Evaluation of the Enzymatic Production and Prebiotic Activity of Galactomannan Oligosaccharides Derived from Gleditsia microphylla
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Gleditsia microphylla Galactomannans
2.3. Component Analysis of Gleditsia microphylla Galactomannans
- Cgal: Concentration of galactose.
- Cman: Concentration of mannose.
2.4. Quantification of Gleditsia microphylla Galactomannan Oligosaccharides
2.5. Preparation of Gleditsia microphylla Galactomannan Oligosaccharides
- YieldGM: yield of galactomannan.
- CSgal: supernatant galactose concentration.
- CSman: supernatant mannose concentration.
- C′Sgal: substrate galactose concentration.
- C′Sman: substrate galactose concentration.
2.6. Purification of Gleditsia microphylla Galactomannan Oligosaccharides
2.6.1. Removal of Pigment
2.6.2. Monosaccharide Removal
2.7. In Vitro Fermentation of Gleditsia microphylla Galactomannan Oligosaccharides
2.7.1. Collection of Human Fecal Matter and In Vitro Batch Fermentation of GMOS
2.7.2. Basic Medium
2.7.3. OD600 of Intestinal Bacteria
2.7.4. Analysis of Short-Chain Fatty Acids
2.7.5. Analysis of Human Intestinal Bacteria during In Vitro Fermentation
2.8. Utilization of GMOS by Intestinal Bacteria
- COS: original sugar concentration.
- CFS: final sugar concentration.
2.9. Statistical Analysis
3. Results and Discussion
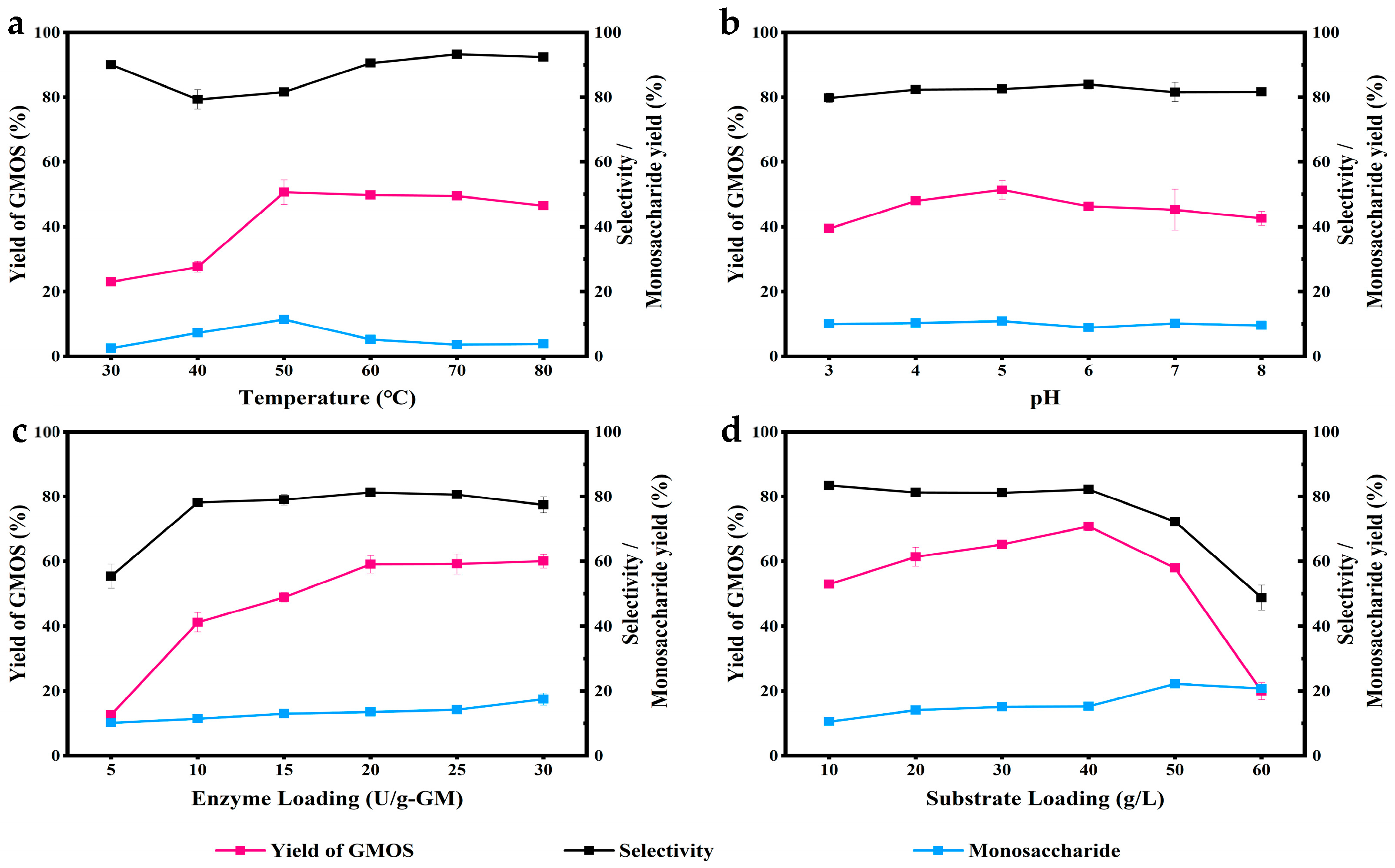
3.1. Optimization of Enzymatic Hydrolysis Conditions for GMOS Production
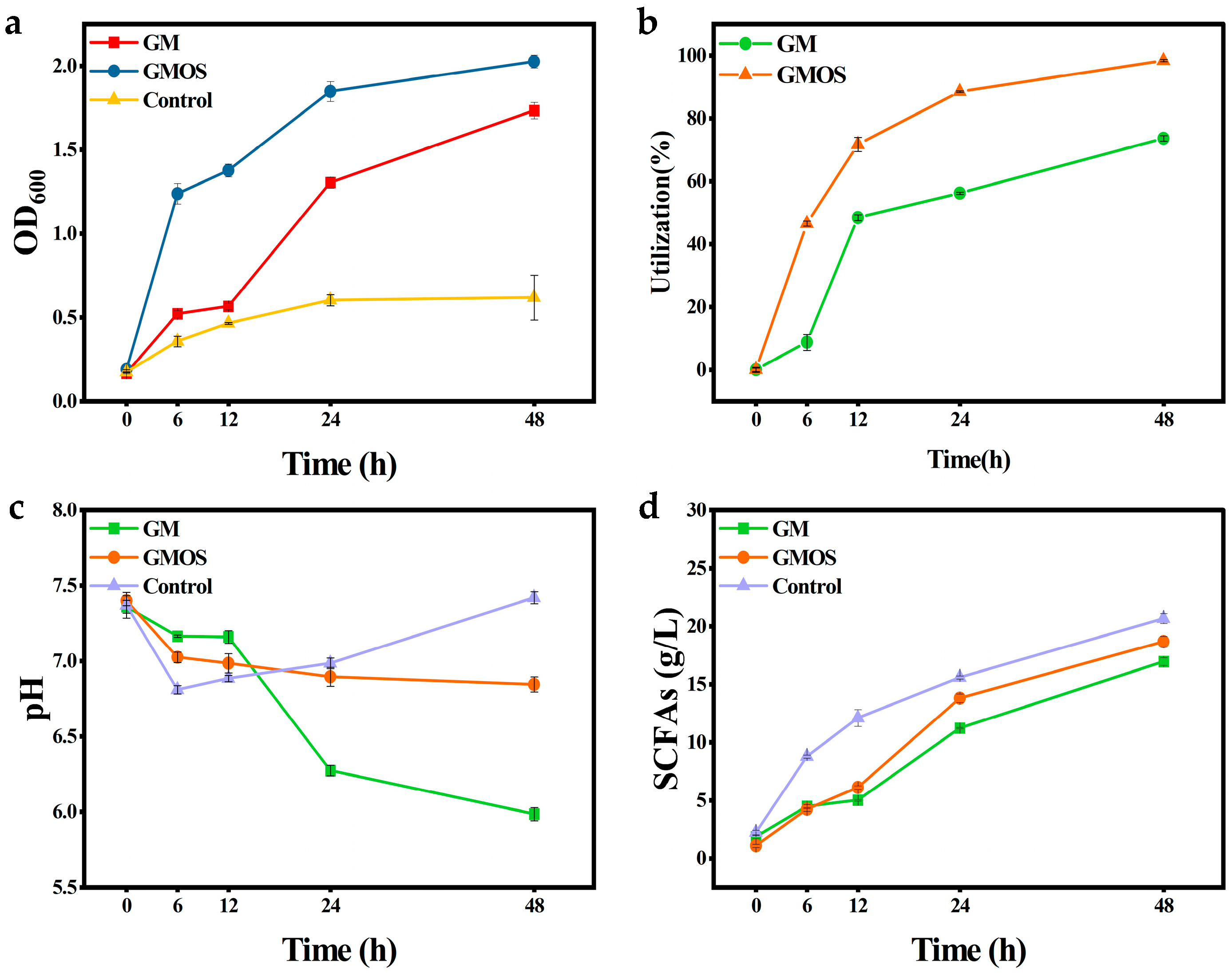
3.2. Effect of GMOS on the Physiological Activity of Intestinal Bacteria
3.3. Diversity Analysis of Human Intestinal Bacteria
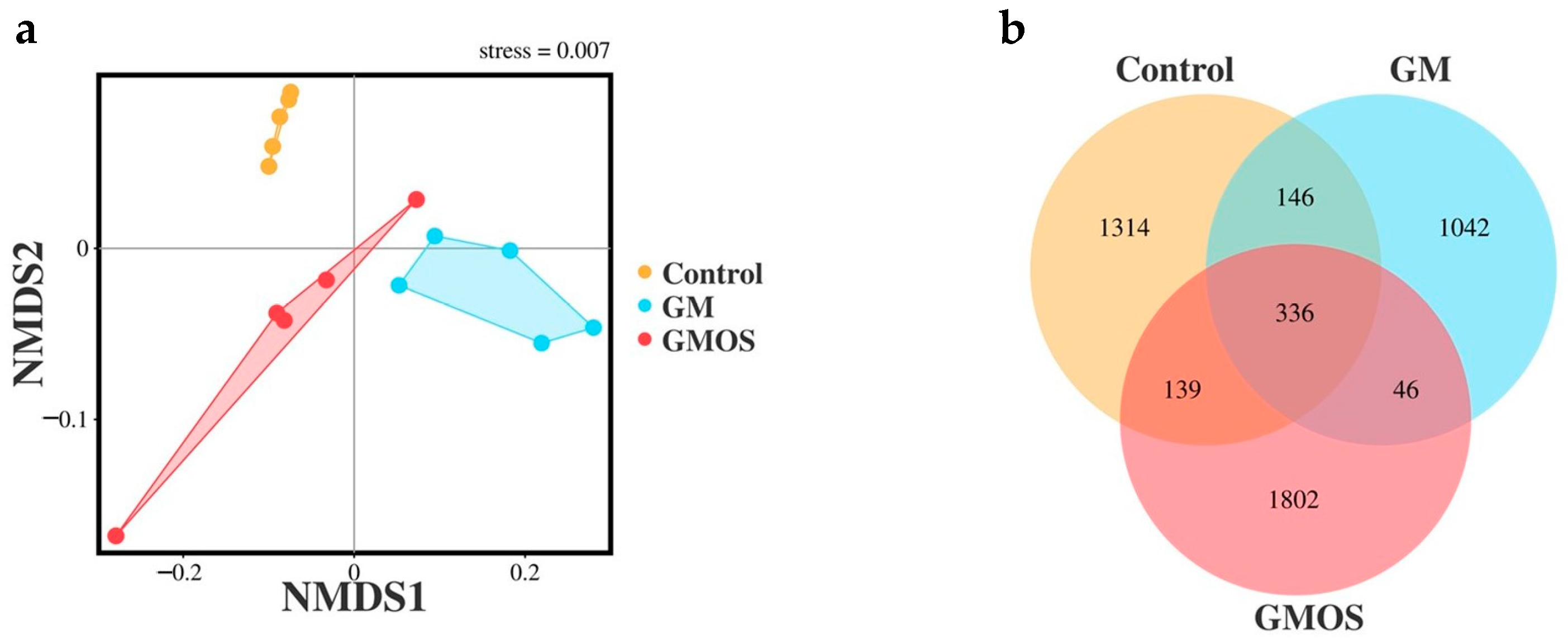
