Effect of Condensed Tannins on Nitrogen Distribution and Metabolome after Aerobic Exposure of Sainfoin Silage
Abstract
1. Introduction
2. Materials and Methods
2.1. Silage Preparation
2.2. Nitrogen Content Analysis of Sainfoin Silage
2.3. Metabolite Analysis
2.4. Statistical Analysis
3. Results
3.1. Nitrogen Distribution in Sainfoin Silage upon Aerobic Exposure
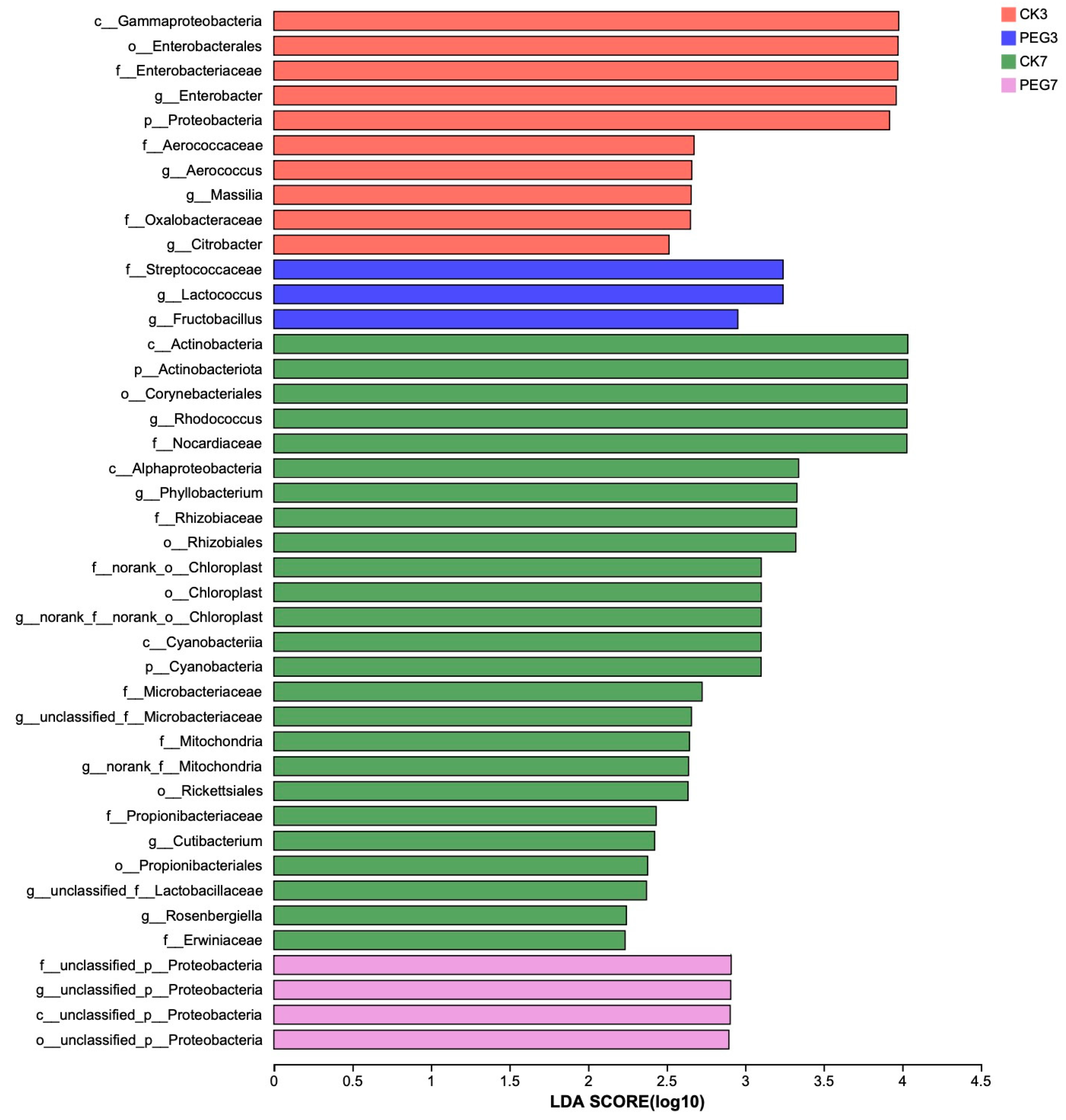
3.2. Bacterial Community Structure of Sainfoin Silage upon Aerobic Exposure
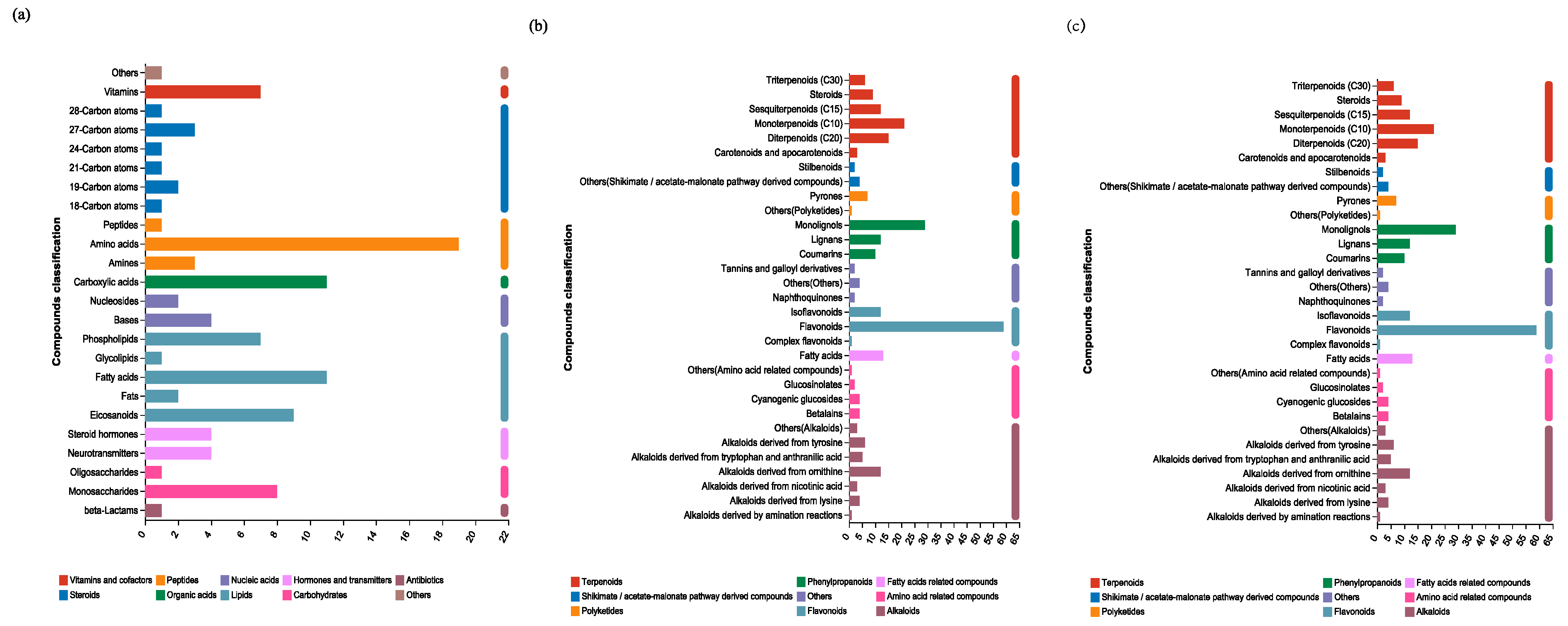
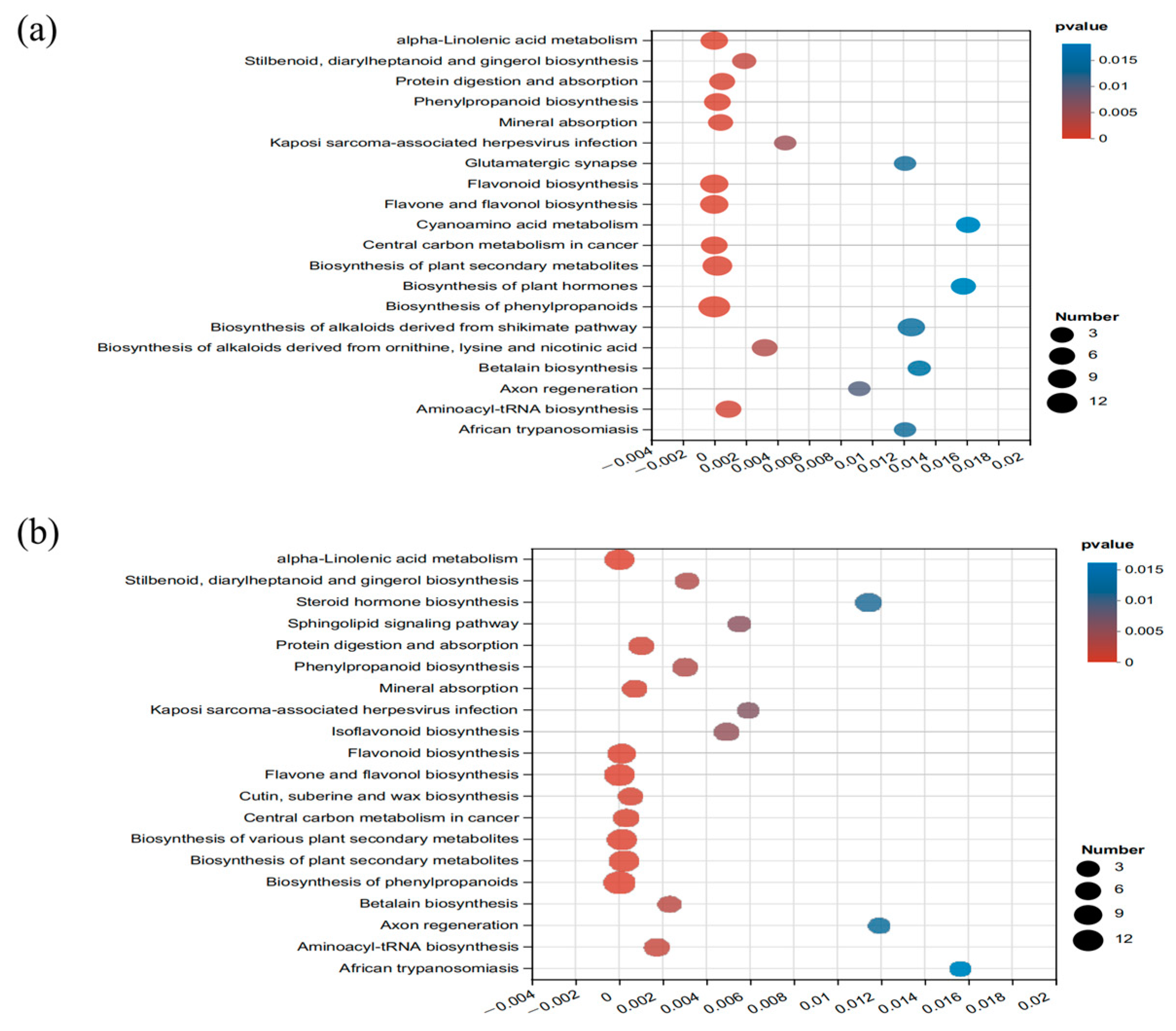
3.3. Metabolomic Profiles
3.4. Correlation between Bacteria Relative Abundance and Metabolites
4. Discussion
4.1. Nitrogen Distribution after Aerobic Exposure of Sainfoin Silage
4.2. Metabolite Analysis after Aerobic Exposure of Sainfoin Silage
4.3. Correlation Analysis between Bacteria and Metabolites after Aerobic Exposure of Sainfoin Silage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonald, P.; Henderson, A.R.; Herson, S. The Biochemistry of Silage; Chalcombe Publications: Kingston, UK, 1991. [Google Scholar]
- Jones, B.A.; Hatfield, R.D.; Muck, R.E. Characterization of Proteolysis in Alfalfa and Red-Clover. Crop Sci. 1995, 35, 537–541. [Google Scholar] [CrossRef]
- Wetherall, J.A.; Armstrong, D.G.; Finlayson, H.J.; Rooke, J.A. Reduction of Proteolysis during Ensilage of Perennial Ryegrass by Protease Inhibitors. J. Sci. Food Agric. 1995, 68, 497–505. [Google Scholar] [CrossRef]
- Wang, Y.; Barbier, L.R.; Berg, B.P.; McAllister, T.A. Effects of Mixing Sainfoin with Alfalfa on Ensiling, Ruminal Fermentation and Total Tract Digestion of Silage. Anim. Feed Sci. Technol. 2007, 135, 296–314. [Google Scholar] [CrossRef]
- Zeller, W.E. Activity, Purification, and Analysis of Condensed Tannins: Current State of Affairs and Future Endeavors. Crop Sci. 2019, 59, 886–904. [Google Scholar] [CrossRef]
