Abstract
Owing to the well-established application of prebiotics in human food products, there is a growing interest in their potential as dietary supplements for gut microbiota composition and improvement of the digestive health of dogs. However, targeted studies with dogs as research subjects are still limited. In the present study, an in vitro simulated gut microbiota fermentation system using canine feces from a healthy Border Collie breed was used to investigate the prebiotic effects of five different oligosaccharides and compare their regulatory effects on the gut microbiota structure and the resultant metabolites. Due to the addition of oligosaccharides, the fermented samples had lower pH and higher bacterial proliferation. The oligosaccharide-fermentation selectively boosted Lactobacillus spp., Streptococcus spp., Enterococcus spp., Bacteroides spp., and hindered Escherichia-Shigella spp., Paeniclostridium, spp., and Bacteroides spp. Each oligosaccharide showed distinct characteristics and preferences for regulating gut microbiota structure and abundance. Furthermore, the addition of oligosaccharides increased the production of short-chain fatty acids, particularly butyric acid. This study provides a preliminary basis for the rapid and rational selection of prebiotic oligosaccharides as canine dietary supplements and further explores the function of oligosaccharides and their combinations in canine health.
1. Introduction
Domestic dogs are among the most popular companion animals worldwide. Over 180 million dogs live in households in Europe and the US, and the population is increasing, especially in Asia and Latin America (data from FinancesOnline). Companion dogs are considered family members and establish strong emotional connections with their owners. Therefore, the health and longevity of companion dogs have attracted increasing attention in recent decades [1]. The canine gastrointestinal tract harbors a large and complex microbiota. This microbial ecosystem plays critical roles in nutrient digestion, bioconversion and utilization, resistance to pathogen colonization, and immune modulation. The regulation of gut microbiota composition and metabolism is regarded as an effective strategy for maintaining canine health and preventing diseases [2].
Prebiotics are substrates selectively fermented by the host gut microbiota, thereby providing health benefits to the host. Several studies have shown that supplementing prebiotics into dog feed can potentially have beneficial effects on the health of companion dogs [3,4]. These oligosaccharides, including fructo-oligosaccharides (FOS), galacto-oligosaccharides (GOS), mannan oligosaccharides (MOS), and the emerging raffinose (RFN), have been proposed as the most common archetypes of prebiotics and are widely used in human food products and supplements to promote favorable gut microbial balance and enhance wellness [5]. Because of ethical restrictions, entry criteria, and relatively high costs, there are only a few studies regarding the effects of oligosaccharide consumption on canine gut microbiota composition and metabolism [6]. Most previous studies have focused on FOS as a dog feed supplement and determined its positive functions in promoting beneficial microbial growth, inhibiting undesirable bacterial reproduction, and increasing short-chain fatty acid (SCFA) concentrations [7,8]. Recently, Kee et al. found that soybean oligosaccharides have a significant capacity to stimulate butyrate production in a canine in vitro fermentation model [4]. However, limited studies have been conducted on the diverse prebiotic effects of various oligosaccharides as potential dietary supplements for enhancement of canine gastrointestinal health.
To better understand the application prospects of oligosaccharide prebiotics as canine feed supplements, this study focused on five oligosaccharides, FOS, GOS, MOS, RFN, and polydextrose (PD), and compared their effects on gut microbiota derived from the Border Collie breed. The effects of these oligosaccharides on pH changes, SCFA production, gut microbiota composition, and abundance modulation were investigated using an in vitro fermentation model with canine fecal inoculum.
2. Materials and Methods
2.1. Materials
FOS (≥98% purity) and PD (≥90% purity) were purchased from Baolingbao Biotech (Yucheng, China). GOS (≥90% purity) and MOS (≥90% purity) were purchased from New Francisco (Yunfu, China) and Chengdu iMOS Co., Ltd. (Chengdu, China), respectively. RFN (≥98% purity) was purchased from Ever Brilliance Biotech (Tongjiao, China). Acetic, propionic, butyric, and lactic acid were obtained from Macklin Biochemical (Shanghai, China). The ultrapure water used in all the experiments was purchased from Wahaha Co., Ltd. (Hangzhou, China). Chromatography grade acetonitrile and methanol were purchased from Fisher Scientific (Waltham, MA, USA). All other chemicals and reagents were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Canine Feces Collection and Inoculum Preparation
Feces were collected from the Pet Nutrition Research Center of the Gambol Pet Group (Liaocheng, China) according to the guidelines of the Animal Ethics Committee of Liaocheng University. Three healthy adult Border Collies were selected as donors and were fed a standard commercial dog diet (Table 1) for 30 days before fecal sample donation. These dogs had not received antibiotics or probiotic/prebiotic supplements in the past three months. Fresh fecal samples were collected in sterile bags and placed in an anaerobic jar with an AnaeroPack (Mitsubishi Gas Chemical Co. Inc., Tokyo, Japan). They were immediately processed in an LAI-3 anaerobic workstation (LongYue Instrument Co., Ltd., Shanghai, China) filled with anaerobic mixed gas (AMG, 90% N2, 5% CO2, and 5% H2) in the laboratory. An equal amount of feces from each donor (10 g) was mixed and diluted (1:9 w/v) with 0.1 M sterile phosphate-buffered saline (PBS, pH = 7.4) containing cysteine hydrochloride (0.5 g), which was deoxygenated with filtered AMG for 15 min. The supernatant of the fecal slurry was obtained as fecal inoculum by centrifugation at 300× g for 5 min at 4 °C. The resulting fecal inoculum was sealed in serum bottles at 38.5 °C before use.

Table 1.
Analyzed nutrient composition of the commercial diet 1 fed to dogs before feces collection.