3.3.1. NMDS and Venn Analysis of 16S rRNA Sequencing Data
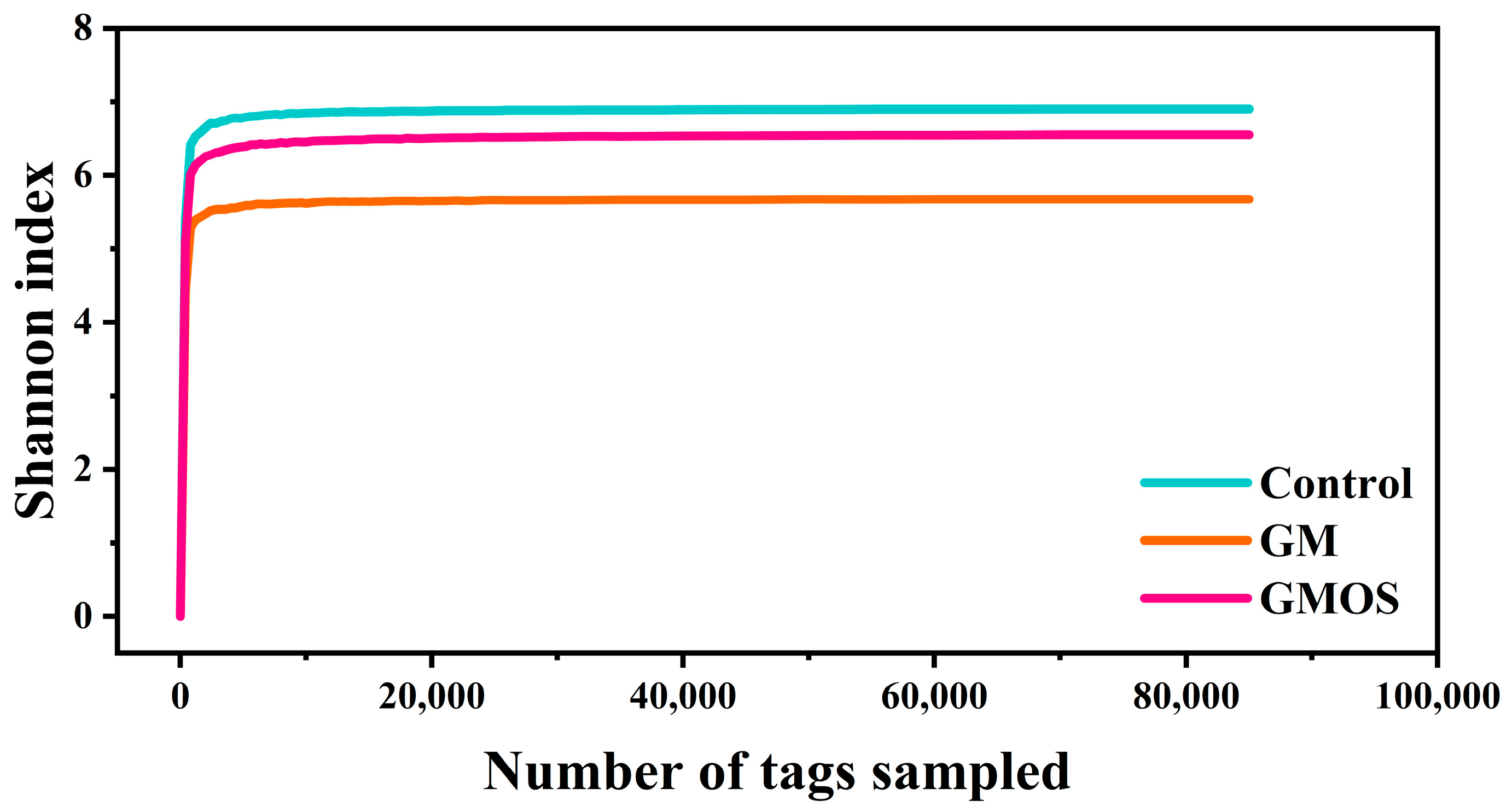
3.3.2. Alpha Diversity of Human Intestinal Bacteria
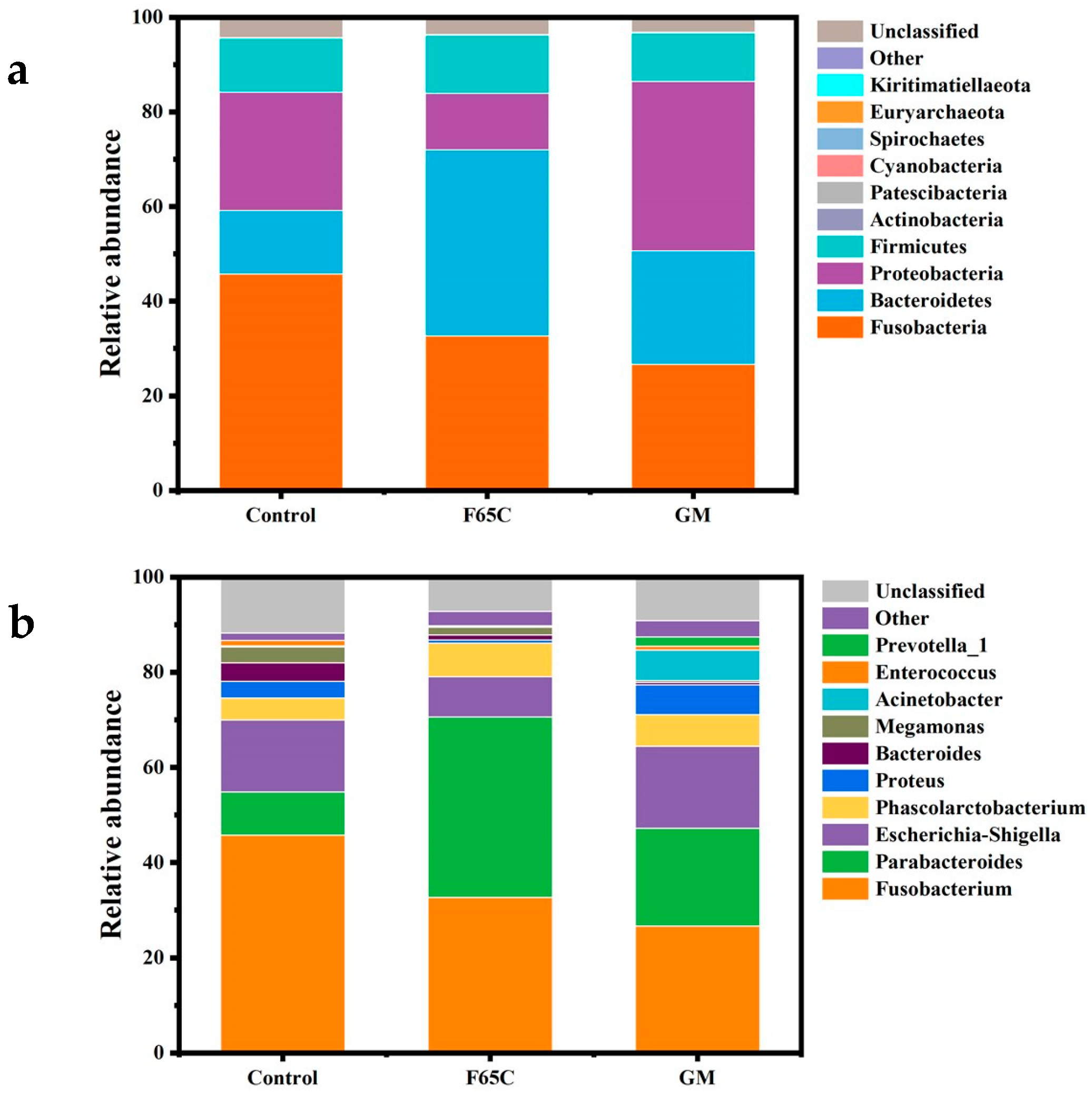
3.3.3. Changes in the Composition of Intestinal Bacteria
3.4. Functional Analysis of Intestinal Bacteria Affected by GMOS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, J.; Zhang, W.; Zhu, L.; Li, X. Exploitation and Distribution of Gleditsia microphylla Resources. Chin. Wild Plant Resour. 2015, 22–23+33. [Google Scholar]
- Takagi, T.; Ducros, V.M.-A.; Flint, J.E.; Roberts, S.M.; Morland, C.; Zechel, D.L.; Smith, N.; Bjørnvad, M.E.; Borchert, T.V.; Wilson, K.S.; et al. Partially hydrolysed guar gum ameliorates murine intestinal inflammation in association with modulating luminal microbiota and SCFA. Br. J. Nutr. 2016, 116, 1199–1205. [Google Scholar] [CrossRef]
- Zhou, M.; Tao, Y.; Wang, T.; Wang, R.; Yong, Q. Assessing the in vitro digestion of Sesbania gum, a galactomannan from S. cannabina, and subsequent impact on the fecal microbiota. J. Funct. Foods 2021, 87, 104766. [Google Scholar] [CrossRef]
- Mathur, N.K. Industrial Galactomannan Polysaccharides; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Tao, Y.; Wang, T.; Huang, C.; Lai, C.; Ling, Z.; Zhou, Y.; Yong, Q. Incomplete degradation products of galactomannan from Sesbania cannabina modulated the cecal microbial community of laying hens. J. Anim. Sci. 2022, 100, skac087. [Google Scholar] [CrossRef] [PubMed]
- Jana, U.K.; Kango, N.; Pletschke, B. Hemicellulose-Derived Oligosaccharides: Emerging Prebiotics in Disease Alleviation. Front. Nutr. 2021, 8, 670817. [Google Scholar] [CrossRef] [PubMed]
