Valorization of Mexican Rambutan Peel through the Recovery of Ellagic Acid via Solid-State Fermentation Using a Yeast
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Microorganisms
2.3. Material Characterization
2.3.1. Water-Absorption Index and Maximum Moisture
2.3.2. Adaptation of the Microorganisms on Rambutan Peel
2.3.3. Determination of the Time of Maximum Accumulation of Ellagic Acid via Solid-State Fermentation
2.3.4. Selection of the Best Conditions
2.3.5. Determination of Total Polyphenols
2.3.6. Antioxidant Activity
ABTS●+ Assay
DPPH● Assay
2.3.7. Ellagic Acid HPLC/ESI/MS Analysis
2.4. Statistical Analysis
3. Results
3.1. Evaluated Parameters of Rambutan Peel
3.2. Growth of Microorganisms on Rambutan Peel
3.3. Solid-State Fermentation
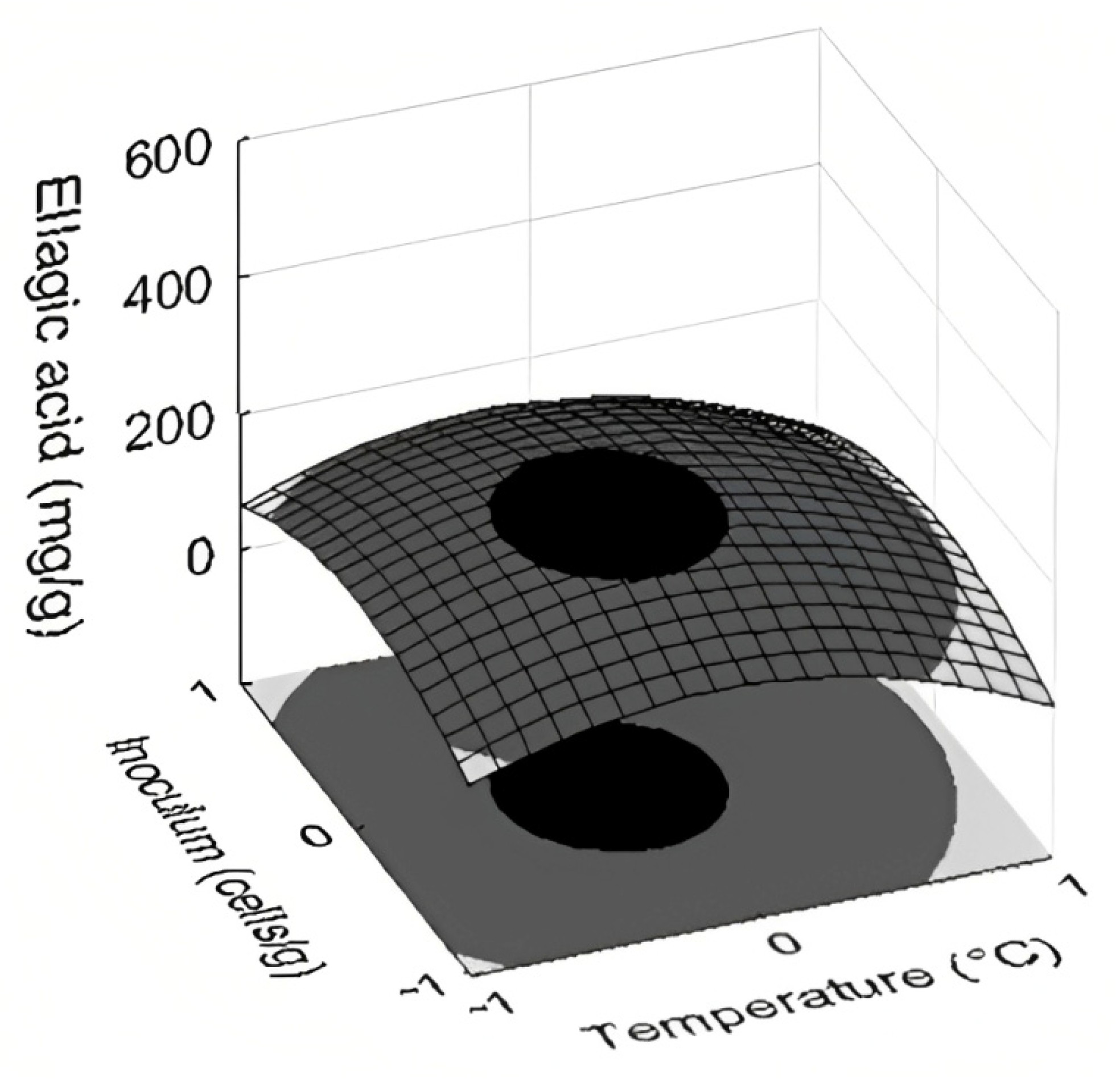
3.4. Selection of the Best Conditions and Statical Analysis
3.5. Identification of Ellagic Acid via HPLC/ESI/MS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Niño, W.R.; Zazueta, C. Ellagic acid: Pharmacological activities and molecular mechanisms involved in liver protection. Pharmacol. Res. 2015, 97, 84–103. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Rajasekar, N.; Sivanantham, A. Therapeutic potential of plant-derived tannins in non-malignant respiratory diseases. J. Nutr. Biochem. 2021, 94, 108632. [Google Scholar] [CrossRef] [PubMed]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions, and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Rønning, S.B.; Voldvik, V.; Bergum, S.K.; Aaby, K.; Borge, G.I.A. Ellagic acid and urolithin A modulate the immune response in LPS-stimulated U937 monocytic cells and THP-1 differentiated macrophages. Food Funct. 2020, 11, 7946–7959. [Google Scholar] [CrossRef] [PubMed]
- Bilawal, A.; Ishfaq, M.; Gantumur, M.A.; Qayum, A.; Shi, R.; Fazilani, S.A.; Anwar, A.; Jiang, Z.; Hou, J. A review of the bioactive ingredients of berries and their applications in curing diseases. Food Biosci. 2021, 44, 101407. [Google Scholar] [CrossRef]
- Dhingra, D.; Jangra, A. Antiepileptic activity of ellagic acid, a naturally occurring polyphenolic compound, in mice. J. Funct. Foods 2014, 10, 364–369. [Google Scholar] [CrossRef]
- Verotta, L.; Panzella, L.; Antenucci, S.; Calvenzani, V.; Tomay, F.; Petroni, K.; Caneva, E.; Napolitano, A. Fermented pomegranate wastes as sustainable source of ellagic acid: Antioxidant properties, anti-inflammatory action, and controlled release under simulated digestion conditions. Food Chem. 2018, 246, 129–136. [Google Scholar] [CrossRef]
