Abstract
The use of selected strains of lactic acid bacteria is necessary to produce fermented table olives with high hygiene and quality standards at the industrial level. A current tendency is the use of fermentation adjuvants (nutrients and activators) that can satisfy the nutritional needs of starter strains. In this study, five experimental protocols, different for nutrient and activator presence and addition of Lactiplantibacillus pentosus OM13 in freeze-dried form and after acclimatisation, were tested with the aim of improving the fermentation performances of the commercial starter. The trial inoculated with the starter strain acclimatised in the presence of nutrients and activator showed the most rapid acidification during the first phase of fermentation (third to ninth day), registering a pH loss of 3.40 units. The addition of adjuvants positively influences starter dominance (>89%) and rapid colonisation (>7 Log CFU/mL from third d) by indirectly limiting the presence of undesirable microorganisms. The analysis of volatile organic compounds revealed the presence of 32 chemicals distributed differently in each trial. Sensory evaluation showed that table olives produced with the different treatments were characterised by low bitterness, acidity, and absence of unpleasant odours/flavours. Control production showed slower acidification kinetics and lower sensory pleasantness than the other trials.
1. Introduction
Table olives represent one of the oldest plant-fermented foods in the Mediterranean area. Total world production of table olives in 2020/21 is estimated at around 3 million t [1], and Europe accounts for 68.1%. Spain ranks first (1.3 million t), followed by Italy (basically Puglia, Calabria, and Sicily regions) and Greece, with 366,000 t and 275,000 t, respectively [1].
Olive drupes cannot be consumed without processing because of oleuropein, the glycoside responsible for the bitter taste [2]. Olive debittering can be carried out using different techniques variable among producing countries. The procedure usually applied to process green table olives is the Sevillian method, which requires an alkaline solution (2.0 to 5.0% NaOH (w/v) for 5–10 h) for drupe debittering [3]. NaOH cleaves the oleuropein into three compounds: oleuropein aglycon, elenolic acid, and hydroxytyrosol [4]. When NaOH penetrates into the mesocarp of drupes and reaches 2/3 of the flesh diameter, the treatment is deemed complete [5]. After that, the olives are washed to remove NaOH and to allow the leaching of chemicals generated by hydrolysis. Washing can be performed in several ways; generally, this procedure includes three washes in 12–14 h, during which drupe pH drops to about 7.5–8.0. The drupes are then ready to be fermented in brine. Together with the Sevillan or Spanish style, “natural” or Greek style and Castelvetrano transformation are commonly applied in Italy [6].
The fermentation in brine is basically carried out by lactic acid bacteria (LAB). Selected starter cultures belonging to this group are routinely applied to process table olives at an industrial scale [7]. Lactiplantibacillus plantarum and Lactiplantibacillus pentosus represent the most common species applied in the production of Seville-style table olives [2]. However, starter strain activities can be strongly limited by a non-optimal initial brine pH (due to the presence of soda residues), insufficient nutrients, and inadequate fermentation temperature [8]. The value of brine pH can be easily adjusted with lactic acid [9,10]. Temperature is a process parameter difficult to control during fermentation because of the high volume of the fermentation tanks and the elevated energy required. The selection of starter strains able to grow at high pHs with low nutrient requirements and adapted to room temperature represents a costless solution to the table olive-producing issues [11]. Conte et al. [5] pursued this strategy to produce table olives at the industrial level. The rapid growth of LAB determines fast acidification of the brine, creating a hostile environment for alternative and/or pathogenic microorganisms [7]. Different innovative protocols involving the use of the “pied de cuve” technique, starter strain acclimatisation, and the use of adjuvants have recently been successfully proposed to optimise the rapid increase of LAB cell densities in the early phase of fermentation [9,12]. Although the results obtained were highly valid, it was considered essential to further improve the production protocol in the inoculation phase of the starter strain L. pentosus OM13 by using new nutrient and fermentation activator formulations. To this end, four different experimental fermentations of table olives were studied, using for the first time an activator in combination with the acclimatisation of the starter strain and the use of a specific nutrient previously tested in vitro. The results obtained will make it possible to develop innovative protocols that can be applied to the processing industries of the table olive chain, improving the quality of the product and offering a guarantee to the consumer.
2. Materials and Methods
2.1. Fermentation Process and Brine Sampling
The table olive production process was performed with PDO Nocellara del Belice olives at the industrial plant Geolive Belice s.r.l, located in Castelvetrano (Italy). The drupes were harvested at the green stage of ripeness, and after calibration, they were selected and washed to eliminate vegetation and soil residues. The Sevillan-style production process was applied. After treatment with lye (2.6 °Be for 8 h), 216 kg of olives were subjected to three successive washes with water to remove lye residues and were aliquoted into 12 plastic 25 L volume tanks containing 18 kg each. Five trials were prepared: (i) inoculation of the Lal’Olive Crispy L. pentosus OM13 (Lallemand Inc., Castel D’Azzano, Italy) in freeze-dried form, containing approximately 1.1 × 109 colony-forming units (CFU)/g and food grade maltodextrin as a carrier; (ii) addition of Lal’Olive Activator (Lallemand Inc., Fredericia, Danmark) and inoculation of the Lal’Olive Crispy L. pentosus OM13; (iii) acclimatisation of the Lal’Olive Crispy L. pentosus OM13 starter strain in brine (6% w/v NaCl) for 12 h supplemented with the Lal’Olive Activator; (iv) addition of Lal’Olive Activator, Lal’Olive Nutrient (Lallemand Inc., Fredericia, Danmark) and inoculation of the Lal’Olive Crispy L. pentosus OM13; (v) acclimatisation of the Lal’Olive Crispy L. pentosus OM13 starter strain in brine (6% w/v NaCl) for 12 h supplemented with Lal’Olive Activator and addition of the Lal’Olive Nutrient at inoculation. The dosages used for the activator, starter strain, and nutrient were as follows: 0.0083 g of freeze-dried cells of Lal’Olive Crispy L. pentosus OM13 per Kg olives + brine; 0.033 g of Lal’Olive Activator per kg olives + brine; 0.66 g of Lal’Olive Nutrient per kg olives + brine. Each tank was filled with 4.5 L of brine (reaching a final concentration of 10% w/v NaCl) and closed with a cap. Lal’Olive Nutrient consisted of glucose (400 g/kg), maltodextrin (500 g/kg), and inactivated yeasts (100 g/kg), whereas Lal’Olive Activator consisted of glucose (400 g/kg) and inactivated yeasts (600 g/kg). The entire process was monitored for 195 d (22.0 ± 1.0 °C). This experiment was performed in triplicate (three vats per treatment). The sampling programme included the collection of brine (≈100 mL) at the following times: 0, 3, 6, 9, 15, 35, 65, 143, and 195 d of fermentation. The experimental plan is shown in Figure 1.
