Reducing Carbon Intensity of Food and Fuel Production Whilst Lowering Land-Use Impacts of Biofuels
Abstract
1. Introduction
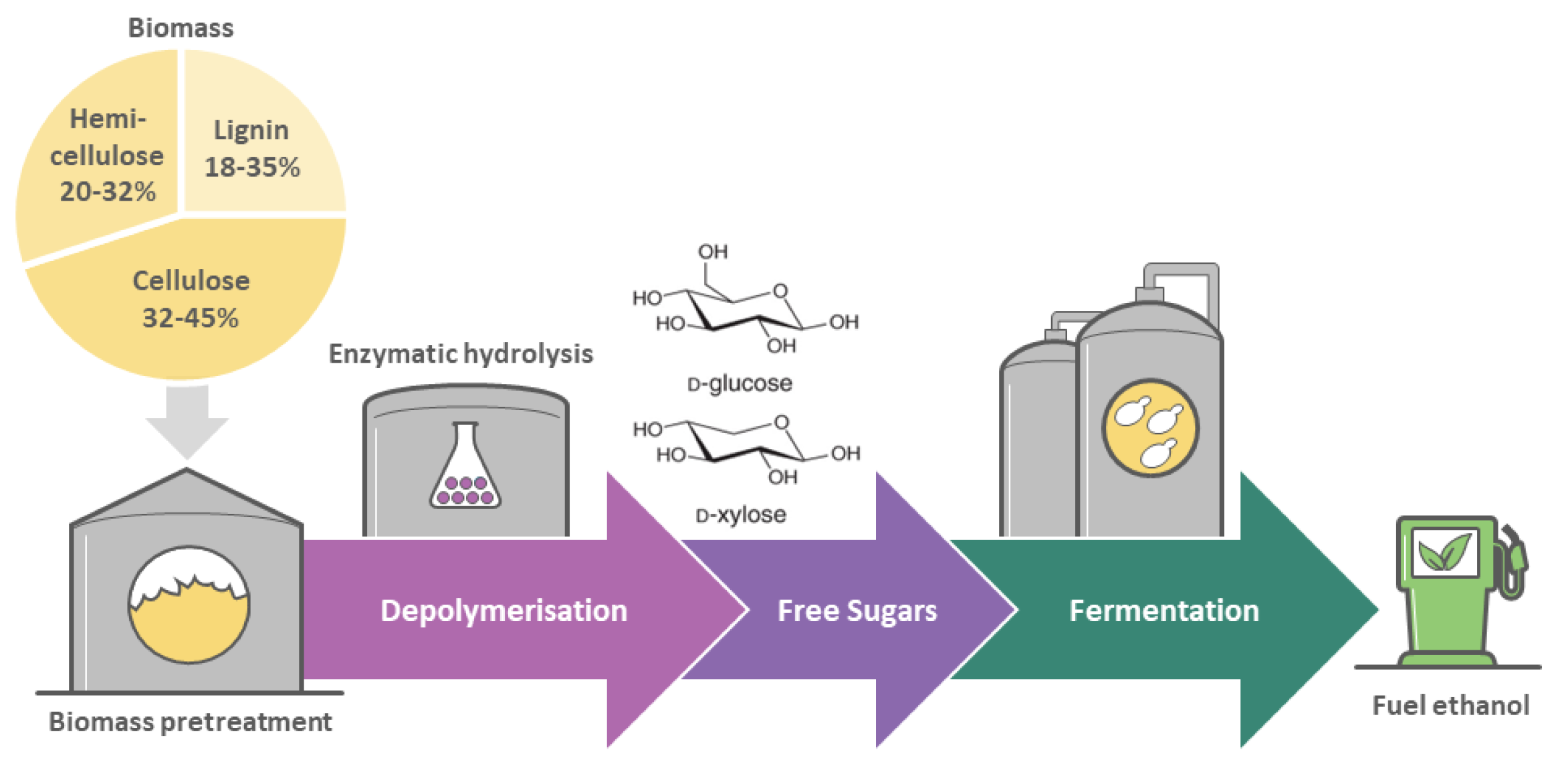
2. Conventional Second-Generation Bioethanol from Biomass Only Partially Addresses Environmental Issues
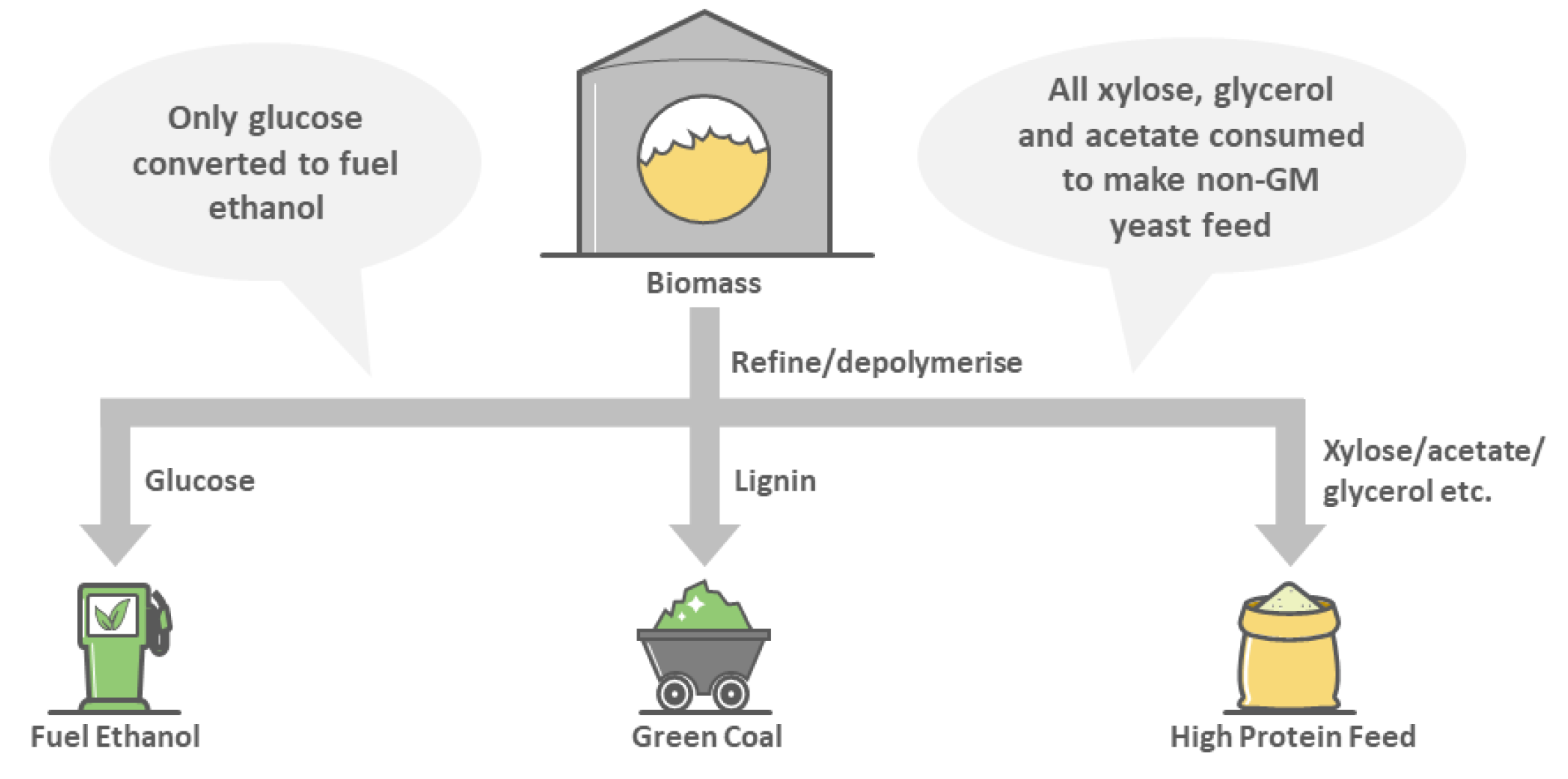
3. A Food and Fuel Lignocellulosic Biorefinery That Sequesters Carbon
4. A Model Lignocellulosic Bio/Chemicals Refinery Delivering Food and Fuel and Power-to-X
5. Consideration of Microbial Functions in Lignocellulosic and Bio/Chemical Refineries
6. Final Comments and Recommendations for Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, J.E. Population and climate change. Proc. Am. Philos. Soc. 2010, 154, 158–182. [Google Scholar] [PubMed]
- Lutz, W. How population growth relates to climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 12103–12105. [Google Scholar] [CrossRef] [PubMed]
- Muttarak, R. Demographic perspectives in research on global environmental change. Popul. Stud. 2021, 75, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, I. Securing a sustainable biomass supply in a growing bioeconomy. Glob. Food Sec. 2015, 6, 34–42. [Google Scholar] [CrossRef]
- Lenaerts, B.; Collard, B.C.Y.; Demont, M. Improving global food security through accelerated plant breeding. Plant Sci. 2019, 287, 110207. [Google Scholar] [CrossRef]
- Jaiswal, K.K.; Chowdry, C.R.; Yadav, D.; Verma, R.; Dutta, S.; Jaiswal, K.S.; Selvakumar, S.K. Renewable and sustainable clean energy development and impact on social, economic, and environmental health. Energy Nexus 2022, 7, 100118. [Google Scholar] [CrossRef]
- Martin-Roberts, E.; Scott, V.; Flude, S.; Johnson, G.; Haszeldine, R.S.; Gilfillan, S.M. Carbon capture and storage at the end of a lost decade. One Earth 2021, 4, 1569–1584. [Google Scholar] [CrossRef]