- Athiappan, M.; Srinivasan, S.; Anandan, R.; Rajaram, J. Novel Process of Ellagic Acid Synthesis from Waste Generated from Mango Pulp Processing Industries. In Emerging Technologies in Environmental Bioremediation; Shah, M.P., Rodriguez-Couto, S., Sevinç, Ş.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 443–454. [Google Scholar] [CrossRef]
- Méndez-Flores, A.; Hérnandez-Almanza, A.; Sáenz-Galindo, A.; Morlett-Chávez, J.; Aguilar, C.N.; Ascacio-Valdés, J. Ultrasound-assisted extraction of antioxidant polyphenolic compounds from Nephelium lappaceum L. (Mexican variety) husk. Asian Pac. J. Trop Med. 2018, 11, 676–681. [Google Scholar] [CrossRef]
- Hernández, C.; Ascacio-Valdés, J.; De la Garza, H.; Wong-Paz, J.; Aguilar, C.N.; Martínez-Avila, G.C.; Castro-Lopez, C.; Aguilera-Carbó, A. Polyphenolic content, in vitro antioxidant activity and chemical composition of extract from Nephelium lappaceum L. (Mexican rambutan) husk. Asian Pac. J. Trop Med. 2017, 10, 1201–1205. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Azzatul, F.S.; Sharifundin, M.S.; Norliza, M.J.; Hasmadi, M.; Lee, J.S.; Patricia, M.; Jinap, S.; Rahlam George, M.R.; Firoz Khan, M.; et al. Functional and nutritional proprieties of rambutan (Nephelium lappaceum L.) seed and its industrial application: A review. Trends Food Sci. Technol. 2020, 99, 367–374. [Google Scholar] [CrossRef]
- Peixoto Araujo, N.M.; Arruda, H.S.; Paixao Marques, D.R.; de Oliveira, W.Q.; Araujo Pereira, G.; Pastore, G.M. Functional and nutritional properties of selected Amazon fruits: A review. Food Res. Int. 2021, 147, 110520. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Pinela, J.; Días, M.I.; Pereira, C.; Petrovic, J.; Sokovic, M.; Calhelha, R.C.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R.; Barros, L. Valorization of rambutan (Nephelium lappaceum L.) peel: Chemical composition, biological activity, and optimized recovery of anthocyanins. Food Res. Int. 2023, 165, 112574. [Google Scholar] [CrossRef] [PubMed]
- De León-Medina, J.C.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Aguilar, C.N.; Ascacio-Valdés, J.A. Fungal biodegradation of ellagitannins extracted from rambutan peel. Food Bioprod. Process. 2023, 1–35. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Aguilar, C.N.; Rodríguez-Herrera, R.; Flores-Gallegos, A.C.; Morlett-Chavéz, J.; Govea-Salas, M.; Ascacio-Valdés, J.A. Rambutan (Nephelium lappaceum L.): Nutritional and functional properties. Trends Food Sci. Technol. 2019, 85, 201–210. [Google Scholar] [CrossRef]