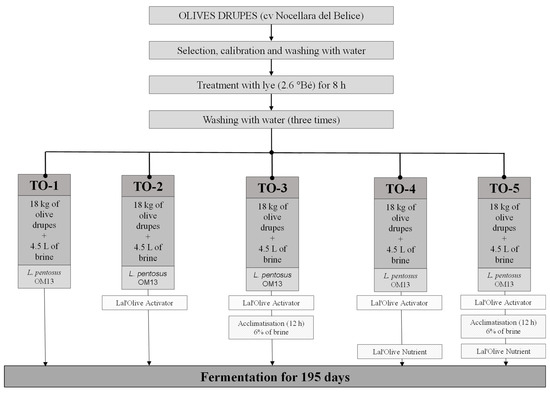
Figure 1.
Experiment plan for green table olive production. Abbreviation: °Bé, Baumè degree; TO-1, code refers to the control trial; TO-2, TO-3, and TO-4 codes refer to the experimental trials; L., Lactiplantibacillus.
2.2. Brine Acidification Kinetics and Microbial Population Monitoring
Brine pH was measured with a Hanna Instruments HI98165 pH meter (Ronchi di Villafranca Padovana, Italy) by direct immersion. All measurements were carried out in duplicate on each sample. Microbiological monitoring was performed by enumerating the following groups: rod LAB on de Man-Rogosa-Sharpe (MRS) agar (Condalab, Torrejón de Ardoz, Spain), total yeast (TY) on dichloran rose bengal chloramphenicol (DRBC) agar (Microbiol, Uta, Italy), Enterobacteriaceae on violet red bile glucose agar (VRBGA; Microbiol, Uta, Italy), Staphylococcaceae on Baird Parker (BP) and coagulase-positive staphylococci (CPS) on BP added with RPF supplement (Oxoid, Milan, Italy), and Pseudomonadaceae on Pseudomonas agar base (PAB) supplemented with CFC supplement (Oxoid, Milan, Italy). Microbial counts were determined according to the methodology reported by Martorana et al. [12]. All samples were analysed in triplicate.
2.3. Dominance of Starter Strain
Presumptive LAB were collected according to the methodology described by Alfonzo et al. [13], and the phenotypic grouping was performed as reported by Martorana et al. [12]. Overnight-grown LAB cells were harvested by centrifugation from MRS broth cultures (1.5 mL) at 10,000 RPM for 5 min. DNA extraction was performed using InstaGene Matrix (Bio-Rad Laboratories, Hercules, CA, USA). The dominance of the L. pentosus OM13 strain was confirmed throughout fermentation by random amplification of polymorphic DNA-PCR (RAPD-PCR) using the procedure described by Rossetti and Giraffa [14]. RAPD-PCR (Swift max PRO, Esco Healthcare, Singapore) was carried out using DreamTaq Green PCR Master Mix (Thermo Fisher Scientific, Rodano, Italy) and the primer M13 (Thermo Fisher Scientific, Rodano, Italy). The PCR conditions for every single cycle were 2 min of initial denaturation at 94 °C, continued up to 40 cycles of amplification. Each cycle consisted of denaturation at 94 °C for 1 min, annealing at 42 °C for 20 s, and extension at 72 °C for 2 min. The last cycle was 10 min at 72 °C for a final extension. Electrophoresis (1.5% agarose gel) was conducted using 1.0X TBE buffer and GeneRuler 100 bp Plus DNA Ladder (M-Medical S.r.l, Milan, Italy), and the gels were visualised with the SYBR® Safe DNA gel stain (Molecular Probes, Eugene, OR, USA) on a UV transilluminator (ECX-F.20M V1, Vilber Lourmat, Germany). All gels were acquired using the KODAK Gel Logic 100 system (Kodak, Rochester, NY, USA). The RAPD-PCR patterns of the isolates were compared to that of the pure culture of L. pentosus OM13 through the GelCompar II software (v. 6.5. Applied-Maths, Sin Marten Latem, Belgium).
2.4. Volatile Organic Compounds
VOCs were identified after 195 d of fermentation using the Solid Phase Micro-Extraction method in Head Space, followed by Gas Chromatography/Mass Spectrometry (HS-SPMEGC/MS) [15]. Sample preparation for analysis was performed as reported by Alfonzo et al. [13]. Fifty microliters of a 2-pentanol-4-methylmethanol (0.981 mg/mL) solution was used as an internal standard. Both fibers used and the SPME (Supelco, Bellefonte, PA, USA) fiber receptacle were treated with divinylbenzene/carboxen/polydimethylsiloxane. Vials were heated at a defined temperature (40 ± 0.5 °C) for 30 min to establish equilibrium. GC-MS parameters applied were those reported by Corona et al. [16]. VOCs were identified by comparing their mass spectra and GC retention times with pure reference chemicals and the NIST/EPA/NIH Mass Spectra Library (version 2.0d, build 2005). In the absence of a commercially available standard, VOCs were identified by comparing their mass spectra with those in the NIST library or published in the literature. The identified compounds were quantified according to the internal standard used. All analyses were carried out in triplicate.
2.5. Sensory Analysis
Sensory characteristics of table olives processed in the different trials were evaluated following the methodology described in ISO 13299:2016 [12,17,18,19]. Olive samples for sensory analysis were taken from the tanks after 195 d and rinsed in water to remove excess salt. The tasting panel consisted of 20 judges (10 men and 10 women, aged between 29 and 64). In order to establish a common method for describing sensory attributes, the panel first tasted commercial samples of Nocellara del Belice table olives produced by the Sevillan-style method. Each sample was coded with a five-digit alphanumeric code and served in odourless plastic trays at room temperature in individual soundproof booths with white incandescent lighting. During the preliminary sessions, 13 descriptors relating to appearance (intensity and brightness of the green colour), odour/aroma (green olives), rheological characteristics (crispiness), taste (juicy, acid, bitter, salty, astringent, sweet, and overall acceptability), unpleasant odours/off-odour, and unpleasant flavours/off-flavour were identified. The quantitative definition of each sensory attribute was carried out using a 9-point hedonic scale, as reported by Randazzo et al. [20].