- Alhindawi, R.; Abu Nahleh, Y.; Kumar, A.; Shiwakoti, N. Projection of greenhouse gas emissions for the road transport sector based on multivariate regression and the double exponential smoothing model. Sustainability 2020, 12, 9152. [Google Scholar] [CrossRef]
- IEA Renewables 2020 Analysis and Forecast to 2025. Available online: https://www.iea.org/reports/renewables-2020/transport-biofuels (accessed on 26 June 2023).
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B. Bioethanol production from renewable raw materials and its separation and purification. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Mohr, A.; Raman, S. Lessons from first generation biofuels and implications for the sustainability appraisal of second generation biofuels. Energy Policy 2013, 63, 114–122. [Google Scholar] [CrossRef]
- Lewandrowski, J.; Rosenfeld, J.K.; Pape, D.; Hendrickson, T.P.; Jaglo, K.; Moffroid, K. The greenhouse gas benefits of corn ethanol—Assessing recent evidence. Biofuels 2020, 11, 361–375. [Google Scholar] [CrossRef]
- Oliveira, M.D.D. Sugarcane and ethanol production and carbon dioxide balances. In Biofuels, Solar and Wind as Renewable Energy Systems; Pimentel, D., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 215–230. [Google Scholar] [CrossRef]
- Nakashima, T.; Ishikawa, S. Energy inputs and greenhouse gas emissions associated with small-scale farmer sugarcane cropping systems and subsequent bioethanol production in Japan. NJAS–Wageningen J. Life Sci. 2016, 76, 43–53. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Chilvers, A.; Azapagic, A. Environmental sustainability of biofuels: A review. Proc. Roy. Soc. A 2020, 476, 20200351. [Google Scholar] [CrossRef]
- Graham-Rowe, D. Agriculture: Beyond food versus fuel. Nature 2011, 474, S6–S8. [Google Scholar] [CrossRef]