- Abbas, M.; Ahmed, D.; Qamar, M.T.; Ihsan, S.; Noor, Z.I. Optimization of ultrasound-assisted, microwave-assisted and Soxhlet extraction of bioactive compounds from Lagenaria siceraria: A comparative analysis. Bioresour. Technol. Rep. 2021, 15, 100746. [Google Scholar] [CrossRef]
- Rubio, J.; Rodriguez, S. Extracción de compuestos bioactivos mediante pre- tratamiento con microondas de subproductos vitivinícolas. Valorización Raspón Uva. UdeV. 2018, 1, 15. [Google Scholar]
- Buenrostro-Figueroa, J.J.; Nevárez-Moorillón, G.V.; Chávez-González, M.L.; Sepúlveda, L.; Ascacio-Valdés, J.A.; Aguilar, C.N.; Pedroza-Islas, R.; Huerta-Ochoa, S.; Arely Prado-Barragán, L. Improved Extraction of High Value-Added Polyphenols from Pomegranate Peel by Solid-State Fermentation. Fermentation 2023, 9, 530. [Google Scholar] [CrossRef]
- Garro, M.S.; Rivas, F.P.; Garro, O.A. Solid State Fermentation in Food Processing: Advances in Reactor Design and Novel Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Yepes-Betancur, D.P.; Márquez-Cardozo, C.J.; Cadena-Chamorro, E.M.; Saldarriaga, J.M.; Torres-Léon, C.; Ascacio-Valdés, A.; Aguilar, C.N. Solid-state fermentation—Assisted extraction of bioactive compounds from hass avocado seeds. Food Bioprod. Process. 2021, 126, 155–163. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Moccia, F.; Flores-Gallegos, A.C.; Chávez-González, M.L.; Sepulveda, L.; Marzorati, S.; Verotta, L.; Panzella, L.; AscacioValdés, J.A.; Aguilar, C.N.; Napolitano, A. Ellagic acid recovery by solid-state fermentation of pomegranate wastes by aspergillus Niger and saccharomyces cerevisiae: A comparison. Molecules 2019, 24, 3698. [Google Scholar] [CrossRef] [PubMed]
- Robledo, A.; Aguilera-Carbó, A.; Rodriguez, R.; Martinez, J.L.; Garza, Y.; Aguilar, C.N. Ellagic acid production by Aspergillus niger in solid state fermentation of pomegranate residues. Soc. Ind. Microbiol. 2008, 35, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Cejudo, N.D.; Buenrostro-Figueroa, J.J.; Sepúlveda, L.; Torres-Leon, C.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Aguilar, C.N. Recovery of ellagic acid from Mexican rambutan peel by solid-state fermentation-assisted extraction. Food Bioprod. Process. 2022, 134, 86–94. [Google Scholar] [CrossRef]
- Estrada-Gil, L.; Contreras-Esquivel, J.C.; Flores-Gallegos, C.; Zugasti-Cruz, A.; Govea-Salas, M.; Mata-Gomez, M.A.; Rodriguez-Herrera, R.; Ascacio-Valdés, J.A. Recovery of Bioactive Ellagitannins by Ultrasound/Microwave-Assisted Extraction. Molecules 2022, 27, 1592. [Google Scholar] [CrossRef]