- Ganter, J.L.M.; Heyraud, A.; Petkowicz, C.L.; Rinaudo, M.; Reicher, F. Galactomannans from Brazilian seeds: Characterization of the oligosaccharides produced by mild acid hydrolysis. Int. J. Biol. Macromol. 1995, 17, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Pan, B.; Liao, G.; Zhao, Q.; Gao, Y.; Chai, X.; Zhuo, X.; Wu, Q.; Jiao, B.; Pan, W.; et al. Synthesis and immunological studies of β-1, 2-mannan-peptide conjugates as antifungal vaccines. Eur. J. Med. Chem. 2019, 173, 250–260. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Wiemann, M.; Stålbrand, H. β-Mannanase BoMan26B from Bacteroides ovatus produces mannan-oligosaccharides with prebiotic potential from galactomannan and softwood β-mannans. Lebensm.-Wiss. Technol. 2021, 151, 112215. [Google Scholar] [CrossRef]
- Moura, P.; Barata, R.; Carvalheiro, F.; Gírio, F.; Loureiro-Dias, M.C.; Esteves, M.P. In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains. LWT-Food Sci. Technol. 2007, 40, 963–972. [Google Scholar] [CrossRef]
- Ahmad, N.; Zakaria, M.R. Chapter 8-Oligosaccharide From Hemicellulose. In Lignocellulose for Future Bioeconomy; Ariffin, H., Sapuan, S.M., Hassan, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–152. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y.; Li, Z.; Yan, B.; Pei, W.; Wu, H. The preparation technology and application of xylo-oligosaccharide as prebiotics in different fields: A review. Front. Nutr. 2022, 9, 996811. [Google Scholar] [CrossRef]
- Deloule, V.; Boisset, C.; Hannani, D.; Suau, A.; Le Gouellec, A.; Chroboczek, J.; Botté, C.; Yamaryo-Botté, Y.; Chirat, C.; Toussaint, B. Prebiotic role of softwood hemicellulose in healthy mice model. J. Funct. Foods 2020, 64, 103688. [Google Scholar] [CrossRef]