- Wyman, C.E.; Dale, B.E.; Elander, R.T.; Holtzapple, M.; Ladisch, M.R.; Lee, Y.Y. Coordinated development of leading biomass pretreatment technologies. Bioresour. Technol. 2005, 96, 1959–1966. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, A. Lignocellulosic biomass: Understanding recalcitrance and predicting hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of physicochemical properties and analytical characterization of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef]
- Rodionova, M.V.; Bozieva, A.M.; Zharmukhamedov, S.K.; Leong, Y.K.; Lan, J.C.-W.; Veziroglu, A.; Veziroglu, T.N.; Tomo, T.; Chang, J.-S.; Allakhverdiev, S.I. A comprehensive review on lignocellulosic biomass biorefinery for sustainable biofuel production. Int. J. Hydrog. Energy 2022, 47, 1481–1498. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Edeh, I. Bioethanol production: An overview. In Bioethanol Technologies; Inambao, F., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Qurat-ul-Ain; Hussain, A.; Sherzada, S.; Clarke, M.; Sadaf, T.; Hasan, A.; Javid, A.; Balakrishnan, D. Production of ethanol from pretreated biomass of Chlorella sorokiniana raised at lab and pilot scales. Biomass Conv. Bioref. 2023, 1–14. [Google Scholar] [CrossRef]
- Aro, E.M. From first generation biofuels to advanced solar biofuels. Ambio 2016, 45 (Suppl. S1), 24–31. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.T.; Soares, P.O.; Baptista, S.L.; Costa, C.E.; Domingues, L. Engineered Saccharomyces cerevisiae for lignocellulosic valorization: A review and perspectives on bioethanol production. Bioengineered 2020, 11, 883–903. [Google Scholar] [CrossRef]
- Ciamponi, F.E.; Procópio, D.P.; Murad, N.F.; Franco, T.T.; Basso, T.O.; Brandão, M.M. Multi-omics network model reveals key genes associated with p-coumaric acid stress response in an industrial yeast strain. Sci. Rep. 2022, 12, 22466. [Google Scholar] [CrossRef]
- Winkler, K.; Fuchs, R.; Rounsevell, M.; Herold, M. Global land use changes are four times greater than previously estimated. Nat. Commun. 2021, 12, 2501. [Google Scholar] [CrossRef]