- Marais, J.P.J.; Mueller-Harvey, I.; Vincent Brandt, E.; Ferreira, D. Polyphenols, Condensed Tannins, and Other Natural Products in Onobrychis viciifolia (Sainfoin). J. Agric. Food Chem. 2000, 48, 3440–3447. [Google Scholar] [CrossRef] [PubMed]
- McAllister, T.A.; Martinez, T.; Bae, H.; Muir, A.; Yanke, L.; Jones, G. Characterization of Condensed Tannins Purified from Legume Forages: Chromophore Production, Protein Precipitation, and Inhibitory Effects on Cellulose Digestion. J. Chem. Ecol. 2005, 31, 2049–2068. [Google Scholar] [CrossRef]
- Lorenz, M.M.; Eriksson, T.; Uden, P. Effect of Wilting, Silage Additive, PEG Treatment and Tannin Content on the Distribution of N between Different Fractions after Ensiling of Three Different Sainfoin (Onobrychis viciifolia). Grass Forage Sci. 2010, 65, 175–184. [Google Scholar] [CrossRef]
- Wang, M.S.; Chen, M.Y.; Bai, J.; Zhang, J.Y.; Su, R.; Franco, M.; Ding, Z.T.; Zhang, X.; Zhang, Y.; Guo, X.S. Ensiling Characteristics, in Vitro Rumen Fermentation Profile, Methane Emission and Archaeal and Protozoal Community of Silage Prepared with Alfalfa, Sainfoin and Their Mixture. Anim. Feed Sci. Technol. 2022, 284, 115154. [Google Scholar] [CrossRef]
- Peng, K.; Jin, L.; Niu, Y.D.; Huang, Q.; McAllister, T.A.; Yang, H.E.; Denise, H.; Xu, Z.; Acharya, S.; Wang, S.; et al. Condensed Tannins Affect Bacterial and Fungal Microbiomes and Mycotoxin Production during Ensiling and upon Aerobic Exposure. Appl. Environ. Microbiol. 2018, 84, e02274. [Google Scholar] [CrossRef]
- Bueno, A.V.I.; Jobim, C.C.; Daniel, J.L.P.; Gierus, M. Fermentation Profile and Hygienic Quality of Rehydrated Corn Grains Treated with Condensed Tannins from Quebracho Plant Extract. Anim. Feed Sci. Technol. 2020, 267, 114559. [Google Scholar] [CrossRef]
- Huang, R.Z.; Wang, X.Z.; Ma, C.H.; Zhang, F.F. Effects of Intrinsic Tannins on Proteolysis Dynamics, Protease Activity, and Metabolome during Sainfoin Ensiling. Front. Microbiol. 2022, 13, 976118. [Google Scholar] [CrossRef] [PubMed]