- Pangsri, P.; Piwpankaew, Y.; Ingkakul, A.; Nitisinprasert, S.; Keawsompong, S. Characterization of mannanase from Bacillus circulans NT 6.7 and its application in mannooligosaccharides preparation as prebiotic. Springerplus 2015, 4, 771. [Google Scholar] [CrossRef] [PubMed]
- Andrés-Barranco, S.; Vico, J.P.; Grilló, M.J.; Mainar-Jaime, R.C. Reduction of subclinical Salmonella infection in fattening pigs after dietary supplementation with a ß-galactomannan oligosaccharide. J. Appl. Microbiol. 2015, 118, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ghosh, A.; Goyal, A. Manno-oligosaccharides as prebiotic-valued products from agro-waste. In Biosynthetic Technology and Environmental Challenges; Varjani, S.J., Parameswaran, B., Kumar, S., Khare, S.K., Eds.; Springer: Singapore, 2018; pp. 205–221. [Google Scholar] [CrossRef]
- Tao, Y.; Ma, J.; Huang, C.; Lai, C.; Ling, Z.; Yong, Q. The immunomodulatory activity of degradation products of Sesbania cannabina galactomannan with different molecular weights. Int. J. Biol. Macromol. 2022, 205, 530–538. [Google Scholar] [CrossRef]
- Piskulich, Z.A.; Mesele, O.O.; Thompson, W.H. Activation energies and beyond. J. Phys. Chem. A 2019, 123, 7185–7194. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Reaney, M.J.T.; Ghosh, S. Assessment of gelation behaviour of fenugreek gum and other galactomannans by dynamic viscoelasticity, fractal analysis and temperature cycle. Int. J. Biol. Macromol. 2019, 126, 337–344. [Google Scholar] [CrossRef]
- Yang, L.; Shi, G.; Tao, Y.; Lai, C.; Li, X.; Zhou, M.; Yong, Q. The increase of incomplete degradation products of galactomannan production by synergetic hydrolysis of β-Mannanase and α-galactosidase. Appl. Biochem. Biotechnol. 2021, 193, 405–416. [Google Scholar] [CrossRef]