- Global Cellulosic Ethanol Market Research Report 2018. Available online: https://www.businesswire.com/news/home/20180430005659/en/Global-Cellulosic-Ethanol-Market-Research-Report-2018-Featuring-DuPont-Beta-Renewables-GraanBio-BP-Clariant-and-Synata-Bio---ResearchAndMarkets.com (accessed on 26 June 2023).
- Neto, A.C.; Guimarães, M.J.O.C.; Freire, E. Business models for commercial scale second-generation bioethanol production. J. Clean. Prod. 2018, 184, 168–178. [Google Scholar] [CrossRef]
- Macrelli, S.; Galbe, M.; Wallberg, O. Effects of production and market factors on ethanol profitability for an integrated first and second generation ethanol plant using the whole sugarcane as feedstock. Biotechnol. Biofuels 2014, 7, 26. [Google Scholar] [CrossRef]
- Petersen, A.M.; Okoro, O.V.; Chireshe, F.; Moonsamy, T.; Görgens, J.F. Systematic cost evaluations of biological and thermochemical processes for ethanol production from biomass residues and industrial off-gases. Energy Convers. Manag. 2021, 243, 114398. [Google Scholar] [CrossRef]
- Ou, L.; Brown, T.; Tilakaratne, R.; Hu, G.; Brown, R. Techno-economic analysis of co-located corn grain and corn stover ethanol plants. Biofuel. Bioprod. Biorefin. 2014, 8, 412–422. [Google Scholar] [CrossRef]
- Mayyas, M.; Nekouei, R.K.; Sahajwalla, V. Valorization of lignin biomass as a carbon feedstock in steel industry: Iron oxide reduction, steel carburizing and slag foaming. J. Clean. Prod. 2019, 219, 971–980. [Google Scholar] [CrossRef]
- Jędrzejczak, P.; Collins, M.N.; Jesionowski, T.; Klapiszewski, L. The role of lignin and lignin-based materials in sustainable construction—A comprehensive review. Int. J. Biol. Macromol. 2021, 187, 624–650. [Google Scholar] [CrossRef]
- Page, J. Wilmar Sugar Proserpine Mill Production Figures. Available online: https://www.wilmarsugar-anz.com/proserpine-production-reports/1283-proserpine-production-report-wk-29-we-14jan23/file (accessed on 11 May 2023).