2.3. In Vitro Fermentation
In vitro fermentation was performed in an LAI-3 anaerobic workstation according to the procedure reported by Tian et al. [9], with slight modifications. The basal fermentation medium was prepared with the following formulation [10,11]: 2.0 g/L peptone, 2.0 g/L yeast extract, 2.0 g/LNaHCO3, 0.1 g/L NaCl, 40 mg/L K2HPO4, 40 mg/L KH2PO4, 10 mg/L MgSO4·7H2O, 10 mg/L CaCl2·6H2O, 0.5 g/L cysteine hydrochloride, 0.5 g/L bile salts, 25 mg/L hemin, 2 mL/L Tween 80, 10 µL/L vitamin K1, 1.0 mg/L resazurin, and the pH adjusted to 6.8. After being autoclaved at 121 °C for 15 min, the medium was deoxygenated using filtered AMG for 15 min.
Oligosaccharides (0.2 g) were dissolved in a small amount of the fermentation medium and then filter sterilized through 0.22 µm size filters. The filtered solutions were mixed with fresh medium to reach 9.8 mL and then inoculated with 0.2 mL canine fecal supernatant. The mixed cultures were incubated at 39 °C for 24 h under anaerobic conditions [8]. A culture without oligosaccharides was used as the blank group. After 24 h incubation, the fermented cultures were stored at −80 °C for subsequent experiments. Each fermentation formulation was conducted in triplicate.
2.4. pH and Ultraviolet (UV) Measure
The pH of the fermentation culture was determined using a SevenCompact pH meter with an Expect Pro-ISM probe (Mettler Toledo, Columbus, OH, USA). The bacterial growth was evaluated by measuring its optical density at 600 nm (OD 600) using an ultraviolet-visible spectrophotometer (U-3900H, Hitachi, Tokyo, Japan). The fermented media (1 mL) were centrifuged at 10,000× g for 10 min at 4 °C and the supernatants were removed carefully. The bacterial precipitate was resuspended in 16 mL of PBS to obtain the OD 600.
2.5. Microbial Analysis
After 24 h of fermentation, the fermented culture was oscillated thoroughly to disperse the bacterial cells. Then, 1 mL of the mixed culture was centrifuged at 12,000× g for 10 min at 4 °C. The sediment was retained and immediately frozen in liquid nitrogen. Genomic DNA from each fermentation trial was extracted using a magnetic soil and stool DNA kit (DP712; TianGen, Beijing, China). The V3-V4 regions of the 16S rRNA genes were amplified using the 341F/806R primer pair and Phusion® high-fidelity PCR master mix (New England Biolabs, Ipswich, MA, USA). The amplicon mixtures were purified using a universal DNA purification kit (DP214; TianGen, China). A sequencing library was constructed using the Next Ultra II FS DNA PCR-free Library Prep Kit (New England Biolabs) and quantified using a Qubit 3.0 fluorometer (Invitrogen, Carlsbad, CA, USA). The quantified library was pooled and sequenced by the Novogene Institute (Beijing, China) on an Illumina platform. Species annotation was performed using QIIME2 software with the Silva database. All results were analyzed based on sequenced reads and operational taxonomic units (OTUs).
2.6. SCFAs and Lactic Acid Quantitative Analysis
Quantitative analysis of SCFAs in the fermented culture was performed using a Trace 1300 gas chromatographic (GC) system equipped with a flame ionization detector (Thermo Fisher Scientific, Waltham, MA, USA), as described by Ma et al. [12]. The fermented sample (500 μL) was acidified by adding an equal volume of 0.1 M hydrochloric acid, which contained 0.01 M 2-ethyl butyric acid as an internal standard. The mixture was centrifuged at 10,000× g for 10 min at 4 °C and the supernatant (1 μL) was injected into the GC system. The injection port temperature was 200 °C, the split ratio was 10:1, and the mobile phase was nitrogen gas at a 2 mL/min flow rate. The component separation was carried out through an Agilent DB-624 column (30 m × 0.32 mm × 1.8 μm). The initial column temperature was held at 50 °C for 2 min, then raised to 200 °C at 5 °C/min, and finally held at 200 °C for 1 min. The standard curve was established using 1.0–50.0 mM standard solutions of acetic acid, 0.1–5.0 mM solutions of propionic acid, and butyric acid.
Quantitative analysis of lactic acid was performed using a lactic acid concentration assay kit (BC2230; Solarbio, Beijing, China) according to the manufacturer’s instructions, based on visible spectrophotometry. The U-3900H spectrophotometer (Hitachi, Tokyo, Japan) was used for absorbance determination and the 1.0–50.0 mM range of the standard curve was established.
2.7. Statistical Analysis
All experiments were conducted in triplicate. Data are presented as means ± standard deviation (SD). Numerical data were compared using t-tests in Prism software (Version 7.0, GraphPad, San Diego, CA, USA), and microbiological data were analyzed using statistical R software (version 4.0.3). Statistical significance was determined at a p-value < 0.05.
3. Results and Discussion
3.1. pH and Total Bacterial Growth
The pH change and total bacterial count of each fermented medium were measured after 24 h of fermentation to obtain an overview of the fermentation process (Figure 1). Although the pH in the canine small intestinal and colon may fluctuate owing to differences in the colonizing microbiota and physiological status [13], multiple studies have reported a near-neutral pH because of the buffering capacity of pancreatic juice and bile [1]. Thus, pH 6.8 was always chosen in the simulated canine intestinal fermentation system in vitro [1]. As shown in Figure 1A, the presence of the oligosaccharides did not induce a change in the pH of the fermentation media before fermentation, which remained at approximately 6.8. After 24 h of fermentation, the pH of the blank sample decreased slightly from 6.8 to 6.6, whereas the fermentation media containing the oligosaccharides showed a significant decrease in pH. The pH values of the media containing FOS, GOS, MOS, and RFN decreased to approximately 4, whereas the sample containing PD had a higher pH of 6.5. The canine gut microbiota can utilize the oligosaccharides and produce beneficial acidic metabolites (e.g., SCFAs) that lower the pH [14]. Furthermore, acidification of the gut environment has several beneficial effects such as inhibiting pathogen overgrowth and promoting the growth of beneficial bacteria such as Lactobacillus and Bifidobacterium [15].
