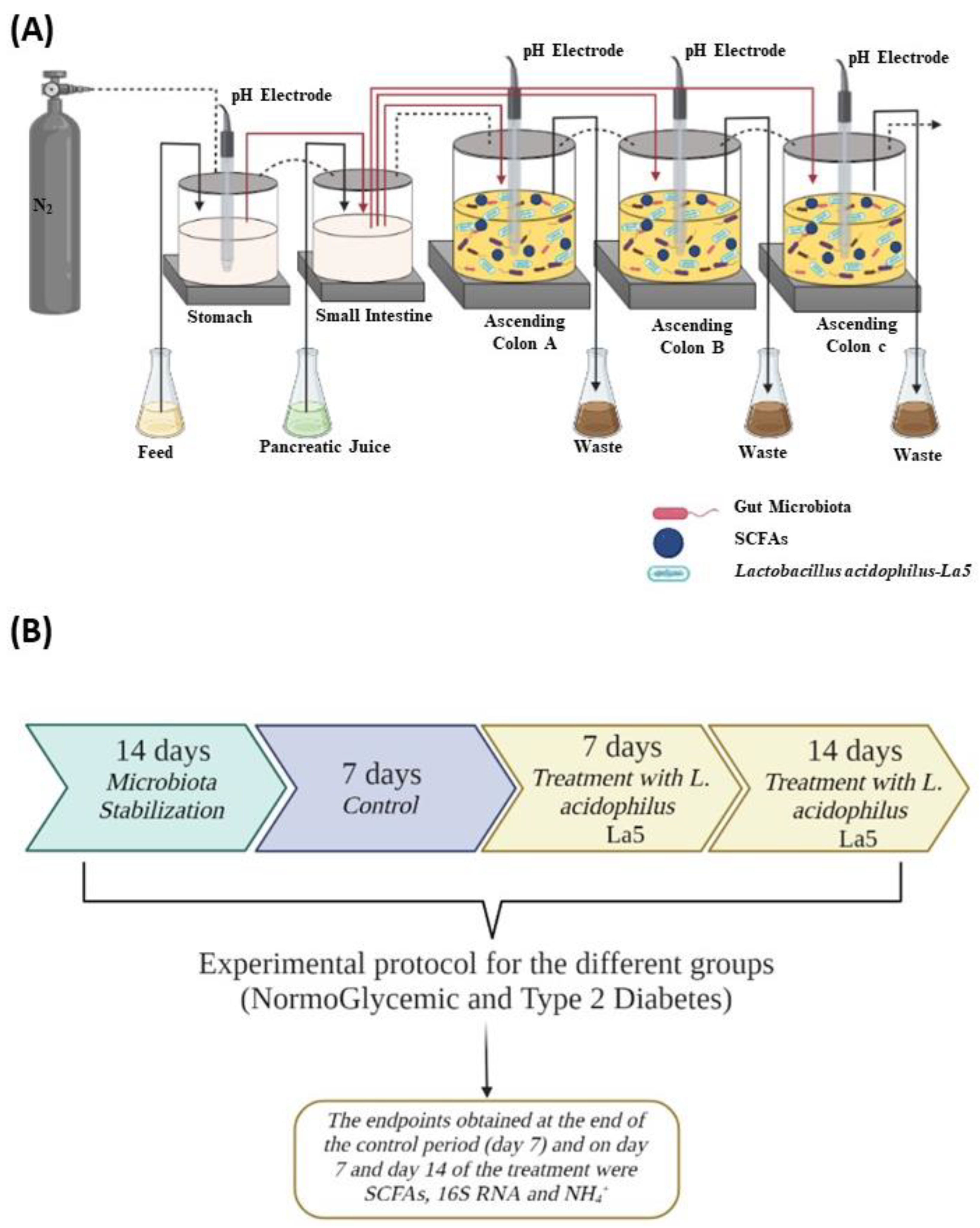

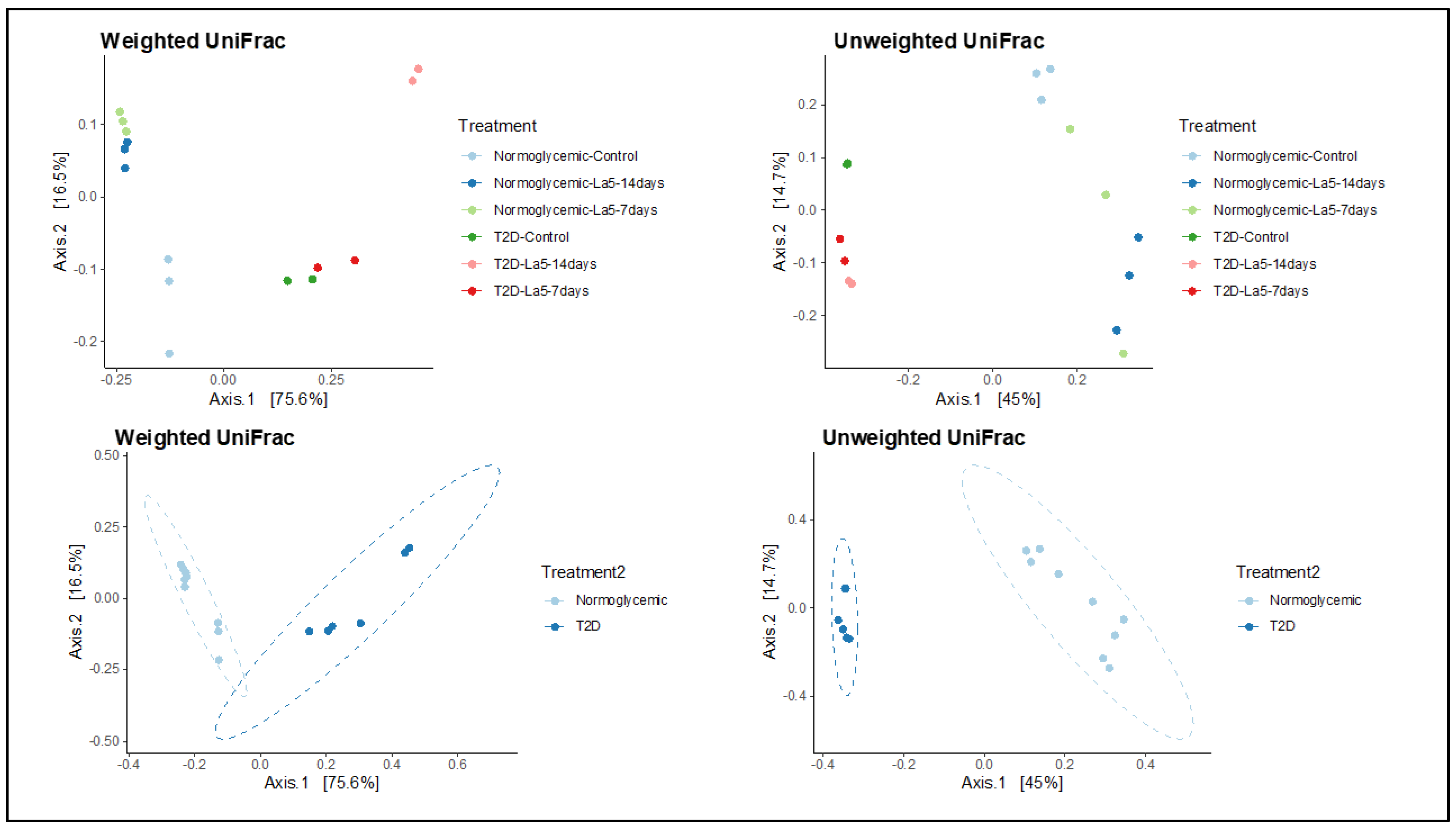

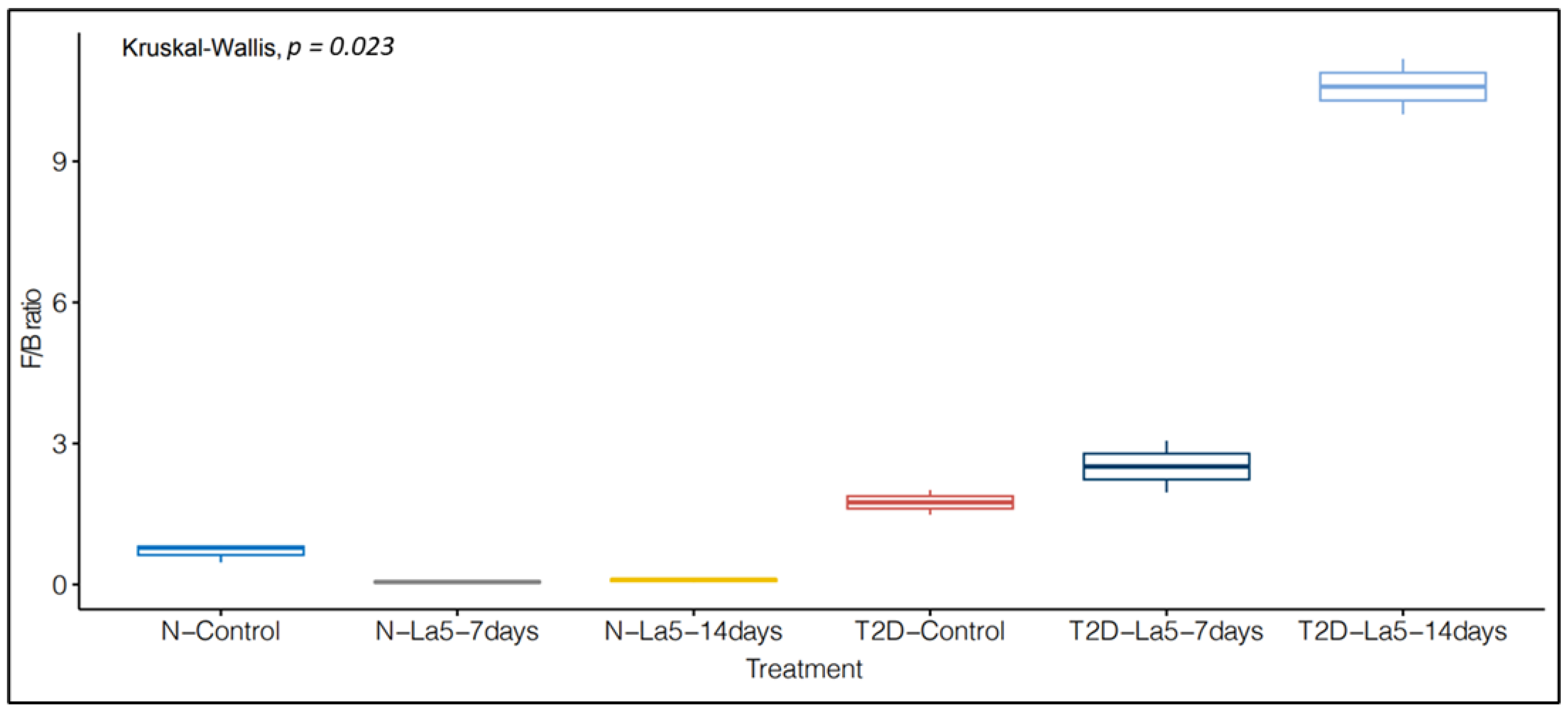
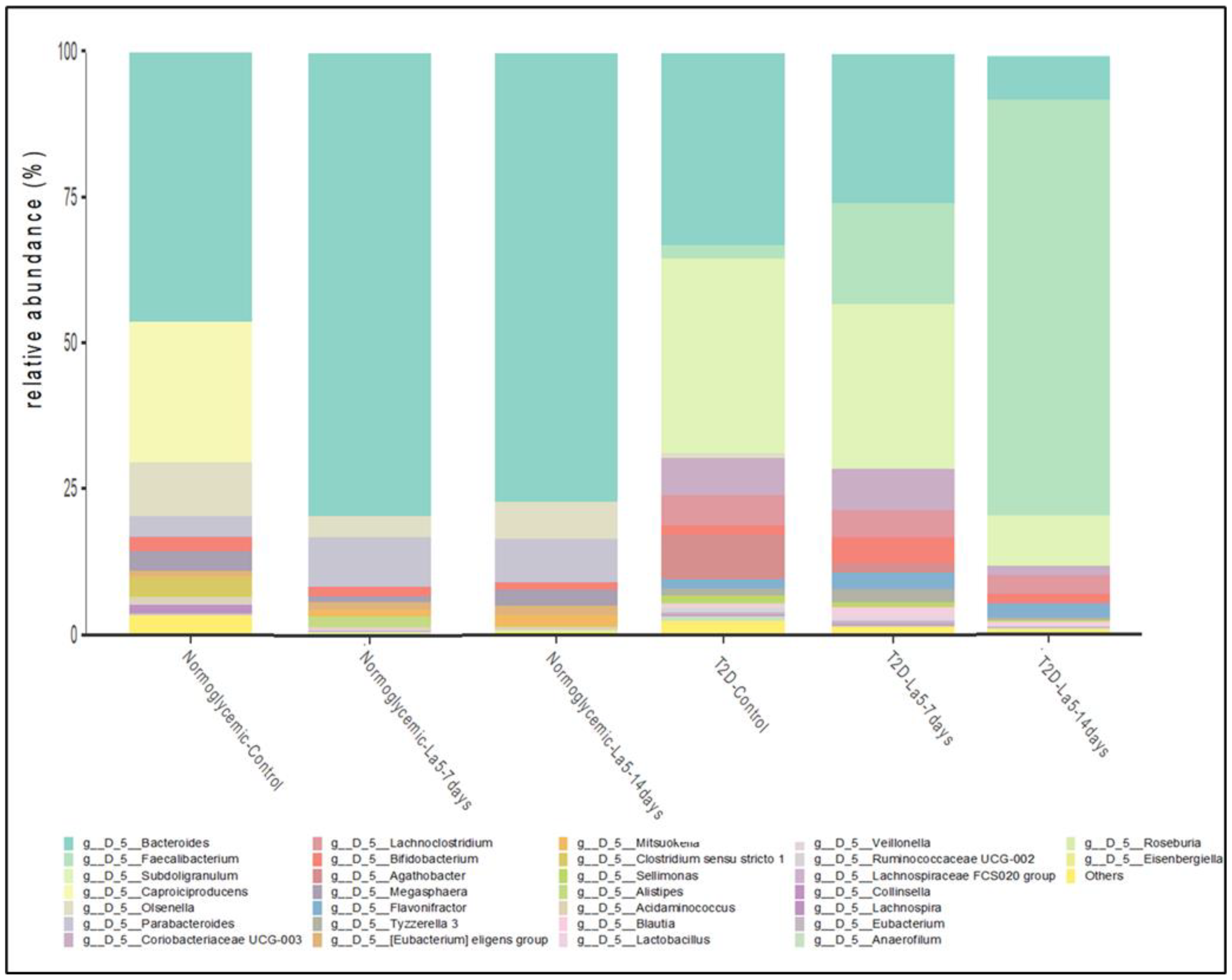
| NormoGlycemic | Type 2 Diabetes | |
|---|---|---|
| Initial Time | 7.98 a ± 0.08 | 8.34 a ± 0.07 |
| Gastric phase | 4.34 b ± 0.03 | 4.54 b ± 0.06 |
| Enteric phase | 4.18 b ± 0.05 | 4.97 b ± 0.04 |
| NormoGlycemic-Group | Type 2 Diabetic Group | |
|---|---|---|
| Control | 529.33 ± 47.64 a | 491.67 ± 2.52 a |
| La 5–7 Days | 255.33 ± 37.81 b | 222.00 ± 2.65 b |
| La 5–14 Days | 246.02 ± 40.70 b | 201.67 ± 3.22 c |