- Reich, L.J.; Kung, L.M. Effects of Combining Lactobacillus buchneri 40788 with Various Lactic Acid Bacteria on the Fermentation and Aerobic Stability of Corn Silage. Anim. Feed Sci. Technol. 2010, 159, 105–109. [Google Scholar] [CrossRef]
- Theodoridou, K.; Aufrere, J.; Andueza, D.; Le Morvan, A.; Picard, F.; Pourrat, J.; Baumont, R. Effects of Condensed Tannins in Wrapped Silage Bales of Sainfoin (Onobrychis viciifolia) on In Vivo and In Situ Digestion in Sheep. Animal 2012, 6, 245–253. [Google Scholar] [CrossRef]
- Guan, H.; Shuai, Y.; Ran, Q.F.; Yan, Y.H.; Wang, X.; Li, D.D.; Cai, Y.M.; Zhang, X.Q. The Microbiome and Metabolome of Napier Grass Silages Prepared with Screened Lactic Acid Bacteria during Ensiling and Aerobic Exposure. Anim. Feed Sci. Technol. 2020, 269, 114673. [Google Scholar] [CrossRef]
- MAKKAR, H.; BLUMMEL, M.; BECKER, K. Formation of Complexes between Polyvinyl Pyrrolidones or Polyethylene Glycols and Tannins, and Their Implication in Gas Production and True Digestibility in in Vitro Techniques. Br. J. Nutr. 1995, 73, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of Procedures for Nitrogen Fractionation of Ruminant Feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Huang, R.Z.; Zhang, F.F.; Wang, T.; Zhang, Y.L.; Li, X.; Chen, Y.C.; Ma, C.H. Effect of Intrinsic Tannins on the Fermentation Quality and Associated with the Bacterial and Fungal Community of Sainfoin Silage. Microorganisms 2022, 10, 844. [Google Scholar] [CrossRef]
- Epifanio, N.M.D.; Cavalcanti, L.R.I.; dos Santos, K.F.; Duarte, P.S.C.; Kachlicki, P.; Ozarowski, M.; Riger, C.J.; Chaves, D.S.D. Chemical Characterization Andin Vivoantioxidant Activity of Parsley (Petroselinum crispum) Aqueous Extract. Food Funct. 2020, 11, 5346–5456. [Google Scholar] [CrossRef]
- Hua, F.; Zhou, P.; Bao, G.H.; Ling, T.J. Flavonoids in Lu’an GuaPian Tea as Potential Inhibitors of TMA-Lyase in Acute Myocardial Infarction. J. Food Biochem. 2022, 46, e14110. [Google Scholar] [CrossRef]