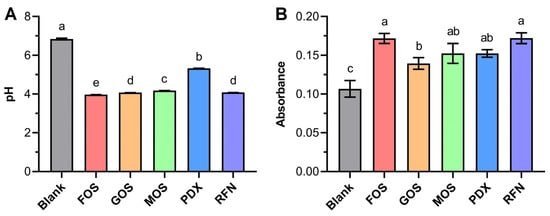
Figure 1.
Changes in pH (A) and OD600 values (B) of in vitro fermented samples by canine fecal microbiota. Blank represents the blank group (not supplemented with oligosaccharides); FOS, GOS, MOS, PDX, and RFN represent the relevant oligosaccharide supplement groups. The different lowercase letters indicate a significant difference (p < 0.05) among different groups.
The proliferation of total bacterial flora in media containing different oligosaccharides was estimated by measuring the OD600 values (Figure 1B). Before fermentation, the OD600 values of the inoculated media were approximately 0.07. After 24 h of fermentation, the OD600 values of all media significantly increased. Compared with the blank sample, the media containing the oligosaccharides showed a 1.29- to 1.60- fold OD600 increase, indicating that the oligosaccharides favored bacterial reproduction.
3.2. Gut Microbial Diversity
Bacterial 16S rRNA pyrosequencing of fermented samples was performed to explore the effects of different oligosaccharides on the diversity and composition of the gut microbiota. The rarefaction and Shannon curves reflected changes in the operational taxonomic units (OTUs) and Shannon indices with an increase in the number of extracted sequences. As shown in Figure 2A,B, rarefaction curves flattened as sequencing depth and sample size increased, suggesting that credible sequencing information had been obtained. Meanwhile, the Shannon indices of all samples reached a stable plateau, suggesting that the volume of sequencing information was sufficient to analyze the gut microbial diversity.
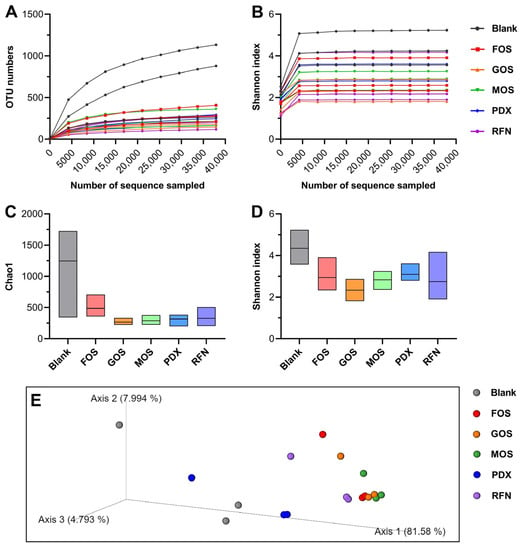
Figure 2.
Effects of different oligosaccharide interventions on canine gut microbiota diversity. (A) Rarefaction curves; (B) Shannon curves; (C) Chao1 indices; (D) Shannon indices; (E) PCoA of microbiota at OTU level. Blank represents the blank group (not supplemented with oligosaccharides); FOS, GOS, MOS, PDX, and RFN represent the relevant oligosaccharide supplement groups.
The α-diversity of fermented samples from the different treatment groups is displayed as Chao1 (Figure 2C) and Shannon indices (Figure 2D). The indices revealed that the microbiota diversity of the samples fermented with oligosaccharides decreased in the blank group. A decrease in diversity caused by carbohydrate prebiotics, including FOS and carrageenan oligosaccharides, has been observed in previous studies [16,17,18]. This phenomenon may be because the oligosaccharides could be utilized by different microbiota and transformed to generate functional metabolites, such as SCFAs, triggering competition for dominant microbiota and reducing microbiota diversity.
Principal coordinate analysis (PCoA) based on the weighted UniFrac distance was used to evaluate the β-diversity of the bacterial communities in each sample [18]. As shown in Figure 2E, 94.37% of the total variance could be explained by the three axes, each having contributed 81.58, 7.99, and 4.79% of the variation. The PCoA results showed that, except for the PDX group, the other four oligosaccharide intervention groups were close to each other and further along the blank group, whereas the distance between the PDX and blank groups was relatively small. The results indicated that oligosaccharide incorporation had different effects on canine gut microbiota, and that four of these oligosaccharides induced a more significant change in microbial composition relative to the blank [19].
3.3. Microbial Composition
The regulatory effect of different oligosaccharides on the taxonomic composition of the microbiota after fermentation was evaluated at the phylum and genus levels (Figure 3A,B). The majority of microbiota within all fermented groups were identified as belonging to the following phyla: Firmicutes (renamed to Bacillota [20]), Proteobacteria (renamed Pseudomonadota), Bacteroidetes, Actinobacteria, and Fusobacteria, which is consistent with previous reports of canine gut microbiota composition [1,21]. Overall, compared to the blank group, the relative abundance of Firmicutes significantly increased in all oligosaccharide groups, whereas that of Bacteroidetes and Actinomycetes significantly decreased. Furthermore, the relative abundance of Proteobacteria significantly decreased in the oligosaccharide groups, except for the PDX group, which showed no obvious changes in the proportion of Proteobacteria. Several studies have demonstrated that increasing the prebiotic carbohydrates and vegetable fiber content in dog feeds leads to an increase in the relative abundance of Firmicutes and a decrease in Proteobacteria, which is perceived to be beneficial for canine intestinal health [22]. A decrease in some bacterial taxa within Firmicutes (genera Ruminococcus and Megamonas) has been associated with canine inflammatory bowel disease [22]. Salas-Mani et al. [23] reported that higher Firmicutes and lower Proteobacteria abundance were correlated with weight control and obesity prevention in dogs. However, Sanchez et al. [24] found a decrease in the ratio of Firmicutes/Bacteroidetes (F/B) appearing in the fecal microbiota of obese dogs who underwent a weight loss program. The same phenomenon was observed in studies on the human gut microbiota, where it was suggested that a microbiome with a higher F/B ratio favors the energy harvest and storage of the host, thus increasing the risk of obesity [18]. Our results indicate that oligosaccharide intervention may change host energy metabolism by regulating the composition of the intestinal microbiota. In addition, Proteobacteria have been associated with canine obesity, as has been suggested in humans [25].