| (mmol/L) | ||||
|---|---|---|---|---|
| Acetic Acid | Propionic Acid | Butyric Acid | ||
| NormoGlycemic Group | NormoGlycemic-Control | 22.42 ± 0.96 b | 7.15 ± 0.69 b | 12.00 ± 3.49 a |
| NormoGlycemic-La 5–7 Days | 13.57 ± 1.30 c | 4.91 ± 0.15 c | 6.48 ± 1.03 b | |
| NormoGlycemic-La 5–14 Days | 24.71 ± 1.79 a | 9.25 ± 1.31 a | 8.78 ± 1.35 a | |
| Type 2 Diabetics Group | T2D-Control | 39.59 ± 3.31 a | 5.73 ± 0.51 a | 4.08 ± 1.30 c |
| T2D-La 5–7 Days | 21.91 ± 1.25 b | 2.15 ± 0.20 b | 4.90 ± 0.31 b | |
| T2D-La 5–14 Days | 20.94 ± 0.65 b | 2.26 ± 0.20 b | 8.56± 0.12 a | |
| Chao1 | Shannon | ||
|---|---|---|---|
| NormoGlycemic Group | NormoGlycemic-Control | 53.00 | 2.38 |
| NormoGlycemic-La 5–7 Days | 50.00 | 2.41 | |
| NormoGlycemic-La 5–14 Days | 36.00 | 2.37 | |
| Type 2 Diabetics Group | T2D-Control | 50.00 | 2.28 |
| T2D-La 5–7 Days | 51.00 | 2.32 | |
| T2D-La 5–14 Days | 44.00 | 2.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgaço, M.K.; de Oliveira, F.L.; Sartoratto, A.; Mesa, V.; Mayer, M.P.A.; Sivieri, K. Impact of Lactobacillus acidophilus—La5 on Composition and Metabolism of the Intestinal Microbiota of Type 2 Diabetics (T2D) and Healthy Individuals