2.6. Statistical and Multivariate Analysis
The ANOVA test was used to analyse data on pH, microbiological counts, and VOCs. Tukey’s test was used to compare the mean. The level of significance was set at p < 0.001. Principal component analysis (PCA) was used to determine the distribution of the different trials in relation to the concentration of each VOC. The bootstrap hulls option was used to obtain the confidence areas for each trial [21]. Sensory product characterisation was used to discriminate trials in terms of the descriptors [22]. A model representing the effect of product, judge, and session was used to score each attribute. The analyses were carried out using the XLStat software version 2019.2.2 (Addinsoft, New York, NY, USA).
3. Results and Discussion
3.1. Microbiological and Physicochemical Analysis
3.1.1. Brine Acidification
Before fermentation, the average pH of the brine in the five trials was 8.48 ± 0.10. These high pH values are justified by the lye debittering treatment, which inevitably raises the pH of the brine, even when rinsed with water [23]. From the third to the ninth day of fermentation, trial TO-5 showed a significant loss of pH units (3.40), whereas the combined use of activator/acclimatisation period (TO-3) and activator/nutrient (TO-4) had no different effect on the acidification kinetics (Figure 2).
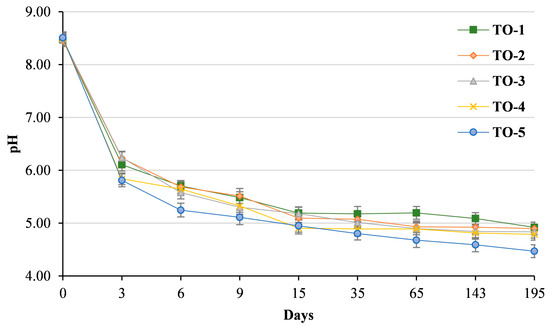
Figure 2.
Brine acidification dynamics observed during the Seville-style table olive production process. Treatments: TO-1, control trial (without activator, nutrient, or acclimatisation time); TO-2, Lal’Olive Activator; TO-3, Lal’Olive Activator and acclimatisation (12 h) in brine (6% NaCl); TO-4, Lal’Olive Activator and Lal’Olive Nutrient; TO-5, Lal’Olive activator, and acclimatisation (12 h) in brine (6% NaCl) and Lal’Olive Nutrient.
From day 15 to day 35, the pH values did not show consistent differences, but from the 65th day to the end of the fermentation process, trial TO-5 was characterised by the lowest pH values (4.47 at day 195). In the control trial (TO-1), the registered decrease in pH showed a similar trend to that described by Alfonzo et al. [13]. The use of the activator without (TO-2) or with acclimatisation treatment (TO-3) showed no benefit in terms of brine acidification. The pH value below 4.5 registered for trial TO-5 is important to keep the microbiological safety of the final product because the growth of most pathogenic and alternative microorganisms is inhibited at this pH level [24].
3.1.2. Lactic Acid Bacteria
The population dynamics of LAB are shown in Figure 3A. The treatment involving the use of an activator, nutrient, and acclimatisation period (TO-5) achieved the highest microbial load (7.1 Log CFU/mL; third day) compared to the other experimental productions. From the 6th until the 15th day of fermentation, the control trial (TO-1) showed the lowest counts ranging from 6.0 Log CFU/mL (0 d) to 7.2 Log CFU/mL (15 d). The highest LAB population levels were recorded in TO-5 up to day 195. At 35, 65, and 195 days, no significant differences were found between the other trials. The cell acclimatisation procedure in the presence of the activator and nutrient resulted in improved LAB levels within the first three days of fermentation. The higher counts, varying between 0.2 and 1.2 log cycles, clearly demonstrate the importance of using these adjuvants to improve the fermentation performance of the starter strain OM13. LAB dynamics showed a slightly different trend, probably due to the absence of adjustment of the initial brine pH with lactic acid [5]; a similar trend was reported by Alfonzo et al. [9]. The practice of acidification of brine through the addition of lactic acid or hydrochloric acid is used to improve the survival and dominance of the inoculated starter strain [5,25]. In this case, the use of a nutrient, activator, and acclimatisation period positively influenced LAB population levels, especially in the first 15 d of fermentation. LAB dynamics were comparable to those of table olive production with artificially acidified brine [9,26].
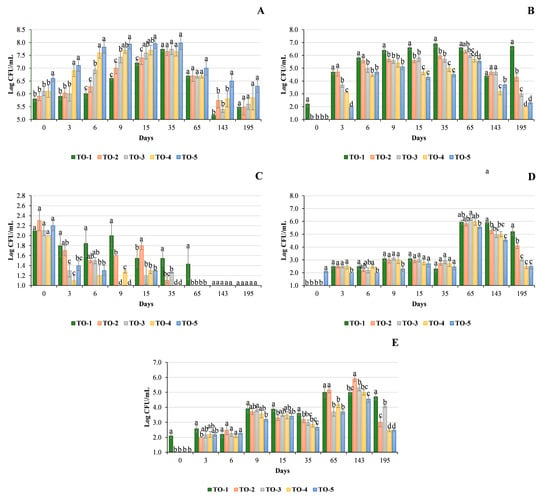
Figure 3.
Microbial load (Log CFU/mL) of samples during the production of table olives: (A) Lactic acid bacteria; (B) Yeasts; (C) Enterobacteriaceae; (D) Pseudomonadaceae; (E) Staphylococcaceae. Treatments: TO-1, control trial (without activator, nutrient, or acclimatisation time); TO-2, Lal’Olive Activator; TO-3, Lal’Olive Activator, and acclimatisation (12 h) in brine (6% NaCl); TO-4, Lal’Olive Activator, and Lal’Olive Nutrient; TO-5, Lal’Olive activator, and acclimatisation (12 h) in brine (6% NaCl) and Lal’Olive Nutrient. Results indicate mean values ± standard deviation of three experimental productions. Different superscript letters indicate significant differences in microbial concentrations were performed at each sampling time according to Tukey’s test between table olive productions for p < 0.05.