- Coutiño-Laguna, B.d.C.; Flores-Gallegos, A.C.; Ascacio-Valdés, J.A.; Iliná, A.; Sáenz-Galindo, A.; Castañeda-Facio, A.O.; Esparza-González, S.C.; Rodriguez-Herrera, R. Biocatalysis and agricultural biotechnology physicochemical and functional properties of the undervalued fruits of cactus Cylindropuntia imbricate (“xoconostle”) and antioxidant potential. Biocatal. Agric. Biotechnol. 2022, 39, 102245. [Google Scholar] [CrossRef]
- Espitia-Hernández, P.; Ruelas-Chacón, X.; Chávez-González, M.L.; Ascacio-Valdés, J.A.; Flores-Nevada, A.; Sepúlveda-Torre, L. Solid state fermentation of sorghum by Aspergillus oryzae and Aspergillus niger: Effects on tannin content, phenolic profile, and antioxidant Activity. Foods 2022, 11, 3121. [Google Scholar] [CrossRef]
- Ordoñez-Torres, A.; Torres-León, C.; Hernández-Almanza, A.; Flores-Guía, T.; Luque-Contreras, D.; Aguilar, C.N.; Ascacio-Valdés, J. Ultrasound-microwave-assisted extraction of polyphenolic compounds from Mexican “Ataulfo” mango peels: Antioxidant potential and identification by HPLC/ESI/MS. Phytochem. Anal. 2021, 32, 495–502. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Aguilar, C.N.; Rodrigues, L.R.; Teixeira, J.A. Colonization of Aspergillus japonicus on synthetic materials and application to the production of fructooligosaccharides. Carbohydr. Res. 2009, 344, 795–800. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larios-Cruz, R.; Buenrostro-Figueroa, J.; Prado-Barragán, A.; Rodríguez-Jasso, R.M.; Rodríguez-Herrera, R.; Montañez, J.C.; Aguilar, C.N. Valorization of grapefruit by-products as solid support for solid-state fermentation to produce antioxidant bioactive extracts. Waste Biomass. Valorization 2019, 10, 763–769. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Aguilera-Carbó, A.F.; Rodríguez-Herrera, R.; Contreras-Esquivel, J.C.; Aguilar, C.N. Submerged culture for production of ellagic acid from pomegranate husk by Aspergillus niger GH1. Micol. Apl. Int. 2014, 26, 27–35. [Google Scholar]
- Sepúlveda, L.; Laredo-Alcalá, E.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Genisheva, Z.; Aguilar, C.; Texeira, J. Ellagic acid production using polyphenols from orange peel waste by submerged fermentation. Electron. J. Biotechnol. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Muhtadi, M.; Haryoto; Sujono, T.A.; Indrayudha, P.; Suhendi, A.; Heng-Yen, K. Antioxidant activity and chemical constituents of some Indonesian fruit peels. Med. Plants 2014, 6, 43–46. [Google Scholar] [CrossRef]