Using a Microbiome Model. Fermentation 2023, 9, 740. https://doi.org/10.3390/fermentation9080740
Salgaço MK, de Oliveira FL, Sartoratto A, Mesa V, Mayer MPA, Sivieri K. Impact of Lactobacillus acidophilus—La5 on Composition and Metabolism of the Intestinal Microbiota of Type 2 Diabetics (T2D) and Healthy Individuals Using a Microbiome Model. Fermentation. 2023; 9(8):740. https://doi.org/10.3390/fermentation9080740
Chicago/Turabian StyleSalgaço, Mateus Kawata, Fellipe Lopes de Oliveira, Adilson Sartoratto, Victoria Mesa, Marcia Pinto Alves Mayer, and Katia Sivieri. 2023. "Impact of Lactobacillus acidophilus—La5 on Composition and Metabolism of the Intestinal Microbiota of Type 2 Diabetics (T2D) and Healthy Individuals Using a Microbiome Model" Fermentation 9, no. 8: 740. https://doi.org/10.3390/fermentation9080740
APA StyleSalgaço, M. K., de Oliveira, F. L., Sartoratto, A., Mesa, V., Mayer, M. P. A., & Sivieri, K. (2023). Impact of Lactobacillus acidophilus—La5 on Composition and Metabolism of the Intestinal Microbiota of Type 2 Diabetics (T2D) and Healthy Individuals Using a Microbiome Model. Fermentation, 9(8), 740. https://doi.org/10.3390/fermentation9080740