- Alokika, A.; Kumar, A.; Kumar, V.; Singh, B. Cellulosic and hemicellulosic fractions of sugarcane bagasse: Potential, challenges, and future perspective. Int. J. Biol. Macromol. 2021, 169, 564–582. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, I.H. Fish meal—Nutritive value. J. Anim. Physiol. Anim. Nutr. 2011, 95, 685–692. [Google Scholar] [CrossRef]
- Attfield, P.V.; Bell, P.J.L. Use of population genetics to derive non-recombinant Saccharomyces cerevisiae that grow using xylose as a sole carbon source. FEMS Yeast Res. 2006, 6, 862–868. [Google Scholar] [CrossRef]
- Kollaras, A.; Koutouridis, P.; Moore, M.; Bell, P.; Attfield, P. Adding value to sugarcane bagasse using a xylose metabolising yeast. Int. Sugar J. 2011, 113, 777–781. [Google Scholar]
- Gawęda, D.; Nowak, A.; Haliniarz, M.; Woźniak, A. Yield and economic effectiveness of soybean grown under different cropping systems. Int. J. Plant Prod. 2020, 14, 475–485. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Adeyemo, S.M.; Onilude, A.A. Enzymatic reduction of anti-nutritional factors in fermenting soybeans by Lactobacillus plantarum isolates from fermenting cereals. Niger. Food J. 2013, 31, 84–90. [Google Scholar] [CrossRef]
- Bell, P.J.L.; Paras, F.E.; Mandarakas, S.; Arcenal, P.; Robinson-Cast, S.; Grobler, A.S.; Attfield, P.V. An electro–microbial process to uncouple food production from photosynthesis for application in space exploration. Life 2022, 12, 1002. [Google Scholar] [CrossRef]
- Kaur, P.; Kunze, G.; Satyanarayana, T. Yeast phytases: Present scenario and future perspectives. Crit. Rev. Biotechnol. 2007, 27, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Bontinck, P.-A.; Grant, T.F. Life Cycle Assessment of Ethanol Production from Bagasse Using MicroBioGen Yeast Strains. Available online: https://microbiogen.com/wp-content/uploads/2021/07/Lifecycles_Microbiogen_ProofofConcept_final_for-public-release-1.pdf (accessed on 11 May 2023).
- Mathews, J.A.; Tan, H.; Moore, M.J.B.; Bell, G. A conceptual lignocellulosic ‘feed+fuel’ biorefinery and its application to the linked biofuel and cattle raising industries in Brazil. Energy Policy 2011, 39, 4932–4938. [Google Scholar] [CrossRef]
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef] [PubMed]
- Broucek, J. Options to methane production abatement in ruminants: A review. J. Anim. Plant Sci. 2018, 28, 348–364. [Google Scholar]
- Trading Economic: Ethanol. Available online: https://tradingeconomics.com/commodity/ethanol (accessed on 27 June 2023).
- Voutilainen, E.; Pihlajaniemi, V.; TParviainen, T. Economic comparison of food protein production with single-cell organisms from lignocellulose side-streams. Bioresour. Technol. Rep. 2021, 14, 100683. [Google Scholar] [CrossRef]
- Yao, B.; Xiao, T.; Makgae, O.A.; Jie, X.; Gonzalez-Cortes, S.; Guan, S.; Kirkland, A.; Dilworth, J.R.; Al-Megren, H.A.; Alshihri, S.M.; et al. Transforming carbon dioxide into jet fuel using an organic combustion-synthesized Fe-Mn-K catalyst. Nat. Commun. 2020, 11, 6395. [Google Scholar] [CrossRef]
- Sanchez, D.L.; Johnson, N.; McCoy, S.T.; Turner, P.A.; Mach, K.J. Near-term deployment of carbon capture and sequestration from biorefineries in the United States. Proc. Natl. Acad. Sci. USA 2018, 115, 4875–4880. [Google Scholar] [CrossRef]
- Jana, A.; Snyder, S.W.; Crumlin, E.J.; Qian, J. Integrated carbon capture and conversion: A review on C2+ product mechanisms and mechanism-guided strategies. Front. Chem. 2023, 16, 1135829. [Google Scholar] [CrossRef]