- Gaisford, S.E.; Harding, S.; Mitchell, J.; Bradley, T. A comparison between the hot and cold water soluble fractions of two locust bean gum samples. Carbohydr. Polym. 1986, 6, 423–442. [Google Scholar] [CrossRef]
- Whistler, R.L.; BeMiller, J.N. Industrial Gums: Polysaccharides and Their Derivatives; Academic Press: Cambridge, MA, USA, 1993. [Google Scholar]
- Malgas, S.; van Dyk, J.S.; Pletschke, B.I. A review of the enzymatic hydrolysis of mannans and synergistic interactions between β-mannanase, β-mannosidase and α-galactosidase. World J. Microbiol. Biotechnol. 2015, 31, 1167–1175. [Google Scholar] [CrossRef]
- Tailford, L.E.; Ducros, V.M.A.; Flint, J.E.; Roberts, S.M.; Morland, C.; Zechel, D.L.; Smith, N.; Bjornvad, M.E.; Borchert, T.V.; Wilson, K.S.; et al. Understanding how diverse β-mannanases recognize heterogeneous substrates. Biochemistry 2009, 48, 7009–7018. [Google Scholar] [CrossRef]
- Yan, B.; Tao, Y.; Huang, C.; Lai, C.; Yong, Q. Using One-pot Fermentation Technology to Prepare Enzyme Cocktail to Sustainably Produce Low Molecular Weight Galactomannans from Sesbania cannabina Seeds. Appl. Biochem. Biotechnol. 2022, 194, 3016–3030. [Google Scholar] [CrossRef] [PubMed]
- Tingirikari, J.M.R. Microbiota-accessible pectic poly- and oligosaccharides in gut health. Food Funct. 2018, 9, 5059–5073. [Google Scholar] [CrossRef] [PubMed]
- Munch Roager, H.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole grain-rich diet reduces body weight and systemic low-grade inflammation without inducing major changes of the gut microbiome: A randomised cross-over trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef]