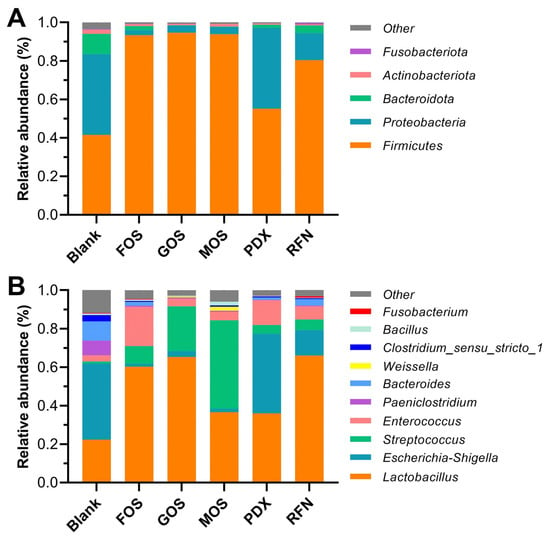
Figure 3.
Responses of the gut microbiota composition to different oligosaccharide interventions at the phylum (A) and genus (B) levels. Blank represents the blank group (not supplemented with oligosaccharides); FOS, GOS, MOS, PDX, and RFN represent the relevant oligosaccharide supplement groups.
The microbiota composition of the fermented samples showed clear differences at the genus level (Figure 3B). The gut microbiota of the blank group was mainly composed of Lactobacillus, Escherichia-Shigella, Streptococcus, Enterococcus, Paeniclostridium, Bacteroides, Weissella, Clostridium, Bacillus, and Parabacteroides, whose relative abundances were over 1% in at least one group. Compared to the blank group, the relative abundances of Lactobacillus, Streptococcus, and Enterococcus in the oligosaccharide groups improved to varying degrees, accompanied by decreases in those of Escherichia-Shigella, Paeniclostridium, and Bacteroides. Differences at the genus level between each oligosaccharide group and the blank group are shown in Figure 4. Intervention with FOS and GOS significantly increased the relative abundance of Lactobacillus and Streptococcus (p < 0.05) and reduced that of Escherichia-Shigella, Bacteroides, and Clostridium_sensu_stricto_1 (p < 0.05). Lactobacillus is a well-known probiotic in the human and animal intestines and produces characteristic metabolites to fortify the intestinal barrier, thus inhibiting colonization by harmful bacteria, and preventing various gastrointestinal disorders [26,27]. Additionally, Lactobacillus confers benefits to the host immunity and enteric nervous systems [28,29]. The maximum effect was seen on Streptococcus in the MOS group, with an increase in its relative abundance from 1.1% in the blank to 45.9%. Meanwhile, the PDX group exhibited higher levels of Enterococcus with a relative abundance of 12.9%, compared to the blank group (3.2%). Some Enterococcus and Streptococcus spp. (e.g., E. faecium and S. salivarius subsp. thermophilus) have been used to treat various canine gastrointestinal disorders such as diarrhea [30], bowel inflammation [31] and chronic enteropathies [32]. However, Garcia-Mazcorro et al. [26] found that, for the probiotic supplement of these bacteria, it is typically difficult to obtain a decent colonization in the gut due to the competition from the already established microbiota. These results revealed that oligosaccharide supplementation may be highly feasible for promoting specific bacterial growth. The RFN provided a moderate regulation in microbial composition, with the promotion of Streptococcus and inhibition for Escherichia-Shigella and Clostridium_sensu_stricto_1. It is noteworthy that, among all the oligosaccharide groups, the PDX group alone did not exhibit an inhibitory effect on Escherichia-Shigella. On similar lines Beloshapka et al. [33] found that polydextrose consumption by dogs did not affect the abundance of Escherichia, lactobacilli, and bifidobacteria. Escherichia-Shigella includes many potentially pathogenic strains (e.g., E. coli, S. flexneri, and S. dysenteriae), and their decrease benefits host health and disease remission [6].
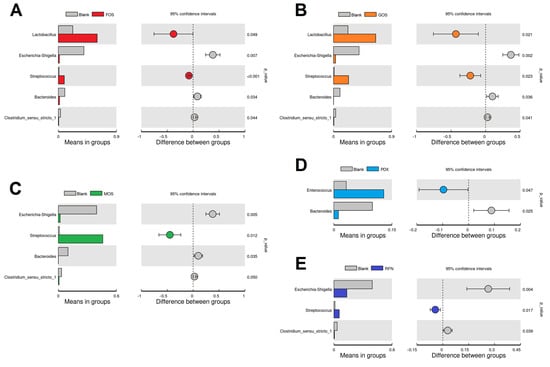
Figure 4.