3.1.3. Yeasts
Initially, yeast load was below the detection limit in all treatments except trial TO-1 trial (2.2 log CFU/mL; Figure 3B). A low presence or absence of yeasts at the beginning of the fermentation process for Nocellara Del Belice table olives processed according to the Sevillan-style method is a common observation [9,26]. Regarding samplings performed during transformation, the growth dynamics of yeast populations showed the same trend reported by Alfonzo et al. [13]. In TO-1, yeast populations were at the highest level at 35 days (6.9 Log CFU/mL), whereas in the other trials, the maximum growth levels were observed at 65 days (TO-2 = 6.3 Log CFU/mL; TO-3 = 6.0 Log CFU/mL; TO-4 = 5.7 Log CFU/mL; TO-5 = 5.5 Log CFU/mL). At 143 days, all experimental groups showed a decline in the yeast population ranging from 1.3 (TO-3) to 2.2 (TO-1) log cycles. At the end of the process, microbial densities showed differences between treatments ranging from 2.0 (TO-4) to 6.7 log CFU/mL (TO-1). The addition of a nutrient and an activator indirectly affected the level of yeast populations, as the yeast microbial load detected in the control treatment (without adjuvants) was higher than in all other treatments. Normally, yeast populations were about 2 log cycles lower than LAB (24). This trend was not observed in the control production (TO-1), where the ratio between LAB/yeast concentrations was slightly shifted towards LAB.
3.1.4. Enterobacteriaceae
The presence of Enterobacteriaceae in table olives represents a serious problem [27]. In the first few days of fermentation, the presence of high levels of this family can cause “gas pockets”, softening, and breaking of the pulp [28]. At the beginning of the fermentation process, Enterobacteriaceae were in the range of 2.0–2.3 log CFU/mL (Figure 3C). TO-3 (1.3 log CFU/mL) and TO-4 (1.1 log CFU/mL) had the lowest levels after three days. The counts varied between trials from 6 to 35 days and never exceeded 2 log cycles.
Except for the control (TO-1), Enterobacteriaceae levels were below the detection limit at 65 days in all trials. The presence of this microbial group was not detected at 195 days. This trend seems to reflect the microbial load determined in other table olive productions using the same style [13,26]. In this case, the presence of the nutrient and activator did not significantly stimulate the growth of Enterobacteriaceae. Probably, lowering the pH effectively controlled the proliferation of this microbial group [28,29].
3.1.5. Pseudomonadaceae
The population of Pseudomondaceae showed an inverse trend to that of Enterobacteriaceae (Figure 3D). Campaniello et al. [30] reported a low presence of Pseudomonas spp. in the first phases of the table olive fermentation process, followed by an increase after 30 d. Indeed, up to day 35, the differences between trials were minimal, and the counts ranged from 2.0 to 3.2 log CFU/mL. At day 65, trials TO-1 and TO-2 showed concentrations around 5 log CFU/mL, and this same trend was observed at day 143 for all trials except T-O5 (4.5 log CFU/mL). At the end of the period, TO-3, TO-4, and TO5 showed the lowest value (2.5–3.0 log CFU/mL). Pseudomonas spp. are a cause of deterioration in table olives when their presence is high [31]. In fact, they are considered to be proteolytic alternative microorganisms that cause a decrease in the acidity of the brine, swelling of the drupes and, indirectly, production of biogenic amines [30,31]. During the 195 days of fermentation, trial TO-5, which included acclimatisation of the starter strain, the use of the activator, and the nutrient, showed the lowest concentrations of pseudomonads.
3.1.6. Staphylococcaceae
The growth dynamics of staphylococci were similar to those of pseudomonads (Figure 3E). Coagulase-positive staphylococci include pathogenic species for humans [32]. Up to 6 d, their presence ranged from 2.0 to 2.6 log CFU/mL with negligible differences between treatments. The load of total staphylococci was approximately 5.0 log CFU/mL on day 65 for trials TO-1 and TO-2 and on day 143 for the other trials. At the end of the fermentation process, trials TO-4 and TO-5 showed the lowest values (2.5 log CFU/mL). The presence of coagulase-positive staphylococci was not detected in any sampling. Due to the low pH and natural suppression by olive phenols, the presence of coagulase-positive staphylococci in Seville-style processed table olives has only occasionally been reported [9,26,33,34,35].
3.1.7. Dominance of the Starter Strain L. pentosus OM13
A total of 871 colonies of presumptive LAB were picked up from Petri dishes containing the highest cell dilutions from olive brines. After purification, they were characterized microscopically. Seven hundred and fifty-eight Gram-positive, catalase-negative, and rod-shaped isolates were considered LAB and grouped according to the experimental trial and day of sampling. All isolates were subjected to RAPD-PCR analysis to assess the similarity of the polymorphic profiles with that of the inoculated starter strain L. pentosus OM13. The presence of LAB isolates with the same profile as the starter strain was variable in relation to the combination of acclimatisation period/activator/nutrient. In the control production (TO-1), the percentage of starter strain detected was 78.7%. When the acclimatisation period was carried out in the presence of the activator (TO-3), the percentage of the dominance of strain OM13 increased to 85.3% compared to the trial where only the activator was used (TO-2; 80.2%). The acclimatisation period in the presence of activator and nutrient (TO-5) further increased the percentage of RAPD profiles similar to strain OM13 (89.8%), whereas, in TO-4 (activator and nutrient), the dominance was only 83.6%. The acclimatisation phase [26] and the use of nutrients [13] allowed to increase the dominance of strain OM13 in previous trials. However, in this experiment, the use of the activator proved essential to further increase the dominance percentage of the starter strain OM13, especially in the first phase of fermentation, thus avoiding an unwanted increase in the indigenous LAB population.
3.2. Volatile Organic Compound Characterisation
The volatile organic compounds (VOCs) for all experimental productions were determined at the end of the monitoring period (195th day; Table 1).

Table 1.
Distribution of volatile organic compounds (μg/kg) in the different treatments after 195 days of fermentation.