- Wang, H.; Yu, Z.; Zhou, J.; Li, C.; Jayanarasimhan, A.; Zhao, X.; Zhang, H. A scientometric review of CO2 electroreduction research from 2005 to 2022. Energies 2023, 16, 616. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, C.; Fang, S.; Zhao, X.; Li, L.; Jiang, H.; Liu, Z.; Fan, Z.; Xu, W.; Xiao, J.; et al. Accelerating electrochemical CO2 reduction to multi-carbon products via asymmetric intermediate binding at confined nanointerfaces. Nat. Commun. 2023, 14, 1298. [Google Scholar] [CrossRef]
- Ram, V.; Salkuti, S.R. An overview of major synthetic fuels. Energies 2023, 16, 2834. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, X.; Wen, X.; Zhou, Y.; Zhou, L.; Li, H.; Tao, L.; Li, Q.; Du, S.; Liu, T.; et al. Coupling N2 and CO2 in H2O to synthesize urea under ambient conditions. Nat. Chem. 2020, 12, 717–724. [Google Scholar] [CrossRef]
- Llorente, B.; Williams, T.C.; Goold, H.D.; Pretorius, I.S.; Paulsen, I.T. Harnessing bioengineered microbes as a versatile platform for space nutrition. Nat. Commun. 2022, 13, 6177. [Google Scholar] [CrossRef]
- Kollaras, A.; Koutouridis, P.; Biddy, M.; McMillan, J.D. Multiple Coproducts Needed to Establish Cellulosic Ethanol Industry. Available online: https://ethanolproducer.com/articles/8926/multiple-coproducts-needed-to-establish-cellulosic-ethanol-industry (accessed on 30 May 2023).
- Benatti, A.L.T.; Polizeli, M.d.L.T.d.M. Lignocellulolytic biocatalysts: The main players involved in multiple biotechnological processes for biomass valorization. Microorganisms 2023, 11, 162. [Google Scholar] [CrossRef]
- Ujor, V.C.; Okonkwo, C.C. Microbial detoxification of lignocellulosic biomass hydrolysates: Biochemical and molecular aspects, challenges, exploits and future perspectives. Front. Bioeng. Biotechnol. 2022, 10, 1061667. [Google Scholar] [CrossRef]
- Parawira, W.; Tekere, M. Biotechnological strategies to overcome inhibitors in lignocellulose hydrolysates for ethanol production: Review. Crit. Rev. Biotechnol. 2011, 31, 20–31. [Google Scholar] [CrossRef]
- McCarty, N.S.; Ledesma-Amaro, R. Synthetic biology tools to engineer microbial communities for biotechnology. Trends Biotechnol. 2019, 37, 181–197. [Google Scholar] [CrossRef]
- Farwick, A.; Bruder, S.; Schadeweg, V.; Boles, E. Engineering of yeast hexose transporters to transport D-xylose without inhibition by D-glucose. Proc. Natl. Acad. Sci. USA 2014, 111, 5159–5164. [Google Scholar] [CrossRef]
- Verhoeven, M.D.; de Valk, S.C.; Daran, J.-M.G.; van Maris, A.J.A.; Pronk, J.T. Fermentation of glucose-xylose-arabinose mixtures by a synthetic consortium of single-sugar-fermenting Saccharomyces cerevisiae strains. FEMS Yeast Res. 2018, 18, foy075. [Google Scholar] [CrossRef]
- Attfield, P.V. Crucial aspects of metabolism and cell biology relating to industrial production and processing of Saccharomyces biomass. Crit. Rev. Biotechnol. 2022, 1–18. [Google Scholar] [CrossRef]
- Parapouli, M.; Vasileiadis, A.; Afendra, A.S.; Hatziloukas, E. Saccharomyces cerevisiae and its industrial applications. AIMS Microbiol. 2020, 6, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, L.N.; Jin, Y.S. In-depth understanding of molecular mechanisms of aldehyde toxicity to engineer robust Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2021, 105, 2675–2692. [Google Scholar] [CrossRef] [PubMed]