Differential abundance analysis of microbiota between the oligosaccharide intervention and blank groups based on t-test. (A–E) represent the results of different oligosaccharide interventions: FOS (A), GOS (B), MOS (C), PDX (D), and RFN (E). Blank represents the blank group (not supplemented with oligosaccharides); FOS, GOS, MOS, PDX, and RFN represent the relevant oligosaccharide supplement groups.
3.4. Linear Discriminant Analysis (LDA)
To reveal the specific bacterial taxa that were significantly influenced by different oligosaccharides during fermentation, LDA and LDA effect size (LEfSe) analyses were performed on the operational taxonomic unit (OUTs) results. As shown in Figure 5A, 13 OUTs from four fermentation groups displayed a marked predominance compared to the other fermentation groups because of their higher LDA scores (log10, >4). The results showed that MOS supplementation was more efficient in increasing the abundance of the genus Streptococcus and that the order Lactobacillales was especially increased by GOS supplementation. In addition, the relative abundance of the phylum Proteobacteria was particularly enriched in the PXD group, where the main contributor was the genus Escherichia-shigella. In the blank group, four OUTs were significantly enriched, including the phylum Proteobacteria and three genera Dorea, Fontibacillus, and Romboutsia belonging to the phylum Firmicutes. This indicated that oligosaccharide addition reduced the abundance of these bacteria. Recent studies have suggested that both Dorea and Romboutsia are harmful to the gut microbiota because of their adverse effects on host weight and serum cholesterol levels [34,35]. The corresponding evolutionary cladogram is shown in Figure 5B. The specifically enriched bacteria between the GOS and MOS groups had a closer evolutionary relationship, while those of the PXD and blank groups partially overlapped.
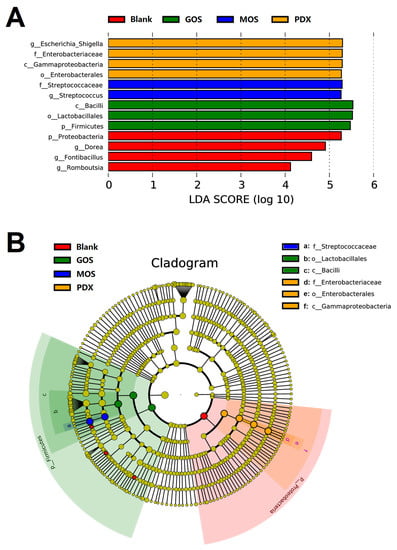
Figure 5.
Differential abundance analysis of microbiota among six different groups based on linear discriminant analysis effect size (LEfSe). (A): LDA score (4.0 or greater in microbiota communities); (B): Cladogram generated from LEfSe analysis. (The different colors represent different groups; circles of different color and colored regions represent taxon with significantly high relative abundance in the corresponding groups).
3.5. Influences on SCFA Production
SCFAs, particularly acetic, propionic, and butyric acids, are important metabolites of various carbohydrates fermented by the anaerobic gut microbiota. SCFAs are easily absorbed through the digestive tract and exert various beneficial effects on the host health [36]. Therefore, SCFA concentrations during fecal fermentation were determined to assess the potential effects of oligosaccharides on the canine gut microenvironment (Figure 6). Acetic acid is the most abundant SCFAs and is mainly produced by Firmicutes, including Lactobacillus and Bifidobacterium [37]. In this study, media containing RFN showed the highest acetic acid concentration after 24 h of fermentation. Higher concentrations of acetic acid not only favor an increase in ghrelin levels and nutrient intake [38] but also inhibit fat synthesis [39]. Unexpectedly, the incorporation of each oligosaccharide caused a steady and significant decrease in propionic acid production compared to that in the blank group. Propionic acid can be produced by putrefactive bacteria, such as Bacteroidetes, Firmicutes, and Propionibacterium species [40], and is considered to promote cholesterol metabolism, reduce lipid storage, and act as an anti-inflammatory [41]. This result differed from that of most previous studies that reported a positive effect of oligosaccharides on propionic acid production [40]. Biagi et al. [42] also found that using chicory (enriched with FOS) as a medium supplement led to reduced propionic acid production during in vitro canine fecal fermentation. Butyric acid is considered the most important SCFA in animal health and provides various health benefits by promoting intestinal mucosal protein production, improving intestinal defense barriers, alleviating oxidative stress injury, and inhibiting colonic carcinogenesis and chronic inflammation [43]. After fermentation, the butyric acid concentration was highest in the media containing RFN (0.63 ± 0.07 mM), followed by GOS (0.52 ± 0.03 mM) and PDX (0.31 ± 0.04 mM), and lowest in the blank group (0.15 ± 0.03 mM). Butyric acid production is closely related to galactose and galacturonic acid metabolism [44], which provides a rational explanation for the high butyric acid production observed in the RFN and GOS groups. In addition, similar to our findings for PDX, Bai et al. [45] reported that β-glucan caused butyric acid production during in vitro fermentation, which is consistent with our results.
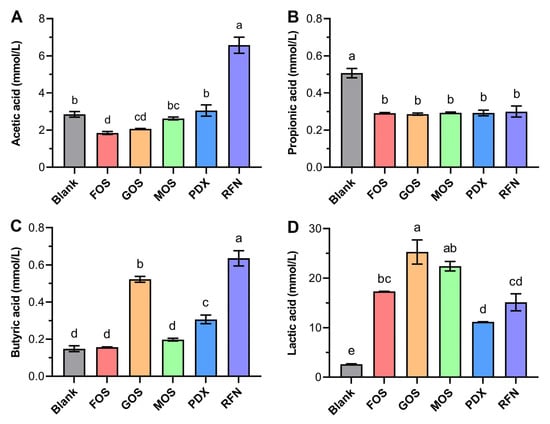
Figure 6.