Thirty-two compounds were identified by HS-SPMEGC/MS. The most abundant class of chemical emitted was that of phenols (35,859.34 μg/kg), followed by the class of acids (29,670.89 μg/kg), alcohols (14,766.97 μg/kg), esters (2059.00 μg/kg), aldehydes (1824.86 μg/kg), ketones (815.77 μg/kg), and aromatic hydrocarbons (729.89 μg/kg). Among phenols, creosol was the most abundant compound, detected at the highest abundance in trial TO-5 and at lower levels in TO-1. A high cresol content is an indication of quality for olives [36]. This compound provides olives with smoky, sweet, and spicy odours, and its content decreases when alternative microorganisms develop at high numbers [37,38]. The other phenols detected at high levels were p-cresol in TO-5, guaiacol, and phenol in TO-4. The presence of guaiacol is associated with the fermentation activity of microorganisms [39]. The odors emitted by this molecule are related to woody and smoky [40]. Acetic acid, the most representative compound among the class of acids, was detected at the highest levels in the control trial (TO-1). High levels of acetic acid are generally produced by heterofermentative LAB [41], and L. pentosus OM13, being facultative heterofermentative, can produce acetic acid under stress conditions [42]. Indeed, the trials added with activator and/or nutrient showed acetic acid levels at almost half of those of the control trial. Phenylethyl alcohol was the most relevant compound among the class of alcohols and was mostly present in trials TO-3 and TO-5. The presence of this alcohol represents a positive feature, since it is responsible for floral and fruity notes [43]. Among esters, the highest concentration was registered for cis-3-hexenyl acetate (in TO-2 and TO-3) and phenylmethyl acetate (in TO-5). Cis-3-Hexenyl acetate has been reported in Manzanilla table olives produced in the Seville style and is associated with notes of ripened fruits [43,44]. For the other groups, the compounds present in high amounts were benzaldehyde, 4-ethyl acetophenone, and alpha-cubebene. Benzaldehyde is one of the most abundant aldehydes in table olives [45]. Its presence is due to enzymatic reactions or fermentation processes by LAB [46,47]. Alpha-cubebene and 4-Ethylacetophenone have been reported at lower levels in Nocellara del Belice [19] and Brandofino [17] table olives. Some compounds, such as benzaldehyde-3-ethyl and ethyl cyclohexanecarboxylate, have been detected in TO-2 and TO-3, respectively. The latter compound is responsible for fruity odours [48].
The concentration of each VOC measured for each trial was subjected to PCA analysis (Figure 4). The total variance (72.92%) was explained by two factors (F1 = 45.71% and F2 = 27.20%), as shown in Figure 4A. F1 correlated positively with 27 compounds (nonal, 4-ethyl acetophenone, phenylacetaldehyde, benzyl alcohol, butanoic acid, and 1-octanol, with correlation > 0.90) and negatively with 5 compounds (methyl cyclohexanoate, phenol, guaiacol, octanal, and acetic acid). F2 correlated positively with 18 compounds and negatively with 14. Trials TO-2, TO-3, and TO-5 were closely and positively associated with F1, showing a correlation with compounds positively correlated with this factor. In contrast, trials TO-1 and TO-4 were negatively correlated with F1, especially for acetic acid. However, trials TO-1, TO-2, and TO-3 were negatively correlated with F2, whereas trials TO-4 and TO-5 were positively correlated with F2. The confidence intervals for each trial are shown in the bootstrap hulls plot (Figure 4B). A higher overlap of areas was observed in trials TO-2 and TO-3 trials. This indicated a similar VOC composition. In contrast, TO-4 was the experimental production with a larger confidence area and less overlap with the other trials.
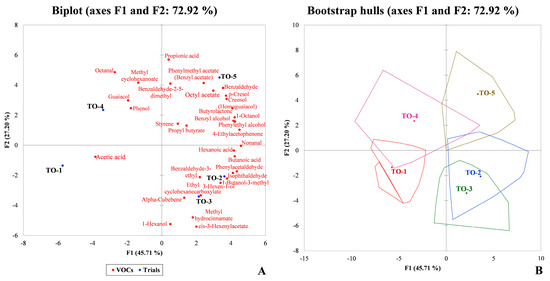
Figure 4.
Biplot (A) and bootstrap hulls plot (B) from principal component analysis (PCA) of VOCs detected in the five experimental table olive productions. Abbreviations: TO-1, control trial (without activator, nutrient, and acclimatisation time); TO-2, Lal’Olive Activator; TO-3, Lal’Olive Activator, and acclimatisation (12 h) in brine (6% NaCl); TO-4, Lal’Olive Activator, and Lal’Olive Nutrient; TO-5, Lal’Olive activator, and acclimatisation (12 h) in brine (6% NaCl) and Lal’Olive Nutrient.
3.3. Sensory Evaluation of Final Products
It is well known that the sensory profile of table olives is strongly influenced by the production protocol and the starters used for fermentation [5]. In this study, a given starter strain (L. pentosus OM13) was tested in different conditions represented by the presence of activator, nutrient, and acclimatisation period in various combinations, and Figure 5 depicts sensory analysis results. The attributes with the highest discriminating power among the 13 descriptors used to establish the sensory profiles of table olives were overall acceptability and odour/aroma of green olives, whereas the attribute with the lowest discriminating power was unpleasant flavours/off-flavour. These results partially support the findings of Martorana et al. [26], who observed that treatments involving brine acidification with lactic acid, nutrient addition, and acclimatisation of LAB starters for 12 h increased the descriptors of green olive aroma and overall acceptability of Nocellara del Belice table olives. Similarly, in the sensory evaluation of Manzanilla table olives prepared in the Seville style, both descriptors were considered discriminative [49].
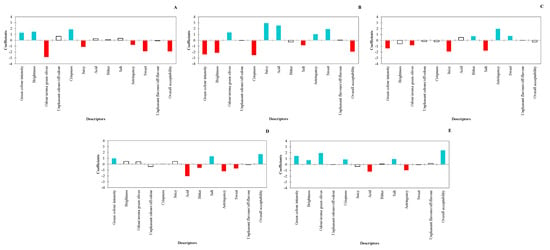
Figure 5.