- Wang, M.; Huang, H.; Wang, L.; Yang, H.; He, S.; Liu, F.; Tu, Q.; He, S. Herbal extract mixture modulates intestinal antioxidative capacity and microbiota in weaning piglets. Front. Microbiol. 2021, 12, 706758. [Google Scholar] [CrossRef]
- Wu, D.T.; He, Y.; Yuan, Q.; Wang, S.; Gan, R.-Y.; Hu, Y.-C.; Zou, L. Effects of molecular weight and degree of branching on microbial fermentation characteristics of okra pectic-polysaccharide and its selective impact on gut microbial composition. Food Hydrocoll. 2022, 132, 107897. [Google Scholar] [CrossRef]
- Li, R.; Tang, N.; Jia, X.; Xu, Y.; Cheng, Y. Antidiabetic activity of galactomannan from Chinese Sesbania cannabina and its correlation of regulating intestinal microbiota. J. Funct. Foods 2021, 83, 104530. [Google Scholar] [CrossRef]
- Abed, J.; Maalouf, N.; Manson, A.L.; Earl, A.M.; Parhi, L.; Emgård, J.E.M.; Klutstein, M.; Tayeb, S.; Almogy, G.; Atlan, K.A.; et al. Colon cancer-associated Fusobacterium nucleatum may originate from the oral cavity and reach colon tumors via the circulatory system. Front. Cell. Infect. Microbiol. 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hu, H.; Zijlstra, R.T.; Zheng, J.; Gänzle, M.G. Metagenomic reconstructions of gut microbial metabolism in weanling pigs. Microbiome 2019, 7, 48. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, H.; Cole, M.; Morris, A.; Martinson, J.; Mckay, H.; Mimiaga, M.; Margolick, J.; Fitch, A.; Methe, B.; et al. Signature changes in gut microbiome are associated with increased susceptibility to HIV-1 infection in MSM. Microbiome 2021, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.N.; Araújo-Pérez, F.; Azcárate-Peril, A.; Yeh, J.J.; Sandler, R.S.; Keku, T.O. Fusobacterium is associated with colorectal adenomas. PLoS ONE 2013, 8, e53653. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Liu, J.-F.; Yang, S.-Z.; Mu, B.-Z. Low-temperature-active and salt-tolerant β-mannanase from a newly isolated Enterobacter sp. strain N18. J. Biosci. Bioeng. 2016, 121, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiang, X.; Gao, Y.; Yuan, L.; Wang, X.; Wu, S.; Xia, Y.; Yao, L.; Yan, J.; Liu, L.; et al. Microbial profiles of patients with antipsychotic-related constipation treated with electroacupuncture. Front. Med. 2021, 8, 737713. [Google Scholar] [CrossRef] [PubMed]
- Ezeji, J.C.; Sarikonda, D.K.; Hopperton, A.; Erkkila, H.L.; Cohen, D.E.; Martinez, S.P.; Cominelli, F.; Kuwahara, T.; Dichosa, A.E.K.; Good, C.E.; et al. Parabacteroides distasonis: Intriguing aerotolerant gut anaerobe with emerging antimicrobial resistance and pathogenic and probiotic roles in human health. Gut Microbes 2021, 13, 1922241. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.R.; Shenvi, S.V.; Widlansky, M.; Suh, J.H.; Hagen, T.M. Lipoic acid as a potential therapy for chronic diseases associated with oxidative stress. Curr. Med. Chem. 2004, 11, 1135–1146. [Google Scholar] [CrossRef]
- Goraca, A.; Skibska, B. Beneficial effect of α-lipoic acid on lipopolysaccharide-induced oxidative stress in bronchoalveolar lavage fluid. J. Physiol. Pharmacol. 2008, 59, 379–386. [Google Scholar]
- Peng, P.; Zhang, X.; Qi, T.; Cheng, H.; Kong, Q.; Liu, L.; Cao, X.; Ding, Z. Alpha-lipoic acid inhibits lung cancer growth via mTOR-mediated autophagy inhibition. FEBS Open Bio 2020, 10, 607–618. [Google Scholar] [CrossRef]