Production of short-chain fatty acids (SCFAs) and lactic acid after in vitro canine fecal microbiota fermentation. (A–D) represent the effects on acetic acid (A), propionic acid (B), butyric acid (C), and lactic acid (D). The different lowercase letters indicate a significant difference (p < 0.05) among different groups.
Lactic acid is also one of the predominant fermentation products of the gut microbiota and plays a positive role in regulating the intestinal pH environment, suppressing harmful microflora, and so on. Moreover, some restricted bacteria utilize lactic acid to produce propionate and butyrate [36]. In this study, the lactic acid content was noticeably increased after treatment with the oligosaccharides (p < 0.05) compared to that in the blank group (2.6 ± 0.1 mM). This result could be attributed to its excellent ability to promote the proliferation of several lactic acid-producing bacteria, such as Lactobacillus, Streptococcus, and Enterococcus spp., belonging to the family Lactobacillales (phylum Firmicutes). The media containing GOS and MOS gave higher lactic acid concentrations with 25.3 ± 4.2 and 22.4 ± 1.7 mM, respectively. Notably, GOS and MOS groups showed lower acetate production. Several studies have demonstrated that relatively higher lactate and a lower acetate yield can allow more ATP generation to improve the competitiveness of Bifidobacterium within the microbiota community [37]. Overall, the results of SCFAs production caused by prebiotics showed some variance compared with previous reports. This may be due to the compositional specificity and complexity of the gut microbiota from different hosts. This finding also suggests that it is imperative to conduct more targeted research on canine gut microbiota and their responses to feed ingredients.
4. Conclusions
The aim of this study was to evaluate and compare the effects of five different oligosaccharides on canine gut microbiota and metabolic environment. During in vitro fecal fermentation, the presence of all the tested oligosaccharides significantly decreased the environmental pH and promoted overall bacterial growth. Oligosaccharide intervention significantly changed the composition of the gut microbiota and the regulation of different oligosaccharides revealed distinct characteristics. FOS and GOS supplements promoted the relative abundance of the probiotic Lactobacillus and inhibited the dysbiotic Escherichia-Shigella. MOS and PDX are more suitable for promoting Streptococcus and Enterococcus growth, respectively. Furthermore, RFN exhibited clear advantages in the production of health-promoting SCFAs, particularly acetic and butyric acids. Our results indicate that each oligosaccharide has a unique effect on the canine gut microbiota. It should be noted that canine gut microbial ecosystems may exhibit significant individual variation due to differences in physiological states, geographic areas, and dietary habits. Thus, the prebiotic effect of oligosaccharides should be further validated through animal feeding studies, specifically focusing on dogs with health conditions. Additionally, this suggests that a combination of several prebiotic ingredients with different kinetic and end-product characteristics can be used to design dog diets to maximize health gains.
Author Contributions
Conceptualization, Z.D. and Z.W.; methodology, Y.Z. and X.C.; software, Y.Z.; formal analysis, M.W.; data curation, M.W.; investigation, Z.D.; writing—original draft preparation, Y.Z. and Z.D.; writing—review and editing, Z.W.; project administration, Z.W.; funding acquisition, Z.D., Q.W. and Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Natural Science Foundation of Shandong Province (No. ZR2021MD123), Shandong Key Research & Development project (No. 2018YYSP008), and Youth Innovation Technology Project of Higher School in Shandong Province (No. 2021KJ099).
Institutional Review Board Statement
The sample collection and experimental protocol was performed in the pet nutrition research center of Gambol Pet Group (Liaocheng, China) in accordance with the guidelines of the animal ethics committee of Liaocheng University and the experiment was approved by the Scientific Research Ethic Committee of Liaocheng University.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Acknowledgments
The authors thank Weipeng Tian and Jinfa Chen for the assistance during the project. The authors appreciate the technical and equipment support from the Pet Nutrition Research Center of Gambol Pet Group and Liaocheng High-Tech Biotechnology Co., Ltd.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deschamps, C.; Denis, S.; Humbert, D.; Zentek, J.; Priymenko, N.; Apper, E.; Blanquet-Diot, S. In vitro models of the canine digestive tract as an alternative to in vivo assays: Advances and current challenges. Altex 2022, 39, 235–257. [Google Scholar] [CrossRef]
- Duysburgh, C.; Ossieur, W.P.; De Paepe, K.; Van den Abbeele, P.; Vichez-Vargas, R.; Vital, M.; Pieper, D.H.; Van de Wiele, T.; Hesta, M.; Possemiers, S.; et al. Development and validation of the Simulator of the Canine Intestinal Microbial Ecosystem (SCIME)1. J. Anim. Sci. 2020, 98, skz357. [Google Scholar] [CrossRef]