- Cedeno, H.; Espinosa, S.; Malagon, Q. Novel Flavonoid Glycosides of Quercetin from Leaves and Flowers of Gaiadendron punctatum G.Don. (Violeta de Campo), Used by the Saraguro Community in Southern Ecuador, Inhibit α-Glucosidase Enzyme. Molecules 2019, 24, 4267. [Google Scholar] [CrossRef] [PubMed]
- Scieszka, S.; Klewicka, E. Influence of the Microalga Chlorella Vulgaris on the Growth and Metabolic Activity of Lactobacillus Spp. Bacteria. Foods 2020, 9, 959. [Google Scholar] [CrossRef] [PubMed]
- Moura, I.B.; Buckley, A.M.; Ewin, D.; Shearman, S.; Clark, E.; Wilcox, M.H.; Chilton, C.H. Omadacycline Gut Microbiome Exposure Does Not Induce Clostridium Difficile Proliferation or Toxin Production in a Model That Simulates the Proximal, Medial, and Distal Human Colon. Antimicrob. Agents Chemother. 2019, 63, e01581-18. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial Membrane Lipids: Diversity in Structures and Pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef] [PubMed]
- Avitabile, C.; Netti, F.; Orefice, G.; Palmieri, M.; Nocerino, N.; Malgieri, G.; D’Andrea, L.D.; Capparelli, R.; Fattorusso, R.; Romanelli, A. Design, Structural and Functional Characterization of a Temporin-1b Analog Active against Gram-Negative Bacteria. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 3767–3775. [Google Scholar] [CrossRef]
- Vera, M.D.; Joullie, M.M. Natural Products as Probes of Cell Biology: 20 Years of Didemnin Research. Med. Res. Rev. 2002, 22, 102–145. [Google Scholar] [CrossRef]
- Guo, X.; Zhou, H.; Yu, Z.; Zhang, Y. Changes in the Distribution of Nitrogen and Plant Enzymatic Activity during Ensilage of Lucerne Treated with Different Additives. Grass Forage Sci. 2007, 62, 35–43. [Google Scholar] [CrossRef]
- Penna, T.C.V.; Moraes, D.A. Optimization of Nisin Production by Lactococcus Lactis. Appl. Biochem. Microbiol. 2002, 98, 775–789. [Google Scholar] [CrossRef]