- Stovicek, V.; Dato, L.; Almqvist, H.; Schöpping, M.; Chekina, K.; Ebdrup Pedersen, L.; Koza, A.; Figueira, D.; Tjosås, F.; Sommer Ferreira, B.; et al. Rational and evolutionary engineering of Saccharomyces cerevisiae for production of dicarboxylic acids from lignocellulosic biomass and exploring genetic mechanisms of the yeast tolerance to the biomass hydrolysate. Biotechnol. Biofuels 2022, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- Haq, I.U.; Qaisar, K.; Nawaz, A.; Akram, F.; Mukhtar, H.; Zohu, X.; Xu, Y.; Mumtaz, M.W.; Rashid, U.; Ghani, W.A.W.A.K.; et al. Advances in valorization of lignocellulosic biomass towards energy generation. Catalysts 2021, 11, 309. [Google Scholar] [CrossRef]
- Brandt, B.A.; García-Aparicio, M.P.; Görgens, J.F.; van Zyl, W.H. Adaptation of Saccharomyces cerevisiae in a concentrated spent sulphite liquor waste stream for increased inhibitor resistance. Appl. Microbiol. Biotechnol. 2022, 106, 455–468. [Google Scholar] [CrossRef]
- Favaro, L.; Jansen, T.; van Zyl, W.H. Exploring industrial and natural Saccharomyces cerevisiae strains for the bio-based economy from biomass: The case of bioethanol. Crit. Rev. Biotechnol. 2019, 39, 800–816. [Google Scholar] [CrossRef]
- Cagnin, L.; Gronchi, N.; Basaglia, M.; Favaro, L.; Casella, S. Selection of superior yeast strains for the fermentation of lignocellulosic steam-exploded residues. Front. Microbiol. 2021, 12, 756032. [Google Scholar] [CrossRef]
- Attfield, P.V.; Bell, P.J.L. Genetics and classical manipulations of industrial yeasts. In Functional Genetics of Yeasts, Topics in Current Genetics 2; de Winde, J.H., Ed.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 17–56. [Google Scholar] [CrossRef]
- Steensels, J.; Snoek, T.; Meersman, E.; Picca Nicolino, M.; Voordeckers, K.; Verstrepen, K.J. Improving industrial yeast strains: Exploiting natural and artificial diversity. FEMS Microbiol. Rev. 2014, 38, 947–995. [Google Scholar] [CrossRef]
- Gibson, B.; Geertman, J.-M.A.; Hittinger, C.T.; Krogerus, K.; Libkind, D.; Louis, E.J.; Magalhães, F.; Sampaio, J.P. New yeasts—New brews: Modern approaches to brewing yeast design and development. FEMS Yeast Res. 2017, 17, fox038. [Google Scholar] [CrossRef]
- Naseeb, S.; Visinoni, F.; Hu, Y.; Hinks Roberts, A.J.; Maslowska, A.; Walsh, T.; Smart, K.A.; Louis, E.J.; Delner, D. Restoring fertility in yeast hybrids: Breeding and quantitative genetics of beneficial traits. Proc. Natl. Acad. Sci. USA 2021, 118, e2101242118. [Google Scholar] [CrossRef]
- Francois, J.M.; Alkim, C.; Morin, N. Engineering microbial pathways for production of bio-based chemicals from lignocellulosic sugars: Current status and perspectives. Biotechnol. Biofuels 2020, 13, 118. [Google Scholar] [CrossRef]
- Tullio, V. Yeast genomics and its applications in biotechnological processes: What is our present and near future? J. Fungi 2022, 8, 752. [Google Scholar] [CrossRef]
- Wegat, V.; Fabarius, J.T.; Sieber, V. Synthetic methylotrophic yeasts for sustainable fuel and chemical production. Biotechnol. Biofuels 2022, 15, 113. [Google Scholar] [CrossRef]
- Patra, P.; Das, M.; Kundu, P.; Ghosh, A. Recent advances in systems and synthetic biology approaches for developing novel cell-factories in non-conventional yeasts. Biotechnol. Adv. 2021, 47, 107695. [Google Scholar] [CrossRef]
- Kawaguchi, H.; Hasunuma, T.; Ogino, C.; Kondo, A. Bioprocessing of bio-based chemicals produced from lignocellulosic feedstocks. Curr. Opin. Biotechnol. 2016, 42, 30–39. [Google Scholar] [CrossRef]