- Kim, H.S.S.; Aldrich, C.G. 111 Organic Matter Disappearance and Production of Short- and Branched-Chain Fatty Acids from Whole Soybeans and Selected Fiber Sources Used in pet Foods by a Canine in Vitro Fermentation Model. J. Anim. Sci. 2022, 100, 51–52. [Google Scholar] [CrossRef]
- Kim, H.S.; Titgemeyer, E.C.; Aldrich, C.G. Evaluation of Fermentability of Whole Soybeans and Soybean Oligosaccharides by a Canine In Vitro Fermentation Model. Fermentation 2023, 9, 414. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Grześkowiak, Ł.; Endo, A.; Beasley, S.; Salminen, S.; Microbiota and probiotics in canine and feline welfare. Anaerobe 2015, 34, 14–23. [Google Scholar] [CrossRef]
- Patra, A.K. Responses of feeding prebiotics on nutrient digestibility, faecal microbiota composition and short-chain fatty acid concentrations in dogs: A meta-analysis. Animal 2011, 5, 1743–1750. [Google Scholar] [CrossRef]
- Belà, B.; Coman, M.M.; Verdenelli, M.C.; Bianchi, C.; Pignataro, G.; Fiorini, D.; Silvi, S. In vitro fermentation of Cucumis sativus fructus extract by canine gut microbiota in combination with two probiotic strains. J. Funct. Foods 2019, 63, 103585. [Google Scholar] [CrossRef]
- Tian, J.; Wang, X.; Zhang, X.; Chen, X.; Rui, X.; Zhang, Q.; Dong, M.; Li, W. Simulated digestion and fecal fermentation behaviors of exopolysaccharides from Paecilomyces cicadae TJJ1213 and its effects on human gut microbiota. Int. J. Biol. Macromol. 2021, 188, 833–843. [Google Scholar] [CrossRef]
- Ogué-Bon, E.; Khoo, C.; Hoyles, L.; McCartney, A.L.; Gibson, G.R.; Rastall, R.A. In vitro fermentation of rice bran combined with Lactobacillus acidophilus 14 150B or Bifidobacterium longum 05 by the canine faecal microbiota. FEMS Microbiol. Ecol. 2011, 75, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ogué-Bon, E.; Khoo, C.; McCartney, A.L.; Gibson, G.R.; Rastall, R.A. In vitro effects of synbiotic fermentation on the canine faecal microbiota. FEMS Microbiol. Ecol. 2010, 73, 587–600. [Google Scholar] [CrossRef][Green Version]
- Ma, G.; Xu, Q.; Du, H.; Muinde Kimatu, B.; Su, A.; Yang, W.; Hu, Q.; Xiao, H. Characterization of polysaccharide from Pleurotus eryngii during simulated gastrointestinal digestion and fermentation. Food Chem. 2022, 370, 131303. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Bollmann, T.; Schäfer, K.J.; Blattner, S.M.; Lotz, R.; Boeck, G.; Weitschies, W. Characterization of the GI transit conditions in Beagle dogs with a telemetric motility capsule. Eur. J. Pharm. Biopharm. 2019, 136, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Vierbaum, L.; Eisenhauer, L.; Vahjen, W.; Zentek, J. In vitro evaluation of the effects of Yucca schidigera and inulin on the fermentation potential of the faecal microbiota of dogs fed diets with low or high protein concentrations. Arch. Anim. Nutr. 2019, 73, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J. Fiber and Prebiotics: Mechanisms and Health Benefits. Nutrients 2013, 5, 1417–1435. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cui, X.; Duan, M.; Ai, C.; Song, S.; Chen, X. In vitro fermentation of κ-carrageenan oligosaccharides by human gut microbiota and its inflammatory effect on HT29 cells. J. Funct. Foods 2019, 59, 80–91. [Google Scholar] [CrossRef]
- Wu, D.-T.; Nie, X.-R.; Gan, R.-Y.; Guo, H.; Fu, Y.; Yuan, Q.; Zhang, Q.; Qin, W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocoll. 2021, 114, 106577. [Google Scholar] [CrossRef]
- Li, X.; Guo, R.; Wu, X.; Liu, X.; Ai, L.; Sheng, Y.; Song, Z.; Wu, Y. Dynamic digestion of tamarind seed polysaccharide: Indigestibility in gastrointestinal simulations and gut microbiota changes in vitro. Carbohydr. Polym. 2020, 239, 116194. [Google Scholar] [CrossRef]
- Yao, H.; Wang, L.; Tang, X.; Yang, Z.; Li, H.; Sun, C.; Wu, X.; Xu, D. Two novel polysaccharides from Solanum nigrum L. exert potential prebiotic effects in an in vitro fermentation model. Int. J. Biol. Macromol. 2020, 159, 648–658. [Google Scholar] [CrossRef]
- Oren, A.; Garrity, G.M. Valid publication of the names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 2021, 71, 005056. [Google Scholar] [CrossRef]
- Vázquez-Baeza, Y.; Hyde, E.R.; Suchodolski, J.S.; Knight, R. Dog and human inflammatory bowel disease rely on overlapping yet distinct dysbiosis networks. Nat. Microbiol. 2016, 1, 16177. [Google Scholar] [CrossRef]
- Pilla, R.; Suchodolski, J.S. The Role of the Canine Gut Microbiome and Metabolome in Health and Gastrointestinal Disease. Front. Vet. Sci. 2019, 6, 498. [Google Scholar] [CrossRef]
- Salas-Mani, A.; Jeusette, I.; Castillo, I.; Manuelian, C.L.; Lionnet, C.; Iraculis, N.; Sanchez, N.; Fernández, S.; Vilaseca, L.; Torre, C. Fecal microbiota composition changes after a BW loss diet in Beagle dogs. J. Anim. Sci. 2018, 96, 3102–3111. [Google Scholar] [CrossRef]
- Bermudez Sanchez, S.; Pilla, R.; Sarawichitr, B.; Gramenzi, A.; Marsilio, F.; Steiner, J.M.; Lidbury, J.A.; Woods, G.R.T.; German, A.J.; Suchodolski, J.S. Fecal microbiota in client-owned obese dogs changes after weight loss with a high-fiber-high-protein diet. PeerJ 2020, 8, e9706. [Google Scholar] [CrossRef]