Sensory profiles of table olives: (A) TO-1, control trial (without activator, nutrient, or acclimatisation time); (B) TO-2, Lal’Olive Activator; (C) TO-3, Lal’Olive Activator, and acclimatisation (12 h) in brine (6% NaCl); (D) TO-4, Lal’Olive Activator, and Lal’Olive Nutrient; (E) TO-5, Lal’Olive activator, and acclimatisation (12 h) in brine (6% NaCl) and Lal’Olive Nutrient. Blue-colored histograms are associated with coefficients that have a significantly positive value, while red-colored histograms are associated with coefficients that have a significantly negative value.
The sensory characterisation of the product-defined coefficients for these 13 descriptors that were quantified by how much the calculated value was significantly above or below the overall average. Processed olives from trial TO-1 showed crispness but did not exhibit odour/aroma of green olives. Both descriptors are usually considered positive for table olive appreciation [5,18]. Olives resulting from trial TO-2 were juicy but did not show crispness. Those from trial TO-3 were astringent and did not display juiciness, while those from trials TO-4 and TO-5 showed high overall acceptability and were not acid. Specifically, trial TO-5 olives showed six descriptors (green color intensity, brightness, crispness, salt, overall acceptability, and odour/aroma of green olives) with values above the global mean and two (acid and astringency) below the global mean, while for those from trial TO-4, three descriptors (green color intensity, salt, and overall acceptability) were above the average and four attributes (bitter, acid, astringency, and sweet) below the global mean. Acid and bitter olives from trials TO-4 and TO-5 reached values below the global mean, while salt in TO-4 reached values above the mean. No unpleasant odours/off-odour or unpleasant flavours/off-flavour were perceived by the panelists in any of the experimental olive productions. These results clearly showed how the sensory profile of fermented table olives is directly affected by parameters considered during the production process [50].
4. Conclusions
This work demonstrated that the acclimatisation of starter LAB before inoculation, as well as the addition of activator and nutrient adjuvants, significantly shortened the acidification process of olive brine during 195 days of observation. These strategies consistently improved the safety and sensory quality of the final products. Indeed, the groups of alterative and/or potentially pathogenic microorganisms (yeasts, Enterobacteriaceae, Staphylococcaceae, and Pseudomonadaceae) showed a significant reduction in microbial load in the protocol involving the use of adjuvants (activator and nutrient) and the acclimatisation period. This study provides useful insights for the production of table olives at a large-scale level; a shorter acidification period means a more rapid transformation of the drupes, lower losses of non-compliant olives, and a reduction of production costs than traditional protocol. Future applications will be carried out in order to develop the optimal fermentation protocol on an industrial scale.
Author Contributions
Conceptualization, A.A. and G.M.; methodology, A.A., O.C. and N.F.; software, A.A. and O.C.; validation, A.A., R.G. and L.S.; formal analysis, V.S. and V.N.; investigation, A.A., F.L.C. and G.M.; resources, A.A., F.L.C. and G.M.; data curation, L.S., V.N., V.S. and N.F.; writing—original draft preparation, A.A.; writing—review and editing, A.A., L.S. and G.M.; visualization, A.A., N.F., F.L.C. and R.G.; supervision, A.A. and G.M.; project administration, G.M.; funding acquisition, G.M. All authors have read and agreed to the published version of the manuscript.
Funding
The publication was made with the co-financing of the European Union-FESR or FSE, PON Research and Innovation 2014-2020-DM 1062/2021. Ministry of University and Research in Italy, CUP B7521002300001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data included in this study are available upon request by contacting the corresponding author.
Acknowledgments
The authors would like to thank: (i) Lallemand Inc. (Montreal, Canada), Paola Vagnoli and Sibylle Krieger-Weber for their financial contribution concerning the adjuvants used in table olive production protocols; (ii) the staff of Geolive Belice S.r.l. (Castelvetrano, Italy) for technical assistance in the production of table olives.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following acronyms are used in this manuscript:
| BP | Baird Parker |
| CFC | Cetrimide–Fucidin–Cephalothin |
| CFU | Colony forming units |
| CPS | Coagulase positive staphylococci |
| DRBC | Dichloran rose bengal chloramphenicol |
| LAB | Lactic acid bacteria |
| MRS | de Man-Rogosa-Sharpe |
| PAB | Pseudomonas agar base |
| PCA | Principal component analysis |
| PCR | Polymerase chain reaction |
| PDO | Protected Designation of Origin |
| RAPD | Random Amplification of Polymorphic DNA |
| RFP | Rabbit Plasma Fibrinogen |
| VOC | Volatile Organic Compounds |
| VRBGA | Violet red bile glucose agar |
References
- Sumrah, M.A.; Jan, M.; Hussain, A.; Akhtar, S.; Nawaz, H.; Afzal, M.; Umar, H. Evaluation of some promising varieties of olive (Olea europaea L.) for growth and yield under Pothwar Regions of Punjab, Pakistan. Pak. J. Agric. Res. 2021, 34, 446–453. [Google Scholar] [CrossRef]
- Perpetuini, G.; Prete, R.; Garcia-Gonzalez, N.; Khairul Alam, M.; Corsetti, A. Table olives more than a fermented food. Foods 2020, 9, 178. [Google Scholar] [CrossRef]
- Gómez, A.H.S.; García, P.G.; Navarro, L.R. Elaboration of table olives. Grasas Aceites 2006, 57, 86–94. [Google Scholar]