- Marugg, J.D.; Meijer, W.; Vankranenburg, R.; Laverman, P.; Bruinenberg, P.G.; Devos, W.M. Medium-Dependent Regulation of Proteinase Gene Expression in Lactococcus Lactis: Control of Transcription Initiation by Specific Dipeptides. J. Bacteriol. 1995, 177, 2982–2989. [Google Scholar] [CrossRef][Green Version]
- Mu, Q.X.; Shi, Y.N.; Li, R.S.; Ma, C.; Tao, Y.; Yu, B. Production of Propionate by a Sequential Fermentation-Biotransformation Process via L-Threonine. J. Agric. Food Chem. 2021, 69, 13895–13903. [Google Scholar] [CrossRef]
- Trip, H.; Mulder, N.L.; Lolkema, J.S. Cloning, Expression, and Functional Characterization of Secondary Amino Acid Transporters of Lactococcus Lactis. J. Bacteriol. 2013, 195, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Tlais, A.Z.A.; Rantsiou, K.; Filannino, P.; Cocolin, L.S.; Cavoski, I.; Gobbetti, M.; Di Cagno, R. Ecological Linkages between Biotechnologically Relevant Autochthonous Microorganisms and Phenolic Compounds in Sugar Apple Fruit (Annona squamosa L.). Int. J. Food Microbiol. 2023, 387, 110057. [Google Scholar] [CrossRef] [PubMed]
- Li, R.R.; Zheng, M.L.; Zheng, M.H.; Cai, R.; Cui, X.Y.; Wang, Y.; Jiang, X.; Xu, C.C. Metagenomic Analysis Reveals the Linkages between Bacteria and the Functional Enzymes Responsible for Potential Ammonia and Biogenic Amine Production in Alfalfa Silage. J. Appl. Microbiol. 2021, 132, 2594–2604. [Google Scholar] [CrossRef]
- Zong, C.; Wu, Q.F.; Wu, A.L.; Chen, S.F.; Dong, D.; Zhao, J.; Shao, T.; Liu, Q.H. Exploring the Diversity Mechanism of Fatty Acids and the Loss Mechanisms of Polyunsaturated Fatty Acids and Fat-Soluble Vitamins in Alfalfa Silage Using Different Additives. Anim. Feed Sci. Technol. 2021, 280, 115044. [Google Scholar] [CrossRef]



| Item | Treatment | Day of Ensiling | Day of Aerobic Exposure | SEM | p Value | |||
|---|---|---|---|---|---|---|---|---|
| 60 | 3 | 7 | Day | Treatment | D*T | |||