- De Leon-Medina, J.C.; Sepulveda, L.; Morlett-Chavez, J.; Melendez-Renteria, P.; Zugasti-Cruz, A.; Ascacio-Valdés, J.A.; Aguilar, C.N. Solid-state fermentation with Aspergillus niger GH1 to enhance polyphenolic content and antioxidative activity of Castilla rose (Purshia plicata). Plants 2020, 9, 1518. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Larroche, C. Current Developments in Solid-State Fermentation; Pandey, A., Soccol, C.R., Larroche, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Crafack, M.; Keul, H.; Eskildsen, C.E.; Petersen, M.A.; Saerens, S.; Blennow, A.; Skovmand-Larsen, M.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Impact of starter cultures and fermentation techniques on the volatile aroma and sensory profile of chocolate. Food Res. Int. 2014, 63, 306–316. [Google Scholar] [CrossRef]



| Run | Temperature (°C) | Moisture (%) | Inoculum (Cells/g) |
|---|---|---|---|
| 1 | −1 | −1 | 0 |
| 2 | −1 | 0 | −1 |
| 3 | −1 | 0 | 1 |
| 4 | −1 | 1 | 0 |
| 5 | 0 | −1 | −1 |
| 6 | 0 | −1 | 1 |
| 7 | 0 | 0 | 0 |
| 8 | 0 | 0 | 0 |
| 9 | 0 | 0 | 0 |
| 10 | 0 | 1 | −1 |
| 11 | 0 | 1 | 1 |
| 12 | 1 | −1 | 0 |
| 13 | 1 | 0 | −1 |
| 14 | 1 | 0 | 1 |
| 15 | 1 | 1 | 0 |
| Factors | Low level (−1) | Medium level (0) | Maximum level (1) |
| Temperature (°C) | 25 | 30 | 35 |
| Moisture (%) | 50 | 60 | 70 |
| Inoculum (cells/g) | 1 × 107 | 1.5 × 107 | 2 × 107 |
| Parameters | Results |
|---|---|
| Moisture (%) | 6.72 ± 1.45 |
| WAI (g of gel/g of dry weight) | 5.44 ± 0.06 |
| Maximum support moisture (%) | 83 ± 0.01 |
| Treatment | Conditions | EA (mg/g) | Total Polyphenols | DPPH (%) | ABTS (%) |
|---|---|---|---|---|---|
| 1 | 25 °C/50%/1.5 × 107 | 14.02 ± 4.4 fg | 58.39 ± 5.14 fg | 62.22 ± 0.62 ab | 95.90 ± 5.90 a |
| 2 | 25 °C/60%/1 × 107 | 72.51 ± 19.12 de | 64.93 ± 6.33 defg | 56.22 ± 7.99 ab | 99.00 ± 1.00 a |
| 3 | 25 °C/60%/2 × 107 | 81.7 ±10.01 d | 67.55 ± 7.45 defg | 63 ± 2.27 a | 94.00 ± 4.83 a |
| 4 | 25 °C/70%/1.5 × 107 | 177.49 ±11.67 b | 80.05 ± 9.58 bcd | 60.86 ± 0.22 ab | 96.95 ± 0.3 a |
| 5 | 30 °C/50%/1 × 107 | 27.84 ± 7.92 efg | 58.05 ± 3.48 fg | 62.35 ± 0.11 ab | 99.38 ± 0.70 a |
| 6 | 30 °C/50%/2 × 107 | 98.88 ± 5.09 cd | 55.33 ± 1.71 g | 62.41 ± 0.4 ab | 100.00 ± 0.52 a |
| 7 | 30 °C/60%/1.5 × 107 | 146.17 ± 5.69 bc | 67 ± 1.16 defg | 62.41 ± 0.73 ab | 94.24 ± 7.18 a |
| 8 | 30 °C/60%/1.5 × 107 | 458.37 ± 44.6 a | 62.06 ± 1.08 efg | 62.41 ± 1.94 ab | 95.33 ± 5.00 a |