- Medina, E.; García, A.; Romero, C.; De Castro, A.; Brenes, M. Study of the anti-lactic acid bacteria compounds in table olives. Int. J. Food Sci. Technol. 2009, 44, 1286–1291. [Google Scholar] [CrossRef]
- Conte, P.; Fadda, C.; Del Caro, A.; Urgeghe, P.P.; Piga, A. Table olives: An overview on effects of processing on nutritional and sensory quality. Foods 2020, 9, 514. [Google Scholar] [CrossRef]
- Ambra, R.; Natella, F.; Bello, C.; Lucchetti, S.; Forte, V.; Pastore, G. Phenolics fate in table olives (Olea europaea L. cv. Nocellara del Belice) debittered using the Spanish and Castelvetrano methods. Food Res. Int. 2017, 100, 369–376. [Google Scholar] [CrossRef]
- Portilha-Cunha, M.F.; Macedo, A.C.; Malcata, F.X. A review on adventitious lactic acid bacteria from table olives. Foods 2020, 9, 948. [Google Scholar] [CrossRef]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozès, N. Lactic acid bacteria from fermented table olives. Food Microbiol. 2012, 31, 1–8. [Google Scholar] [CrossRef]
- Alfonzo, A.; Martorana, A.; Settanni, L.; Matraxia, M.; Corona, O.; Vagnoli, P.; Caruso, T.; Moschetti, G.; Francesca, N. Approaches to improve the growth of the starter lactic acid bacterium OM13 during the early stages of green Spanish-style table olive production. Grasas Aceites 2018, 69, e265. [Google Scholar] [CrossRef]
- Boskou, D.; Camposeo, S.; Clodoveo, M.L. Table Olives as Sources of Bioactive Compounds; AOCS Press: Urbana, IL, USA, 2015. [Google Scholar]
- Ruiz-Barba, J.L.; Jiménez-Díaz, R. A novel Lactobacillus pentosus-paired starter culture for Spanish-style green olive fermentation. Food Microbiol. 2012, 30, 253–259. [Google Scholar] [CrossRef]
- Martorana, A.; Alfonzo, A.; Settanni, L.; Corona, O.; La Croce, F.; Caruso, T.; Moschetti, G.; Francesca, N. An innovative method to produce green table olives based on “pied de cuve” technology. Food Microbiol. 2015, 50, 126–140. [Google Scholar] [CrossRef]
- Alfonzo, A.; Naselli, V.; Gaglio, R.; Settanni, L.; Corona, O.; La Croce, F.; Vagnoli, P.; Krieger-Weber, S.; Francesca, N.; Moschetti, G. Use of different nutrients to improve the fermentation performances of Lactiplantibacillus pentosus OM13 during the production of Sevillian style green table olives. Microorganisms 2023, 11, 825. [Google Scholar] [CrossRef]
- Rossetti, L.; Giraffa, G. Rapid identification of dairy lactic acid bacteria by M13-generated, RAPD-PCR fingerprint databases. J. Microbiol. Methods 2005, 63, 135–144. [Google Scholar] [CrossRef]
- Pawliszyn, J. Solid Phase Microextraction: Theory and Practice; Wiley-VCH: New York, NY, USA, 1997. [Google Scholar]
- Corona, O.; Liguori, L.; Albanese, D.; Di Matteo, M.; Cinquanta, L.; Russo, P. Quality and volatile compounds in red wine atdifferent degrees of dealcoholization by membrane process. Eur. Food Res. Technol. 2019, 245, 2601–2611. [Google Scholar] [CrossRef]
- Aponte, M.; Ventorino, V.; Blaiotta, G.; Volpe, G.; Farina, V.; Avellone, G.; Lanza, C.M.; Moschetti, G. Study of green Sicilian table olive fermentations through microbiological, chemical and sensory analyses. Food Microbiol. 2010, 27, 162–170. [Google Scholar] [CrossRef]
- Aponte, M.; Blaiotta, G.; La Croce, F.; Mazzaglia, A.; Farina, V.; Settanni, L.; Moschetti, G. Use of selected autochthonous lactic acid bacteria for Spanish-style table olive fermentation. Food Microbiol. 2012, 30, 8–16. [Google Scholar] [CrossRef]
- Martorana, A.; Alfonzo, A.; Settanni, L.; Corona, O.; La Croce, F.; Caruso, T.; Moschetti, G.; Francesca, N. Effect of the mechanical harvest of drupes on the quality characteristics of green fermented table olives. J. Sci. Food Agric. 2016, 96, 2004–2017. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Todaro, A.; Pino, A.; Pitino, I.; Corona, O.; Mazzaglia, A.; Caggia, C. Giarraffa and Grossa di Spagna naturally fermented table olives: Effect of starter and probiotic cultures on chemical, microbiological and sensory traits. Food Res. Int. 2014, 62, 1154–1164. [Google Scholar] [CrossRef]
- Piochi, M.; Chiavaro, E.; Cichelli, A.; Torri, L.; Cerretani, L. Sensory propertiers of iodine-biofortified potatoes. Ital. J. Food Sci. 2021, 33, 52–60. [Google Scholar] [CrossRef]
- Olawoye, B.; Gbadamosi, S.O. Sensory profiling and mapping of gluten-free cookies made from blends Cardaba banana flour and starch. J. Food Process. Preserv. 2020, 44, e14643. [Google Scholar] [CrossRef]
- Argyri, A.A.; Nisiotou, A.A.; Mallouchos, A.; Panagou, E.Z.; Tassou, C.C. Performance of two potential probiotic Lactobacillus strains from the olive microbiota as starters in the fermentation of heat shocked green olives. Int. J. Food Microbiol. 2014, 171, 68–76. [Google Scholar] [CrossRef]
- Perricone, M.; Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Use of Lactobacillus plantarum and glucose to control the fermentation of “Bella di Cerignola” table olives, a traditional variety of Apulian region (Southern Italy). J. Food Sci. 2010, 75, M430–M436. [Google Scholar] [CrossRef]
- Rodríguez-Gómez, F.; Romero-Gil, V.; Arroyo-López, F.N.; Roldán-Reyes, J.C.; Torres-Gallardo, R.; Bautista-Gallego, J.; Garcia-Garcia, P.; Garrido-Fernández, A. Assessing the challenges in the application of potential probiotic lactic acid bacteria in the large-scale fermentation of Spanish-style table olives. Front. Microbiol. 2017, 8, 915. [Google Scholar] [CrossRef]
- Martorana, A.; Alfonzo, A.; Gaglio, R.; Settanni, L.; Corona, O.; La Croce, F.; Vagnoli, P.; Caruso, T.; Moschetti, G.; Francesca, N. Evaluation of different conditions to enhance the performances of Lactobacillus pentosus OM13 during industrial production of Spanish-style table olives. Food Microbiol. 2017, 61, 150–158. [Google Scholar] [CrossRef]