| CP (g/kg DM) | CK | 202.93 Aa | 193.77 Ab | 196.02 Ab | 1.407 | <0.01 | <0.01 | <0.01 |
| PEG | 202.11 Aa | 182.92 Bb | 183.72 Bb | |||||
| SOLP (g/kg DM) | CK | 5.00 Ba | 5.42 Ba | 5.57 Ba | 0.249 | 0.065 | <0.01 | 0.950 |
| PEG | 7.77 Aa | 8.04 Aa | 8.31 Aa | |||||
| NDIP (g/kg DM) | CK | 56.54 Ac | 64.04 Ab | 83.19 Aa | 3.944 | <0.01 | <0.01 | <0.01 |
| PEG | 25.36 Ba | 23.38 Ba | 23.68 Ba | |||||
| ADIP (g/kg DM) | CK | 22.57 Ab | 28.59 Aa | 27.99 Aa | 1.049 | <0.01 | <0.01 | 0.0171 |
| PEG | 12.75 Bb | 15.76 Ba | 16.55 Ba | |||||
| NPN (%TN) | CK | 42.74 Ba | 39.67 Bb | 31.67 Bc | 2.203 | <0.01 | <0.01 | <0.01 |
| PEG | 65.50 Aa | 62.57 Aa | 60.45 Ab | |||||
| AA-N (%TN) | CK | 13.39 Ba | 16.11 Aa | 8.19 Bb | 1.108 | <0.01 | <0.01 | <0.01 |
| PEG | 27.58 Aa | 23.07 Ab | 18.75 Ac | |||||
| AN (%TN) | CK | 2.06 Bb | 2.30 Ba | 1.02 Ac | 0.134 | <0.01 | <0.01 | <0.01 |
| PEG | 2.38 Ab | 3.22 Aa | 1.00 Ac | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, R.; Zhang, F.; Wang, X.; Ma, C. Effect of Condensed Tannins on Nitrogen Distribution and Metabolome after Aerobic Exposure of Sainfoin Silage. Fermentation 2023, 9, 739. https://doi.org/10.3390/fermentation9080739
Huang R, Zhang F, Wang X, Ma C. Effect of Condensed Tannins on Nitrogen Distribution and Metabolome after Aerobic Exposure of Sainfoin Silage. Fermentation. 2023; 9(8):739. https://doi.org/10.3390/fermentation9080739
Chicago/Turabian StyleHuang, Rongzheng, Fanfan Zhang, Xuzhe Wang, and Chunhui Ma. 2023. "Effect of Condensed Tannins on Nitrogen Distribution and Metabolome after Aerobic Exposure of Sainfoin Silage" Fermentation 9, no. 8: 739. https://doi.org/10.3390/fermentation9080739
APA StyleHuang, R., Zhang, F., Wang, X., & Ma, C. (2023). Effect of Condensed Tannins on Nitrogen Distribution and Metabolome after Aerobic Exposure of Sainfoin Silage. Fermentation, 9(8), 739. https://doi.org/10.3390/fermentation9080739