| 9 | 30 °C/60%/1.5 × 107 | 90.81 ± 2.58 d | 68.12 ± 1.03 cdefg | 62.41 ± 0.87 ab | 97.14 ± 2.18 a |
| 10 | 30 °C/70%/1 × 107 | 196.30 ± 28.65 b | 75.27 ± 4.75 bcde | 60.93 ± 4.02 ab | 98.71 ± 1.49 a |
| 11 | 30 °C/70%/2 × 107 | 64.13± 14.41 def | 73.13 ± 2.91 cdef | 62.93 ± 0.11 a | 98.19 ± 1.89 a |
| 12 | 35 °C/50%/1.5 × 107 | 88.74 ± 20.12 d | 75.1 ± 4.85 cde | 47.2 ± 14.44 b | 97.38 ± 2.86 a |
| 13 | 35 °C/60%/1 × 107 | 10.19 ± 3.44 g | 90.61 ± 6.85 ab | 57.9 ± 4.41 ab | 94.71 ± 5.32 a |
| 14 | 35 °C/60%/2 × 107 | 9.32 ± 1.67 g | 83.22 ± 9.04 bc | 51.77 ± 7.32 ab | 99.86 ± 0.25 a |
| 15 | 35 °C/70%/1.5 × 107 | 20.11 ± 0.68 fg | 103.66 ± 7.47 a | 52.8 ± 5.07 ab | 99.43 ± 0.74 a |
| ID | Retention Time (min) | Compound | [M-H]− (m/z) | MS2 | Group |
|---|---|---|---|---|---|
| 1 | 7.03 | Gallic acid 4-O-glucoside | 331 | 169, 125 | Hydroxybenzoic acids |
| 2 | 13.22 | Gallic acid | 169 | 125 | Hydroxybenzoic acids |
| 3 | 20.75 | Cyanidin 3-O-(6″-malonyl-3″-glucosyl-glucoside) | 697 | 565, 393, 271, 209, 147 | Anthocyanins |
| 4 | 39.73 | Ellagic acid | 301 | 257, 229, 185 | Hydroxybenzoic acid dimers |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De La Rosa-Esteban, K.; Sepúlveda, L.; Chávez-González, M.L.; Torres-León, C.; Estrada-Gil, L.E.; Aguilar, C.N.; Ascacio-Valdés, J.A. Valorization of Mexican Rambutan Peel through the Recovery of Ellagic Acid via Solid-State Fermentation Using a Yeast. Fermentation 2023, 9, 723. https://doi.org/10.3390/fermentation9080723
De La Rosa-Esteban K, Sepúlveda L, Chávez-González ML, Torres-León C, Estrada-Gil LE, Aguilar CN, Ascacio-Valdés JA. Valorization of Mexican Rambutan Peel through the Recovery of Ellagic Acid via Solid-State Fermentation Using a Yeast. Fermentation. 2023; 9(8):723. https://doi.org/10.3390/fermentation9080723
Chicago/Turabian StyleDe La Rosa-Esteban, Karen, Leonardo Sepúlveda, Mónica L. Chávez-González, Cristian Torres-León, Luis E. Estrada-Gil, Cristóbal N. Aguilar, and Juan A. Ascacio-Valdés. 2023. "Valorization of Mexican Rambutan Peel through the Recovery of Ellagic Acid via Solid-State Fermentation Using a Yeast" Fermentation 9, no. 8: 723. https://doi.org/10.3390/fermentation9080723
APA StyleDe La Rosa-Esteban, K., Sepúlveda, L., Chávez-González, M. L., Torres-León, C., Estrada-Gil, L. E., Aguilar, C. N., & Ascacio-Valdés, J. A. (2023). Valorization of Mexican Rambutan Peel through the Recovery of Ellagic Acid via Solid-State Fermentation Using a Yeast. Fermentation, 9(8), 723. https://doi.org/10.3390/fermentation9080723