- Tzamourani, A.P.; Di Napoli, E.; Paramithiotis, S.; Economou-Petrovits, G.; Panagiotidis, S.; Panagou, E.Z. Microbiological and physicochemical characterisation of green table olives of Halkidiki and Conservolea varieties processed by the Spanish method on industrial scale. Int. J. Food Sci. Technol. 2021, 56, 3845–3857. [Google Scholar]
- Lanza, B. Abnormal fermentations in table-olive processing: Microbial origin and sensory evaluation. Front. Microbiol. 2013, 4, 91. [Google Scholar] [CrossRef]
- Tıraş, Z.E.; Yıldırım, H.K. Application of mixed starter culture for table olive production. Grasas Aceites 2021, 72, e405. [Google Scholar] [CrossRef]
- Campaniello, D.; Bevilacqua, A.; D’Amato, D.; Corbo, M.R.; Altieri, C.; Sinigaglia, M. Microbial characterization of table olives processed according to Spanish and natural styles. Food Technol. Biotechnol. 2005, 43, 289–294. [Google Scholar]
- Botta, C.; Cocolin, L. Microbial dynamics and biodiversity in table olive fermentation: Culture-dependent and-independent approaches. Frontiers Microbiol. 2012, 3, 245. [Google Scholar] [CrossRef]
- Abdeen, E.E.; Mousa, W.S.; Abdelsalam, S.Y.; Heikal, H.S.; Shawish, R.R.; Nooruzzaman, M.; Soliman, M.M.; Batiha, G.E.; Hamad, A.; Abdeen, A. Prevalence and characterization of coagulase positive Staphylococci from food products and human specimens in Egypt. Antibiotics 2021, 10, 75. [Google Scholar] [CrossRef]
- Pereira, A.P.; Pereira, J.A.; Bento, A.; Estevinho, M.L. Microbiological characterization of table olives commercialized in Portugal in respect to safety aspects. Food Chem. Toxicol. 2008, 46, 2895–2902. [Google Scholar] [CrossRef]
- Pino, A.; Vaccalluzzo, A.; Solieri, L.; Romeo, F.V.; Todaro, A.; Caggia, C.; Arroyo López, F.N.; Bautista-Gallego, J.; Randazzo, C.L. Effect of sequential inoculum of beta-glucosidase positive and probiotic strains on brine fermentation to obtain low salt Sicilian table olives. Front. Microbiol. 2019, 10, 174. [Google Scholar] [CrossRef]
- Tassou, C.C.; Nychas, G.J.E. Inhibition of Staphylococcus aureus by olive phenolics in broth and in a model food system. J. Food Prot. 1994, 57, 120–124. [Google Scholar] [CrossRef]
- Sánchez, R.; Fernández, A.; Martín-Tornero, E.; Meléndez, F.; Lozano, J.; Martín-Vertedor, D. Application of Digital Olfaction for Table Olive Industry. Sensors 2022, 22, 5702. [Google Scholar] [CrossRef]
- Sánchez, A.H.; López-López, A.; Cortés-Delgado, A.; Beato, V.M.; Medina, E.; de Castro, A.; Montaño, A. Effect of post-fermentation and packing stages on the volatile composition of Spanish-style green table olives. Food Chem. 2018, 239, 343–353. [Google Scholar] [CrossRef]
- Sánchez, R.; Pérez-Nevado, F.; Martillanes, S.; Montero-Fernández, I.; Lozano, J.; Martín-Vertedor, D. Machine olfaction discrimination of Spanish-style green olives inoculated with spoilage mold species. Food Control 2023, 147, 109600. [Google Scholar] [CrossRef]
- Mikrou, T.; Kasimati, K.; Doufexi, I.; Kapsokefalou, M.; Gardeli, C.; Mallouchos, A. Volatile composition of industrially fermented table olives from Greece. Foods 2021, 10, 1000. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, L.; Cano-Lamadrid, M.; Carbonell-Barrachina, Á.A.; Sendra, E.; Hernández, F. Volatile composition, sensory profile and consumer acceptability of hydrosostainable table olives. Foods 2019, 8, 470. [Google Scholar] [CrossRef]
- Randazzo, C.L.; Todaro, A.; Pino, A.; Pitino, I.; Corona, O.; Caggia, C. Microbiota and metabolome during controlled and spontaneous fermentation of Nocellara Etnea table olives. Food Microbiol. 2017, 65, 136–148. [Google Scholar] [CrossRef]
- Sabatini, N.; Perri, E.; Marsilio, V. An investigation on molecular partition of aroma compounds in fruit matrix and brine medium of fermented table olives. Innov. Food Sci. Emerg. Technol. 2009, 10, 621–626. [Google Scholar] [CrossRef]
- Benítez-Cabello, A.; Rodríguez-Gómez, F.; Morales, M.L.; Garrido-Fernández, A.; Jiménez-Díaz, R.; Arroyo-López, F.N. Lactic acid bacteria and yeast inocula modulate the volatile profile of Spanish-style green table olive fermentations. Foods 2019, 8, 280. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Valli, E.; Bendini, A.; Toschi, T.G.; Simal-Gandara, J. Characterization of virgin olive oils produced with autochthonous Galician varieties. Food Chem. 2016, 212, 162–171. [Google Scholar] [CrossRef]
- Cortés-Delgado, A.; Sánchez, A.H.; de Castro, A.; López-López, A.; Beato, V.M.; Montaño, A. Volatile profile of Spanish-style green table olives prepared from different cultivars grown at different locations. Food Res. Int. 2016, 83, 131–142. [Google Scholar] [CrossRef]
- De Castro, A.; Sánchez, A.H.; Cortés-Delgado, A.; López-López, A.; Montaño, A. Effect of Spanish-style processing steps and inoculation with Lactobacillus pentosus starter culture on the volatile composition of cv. Manzanilla green olives. Food Chem. 2019, 271, 543–549. [Google Scholar] [CrossRef]
- Malheiro, R.; de Pinho, P.G.; Casal, S.; Bento, A.; Pereira, J.A. Determination of the volatile profile of stoned table olives from different varieties by using HS-SPME and GC/IT-MS. J. Sci. Food Agric. 2011, 91, 1693–1701. [Google Scholar] [CrossRef]
- Sansone-Land, A.; Takeoka, G.R.; Shoemaker, C.F. Volatile constituents of commercial imported and domestic black-ripe table olives (Olea europaea). Food Chem. 2014, 149, 285–295. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Hernández, F.; Corell, M.; Burló, F.; Legua, P.; Moriana, A.; Carbonell-Barrachina, Á.A. Antioxidant capacity, fatty acids profile, and descriptive sensory analysis of table olives as affected by deficit irrigation. J. Sci. Food Agric. 2017, 97, 444–451. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Gallardo-Guerrero, L. Characterization and processing of table olives: A special issue. Foods 2020, 9, 1469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).