- Apper, E.; Privet, L.; Taminiau, B.; Le Bourgot, C.; Svilar, L.; Martin, J.-C.; Diez, M. Relationships between Gut Microbiota, Metabolome, Body Weight, and Glucose Homeostasis of Obese Dogs Fed with Diets Differing in Prebiotic and Protein Content. Microorganisms 2020, 8, 513. [Google Scholar] [CrossRef]
- Garcia-Mazcorro, J.F.; Lanerie, D.J.; Dowd, S.E.; Paddock, C.G.; Grützner, N.; Steiner, J.M.; Ivanek, R.; Suchodolski, J.S. Effect of a multi-species synbiotic formulation on fecal bacterial microbiota of healthy cats and dogs as evaluated by pyrosequencing. FEMS Microbiol. Ecol. 2011, 78, 542–554. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, F.; Hou, Q.; Huang, W.; Liu, Y.; Zhang, H.; Sun, Z. Metagenomic analysis revealed beneficial effects of probiotics in improving the composition and function of the gut microbiota in dogs with diarrhoea. Food Funct. 2019, 10, 2618–2629. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Zhang, X.; Aweya, J.J.; Huang, Z.-X.; Kang, Z.-Y.; Bai, Z.-H.; Li, K.-H.; He, X.-T.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. In vitro fermentation of Gracilaria lemaneiformis sulfated polysaccharides and its agaro-oligosaccharides by human fecal inocula and its impact on microbiota. Carbohydr. Polym. 2020, 234, 115894. [Google Scholar] [CrossRef]
- Rose, L.; Rose, J.; Gosling, S.; Holmes, M. Efficacy of a Probiotic-Prebiotic Supplement on Incidence of Diarrhea in a Dog Shelter: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Vet. Intern. Med. 2017, 31, 377–382. [Google Scholar] [CrossRef]
- Rossi, G.; Pengo, G.; Caldin, M.; Palumbo Piccionello, A.; Steiner, J.M.; Cohen, N.D.; Jergens, A.E.; Suchodolski, J.S. Comparison of Microbiological, Histological, and Immunomodulatory Parameters in Response to Treatment with Either Combination Therapy with Prednisone and Metronidazole or Probiotic VSL#3 Strains in Dogs with Idiopathic Inflammatory Bowel Disease. PLoS ONE 2014, 9, e94699. [Google Scholar] [CrossRef]
- Makielski, K.; Cullen, J.; O’Connor, A.; Jergens, A.E. Narrative review of therapies for chronic enteropathies in dogs and cats. J. Vet. Intern. Med. 2019, 33, 11–22. [Google Scholar] [CrossRef]
- Beloshapka, A.N.; Dowd, S.E.; Suchodolski, J.S.; Steiner, J.M.; Duclos, L.; Swanson, K.S. Fecal microbial communities of healthy adult dogs fed raw meat-based diets with or without inulin or yeast cell wall extracts as assessed by 454 pyrosequencing. FEMS Microbiol. Ecol. 2013, 84, 532–541. [Google Scholar] [CrossRef]
- Wang, Y.; Ablimit, N.; Zhang, Y.; Li, J.; Wang, X.; Liu, J.; Miao, T.; Wu, L.; Wang, H.; Wang, Z.; et al. Novel β-mannanase/GLP-1 fusion peptide high effectively ameliorates obesity in a mouse model by modifying balance of gut microbiota. Int. J. Biol. Macromol. 2021, 191, 753–763. [Google Scholar] [CrossRef]
- Sowah, S.A.; Milanese, A.; Schübel, R.; Wirbel, J.; Kartal, E.; Johnson, T.S.; Hirche, F.; Grafetstätter, M.; Nonnenmacher, T.; Kirsten, R.; et al. Calorie restriction improves metabolic state independently of gut microbiome composition: A randomized dietary intervention trial. Genome Med. 2022, 14, 30. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Li, H.; Lane, J.A.; Chen, J.; Lu, Z.; Wang, H.; Dhital, S.; Fu, X.; Huang, Q.; Liu, F.; Zhang, B. In vitro fermentation of human milk oligosaccharides by individual Bifidobacterium longum-dominant infant fecal inocula. Carbohydr. Polym. 2022, 287, 119322. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome–brain–β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Nishimura, Y.; Hishikawa, D.; Tsuzuki, H.; Miyahara, H.; Gotoh, C.; Choi, K.-C.; Feng, D.D.; Chen, C.; Lee, H.-G.; et al. Acetate and Propionate Short Chain Fatty Acids Stimulate Adipogenesis via GPCR43. Endocrinology 2005, 146, 5092–5099. [Google Scholar] [CrossRef]
- Li, J.; Pang, B.; Yan, X.; Shang, X.; Hu, X.; Shi, J. Prebiotic properties of different polysaccharide fractions from Artemisia sphaerocephala Krasch seeds evaluated by simulated digestion and in vitro fermentation by human fecal microbiota. Int. J. Biol. Macromol. 2020, 162, 414–424. [Google Scholar] [CrossRef]
- Al-Lahham, S.H.; Peppelenbosch, M.P.; Roelofsen, H.; Vonk, R.J.; Venema, K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim. Biophys. Acta 2010, 1801, 1175–1183. [Google Scholar] [CrossRef]
- Biagi, G.; Cipollini, I.; Zaghini, G. In vitro fermentation of different sources of soluble fiber by dog faecal inoculum. Vet. Res. Commun. 2008, 32 (Suppl. 1), 335–337. [Google Scholar] [CrossRef]
- Donadelli, R.A.; Titgemeyer, E.C.; Aldrich, C.G. Organic matter disappearance and production of short- and branched-chain fatty acids from selected fiber sources used in pet foods by a canine in vitro fermentation model1. J. Anim. Sci. 2019, 97, 4532–4539. [Google Scholar] [CrossRef]
- Fu, X.; Cao, C.; Ren, B.; Zhang, B.; Huang, Q.; Li, C. Structural characterization and in vitro fermentation of a novel polysaccharide from Sargassum thunbergii and its impact on gut microbiota. Carbohydr. Polym. 2018, 183, 230–239. [Google Scholar] [CrossRef]
- Bai, J.; Li, T.; Zhang, W.; Fan, M.; Qian, H.; Li, Y.; Wang, L. Systematic assessment of oat β-glucan catabolism during in vitro digestion and fermentation. Food Chem. 2021, 348, 129116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).