- Kolisis, N.; Kolisis, F. Synthetic biology: Old and new dilemmas-the case of artificial life. BioTech 2021, 10, 16. [Google Scholar] [CrossRef]
- Saxena, J.; Tanner, R.S. Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogen, Clostridium ragsdalei. J. Ind. Microbiol. Biotechnol. 2011, 38, 513–521. [Google Scholar] [CrossRef]
- Takors, R.; Kopf, M.; Mampel, J.; Bluemke, W.; Blombach, B.; Eikmanns, B.; Bengelsdorf, F.R.; Weuster-Botz, D.; Dürre, P. Using gas mixtures of CO, CO2 and H2 as microbial substrates: The do’s and don’ts of successful technology transfer from laboratory to production scale. Microb. Biotechnol. 2018, 11, 606–625. [Google Scholar] [CrossRef]
- Kerckhof, F.-M.; Sakarika, M.; Van Giel, M.; Muys, M.; Vermeir, P.; De Vrieze, J.; Vlaeminck, S.E.; Rabaey, K.; Boon, N. From biogas and hydrogen to microbial protein through co-cultivation of methane and hydrogen oxidizing bacteria. Front. Bioeng. Biotechnol. 2021, 9, 733753. [Google Scholar] [CrossRef]
- Debabov, V.G. Acetogens: Biochemistry, bioenergetics, genetics, and biotechnological potential. Microbiology 2021, 90, 273–297. [Google Scholar] [CrossRef]
- Liu, X.; Luo, H.; Yu, D.; Tan, J.; Yuan, J.; Li, H. Synthetic biology promotes the capture of CO2 to produce fatty acid derivatives in microbial cell factories. Bioresour. Bioprocess. 2022, 9, 124. [Google Scholar] [CrossRef]
- Jiang, W.; Hernández Villamor, D.; Peng, H.; Chen, J.; Liu, L.; Haritos, V.; Ledesma-Amaro, R. Metabolic engineering strategies to enable microbial utilization of C1 feedstocks. Nat. Chem. Biol. 2021, 17, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Hacquard, P.; Simoën, M.; Hache, E. Is the oil industry able to support a world that consumes 105 million barrels of oil per day in 2025? Oil Gas Sci. Technol. 2019, 74, 88. [Google Scholar] [CrossRef]
- Zhao, Z.; Xian, M.; Liu, M.; Zhao, G. Biochemical routes for uptake and conversion of xylose by microorganisms. Biotechnol. Biofuels 2020, 13, 21. [Google Scholar] [CrossRef]
- Mauser, W.; Klepper, G.; Zabel, F.; Delzeit, R.; Hank, T.; Putzenlechner, B.; Calzadilla, A. Global biomass production potentials exceed expected future demand without the need for cropland expansion. Nat. Commun. 2015, 6, 8946. [Google Scholar] [CrossRef]
- Graham, A.E.; Ledesma-Amaro, R. The microbial food revolution. Nat. Commun. 2023, 14, 2231. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attfield, P.V.; Bell, P.J.L.; Grobler, A.S. Reducing Carbon Intensity of Food and Fuel Production Whilst Lowering Land-Use Impacts of Biofuels. Fermentation 2023, 9, 633. https://doi.org/10.3390/fermentation9070633
Attfield PV, Bell PJL, Grobler AS. Reducing Carbon Intensity of Food and Fuel Production Whilst Lowering Land-Use Impacts of Biofuels. Fermentation. 2023; 9(7):633. https://doi.org/10.3390/fermentation9070633
Chicago/Turabian StyleAttfield, Paul V., Philip J. L. Bell, and Anna S. Grobler. 2023. "Reducing Carbon Intensity of Food and Fuel Production Whilst Lowering Land-Use Impacts of Biofuels" Fermentation 9, no. 7: 633. https://doi.org/10.3390/fermentation9070633
APA StyleAttfield, P. V., Bell, P. J. L., & Grobler, A. S. (2023). Reducing Carbon Intensity of Food and Fuel Production Whilst Lowering Land-Use Impacts of Biofuels. Fermentation, 9(7), 633. https://doi.org/10.3390/fermentation9070633