| Samples | Lactate (g/L) | Acetate (g/L) | Propionate (g/L) | Butyrate (g/L) | Total SCFA (g/L) | p Value |
|---|---|---|---|---|---|---|
| GM | 0.49 c | 4.30 b | 5.51 a | 4.15 b | 13.96 | <0.001 |
| GMOS | 0.29 d | 5.41 b | 7.36 a | 3.88 c | 16.65 | <0.001 |
| Control | 0.00 c | 6.04 b | 8.50 a | 6.13 b | 20.67 | <0.001 |
| Group | Control | GM | GMOS | p Value |
|---|---|---|---|---|
| Sobs | 888 a | 723.2 a | 1504.8 b | <0.001 |
| Shannon | 6.91 a | 5.68 b | 6.56 a | <0.001 |
| Simpson | 0.96 | 0.92 | 0.96 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Lai, C.; Tao, Y.; Zhou, M.; Tang, R.; Yong, Q. Evaluation of the Enzymatic Production and Prebiotic Activity of Galactomannan Oligosaccharides Derived from Gleditsia microphylla. Fermentation 2023, 9, 632. https://doi.org/10.3390/fermentation9070632
Wang H, Lai C, Tao Y, Zhou M, Tang R, Yong Q. Evaluation of the Enzymatic Production and Prebiotic Activity of Galactomannan Oligosaccharides Derived from Gleditsia microphylla. Fermentation. 2023; 9(7):632. https://doi.org/10.3390/fermentation9070632
Chicago/Turabian StyleWang, Hanghong, Chenhuan Lai, Yuheng Tao, Mengyi Zhou, Ruilin Tang, and Qiang Yong. 2023. "Evaluation of the Enzymatic Production and Prebiotic Activity of Galactomannan Oligosaccharides Derived from Gleditsia microphylla" Fermentation 9, no. 7: 632. https://doi.org/10.3390/fermentation9070632
APA StyleWang, H., Lai, C., Tao, Y., Zhou, M., Tang, R., & Yong, Q. (2023). Evaluation of the Enzymatic Production and Prebiotic Activity of Galactomannan Oligosaccharides Derived from Gleditsia microphylla. Fermentation, 9(7), 632. https://doi.org/10.3390/fermentation9070632




