Comprehensive Analysis of Catalytic Characteristics and Molecular Mechanisms in Mutant Trametes versicolor Strains with Enhanced Laccase Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Cultivation Techniques
2.2. Construction of a Mutagenesis Library Using the ARTP Method
2.3. Assays for Laccase Enzyme Activity
2.4. Comprehensive Analysis of Enzymatic Properties of Laccase
2.5. Optimization of Fermentation Conditions Using Single-Factor and Box–Behnken Experiments
2.6. Analysis of Homology Modeling
2.7. Statistical Analysis
3. Results
3.1. ARTP Mutagenesis Enhances Laccase Activity in Trametes versicolor
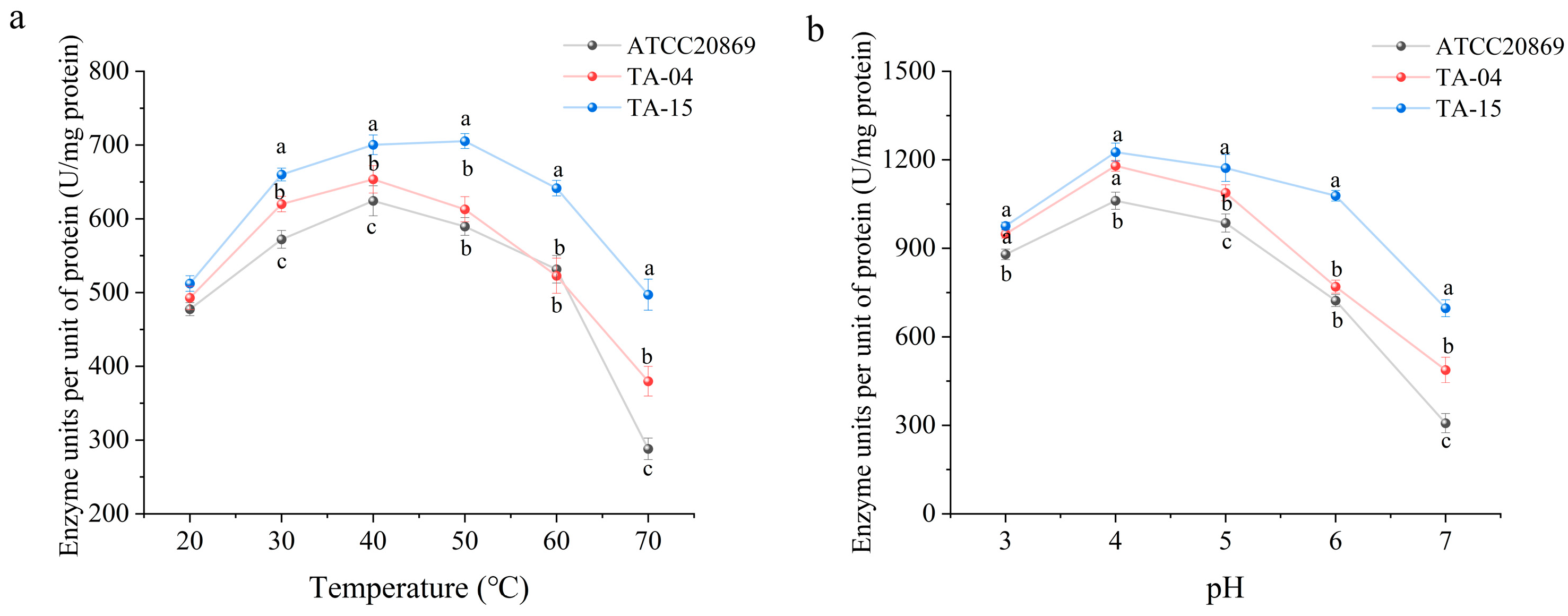
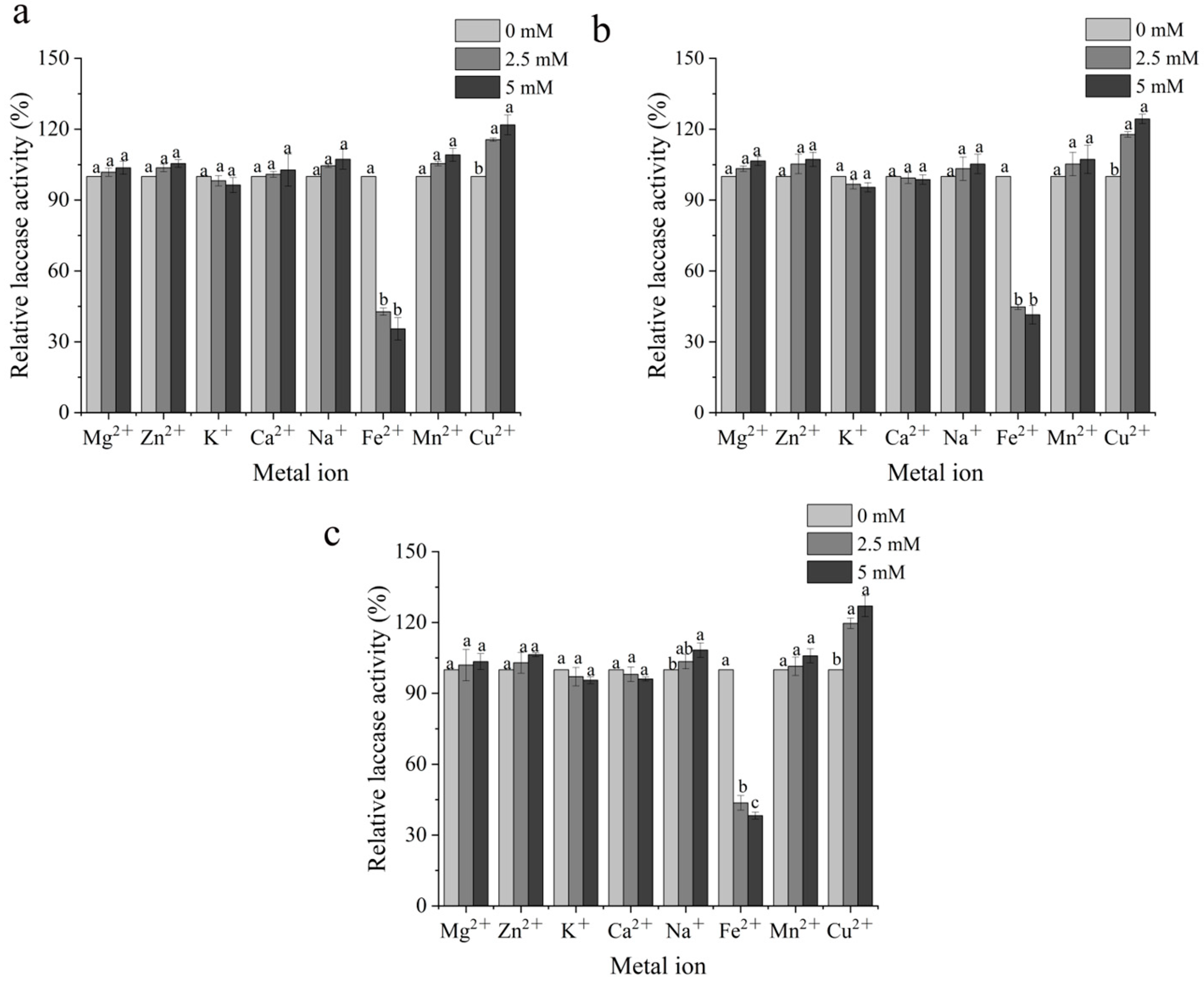
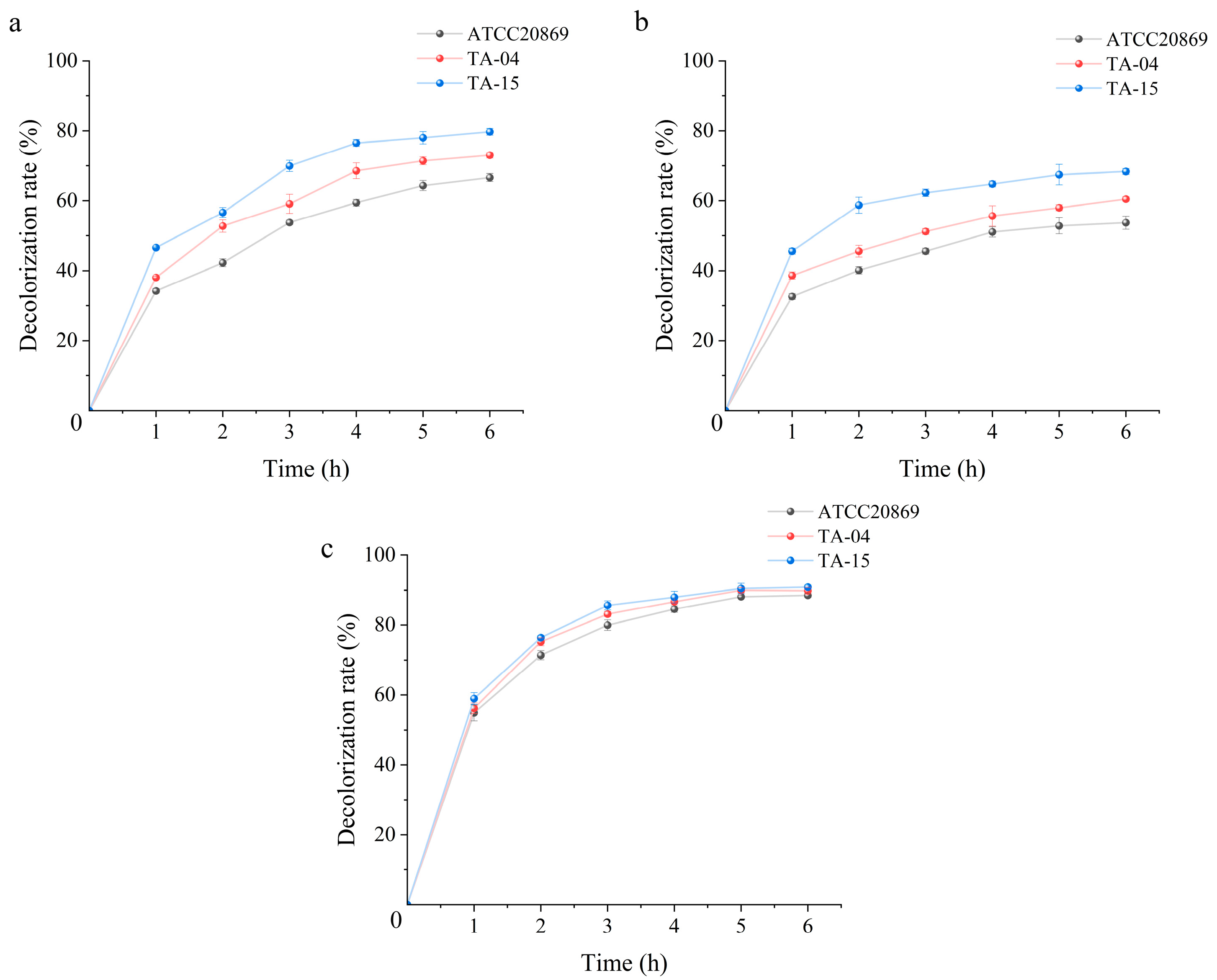
3.2. Analysis of the Enzymological Properties of Mutant Laccases
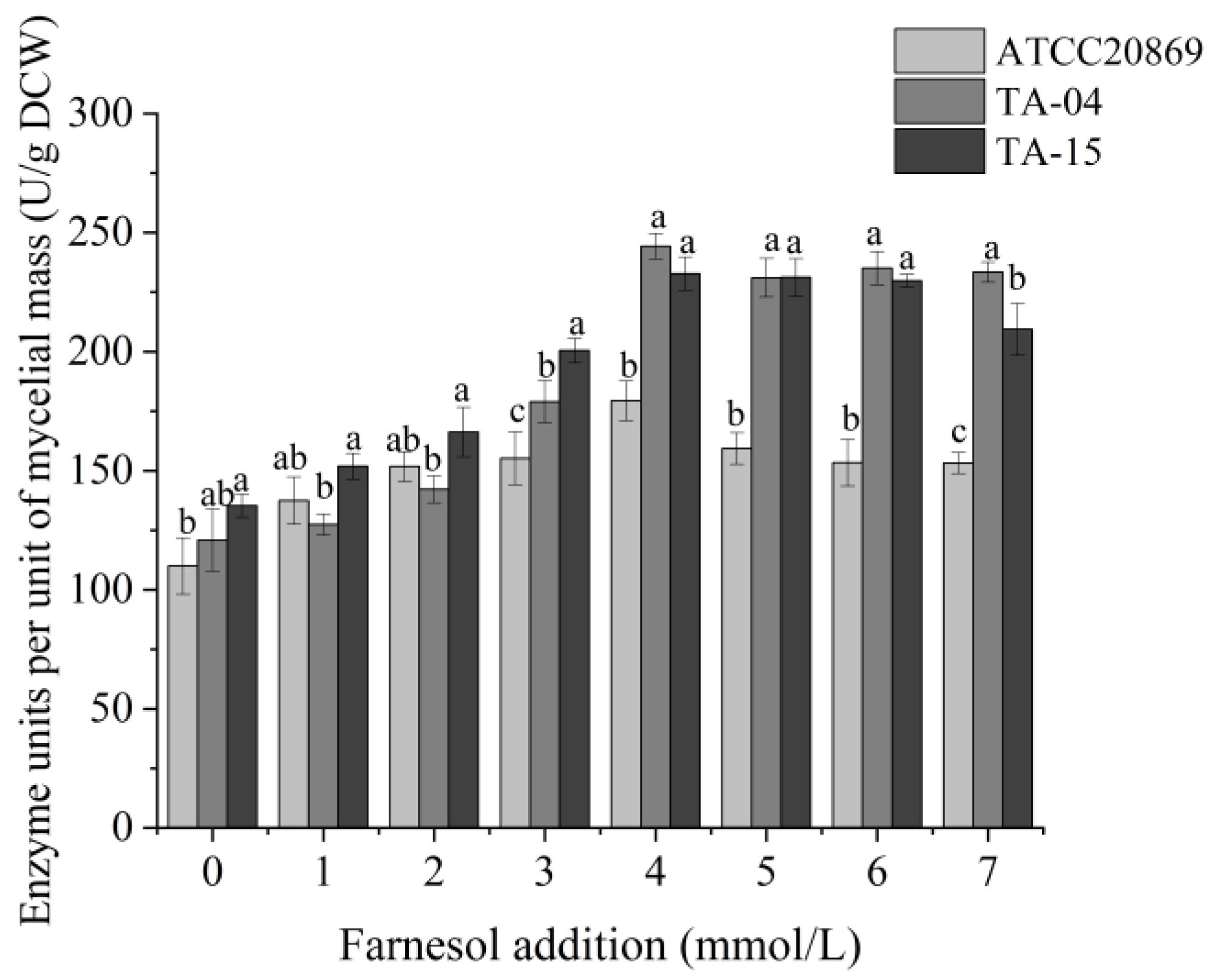
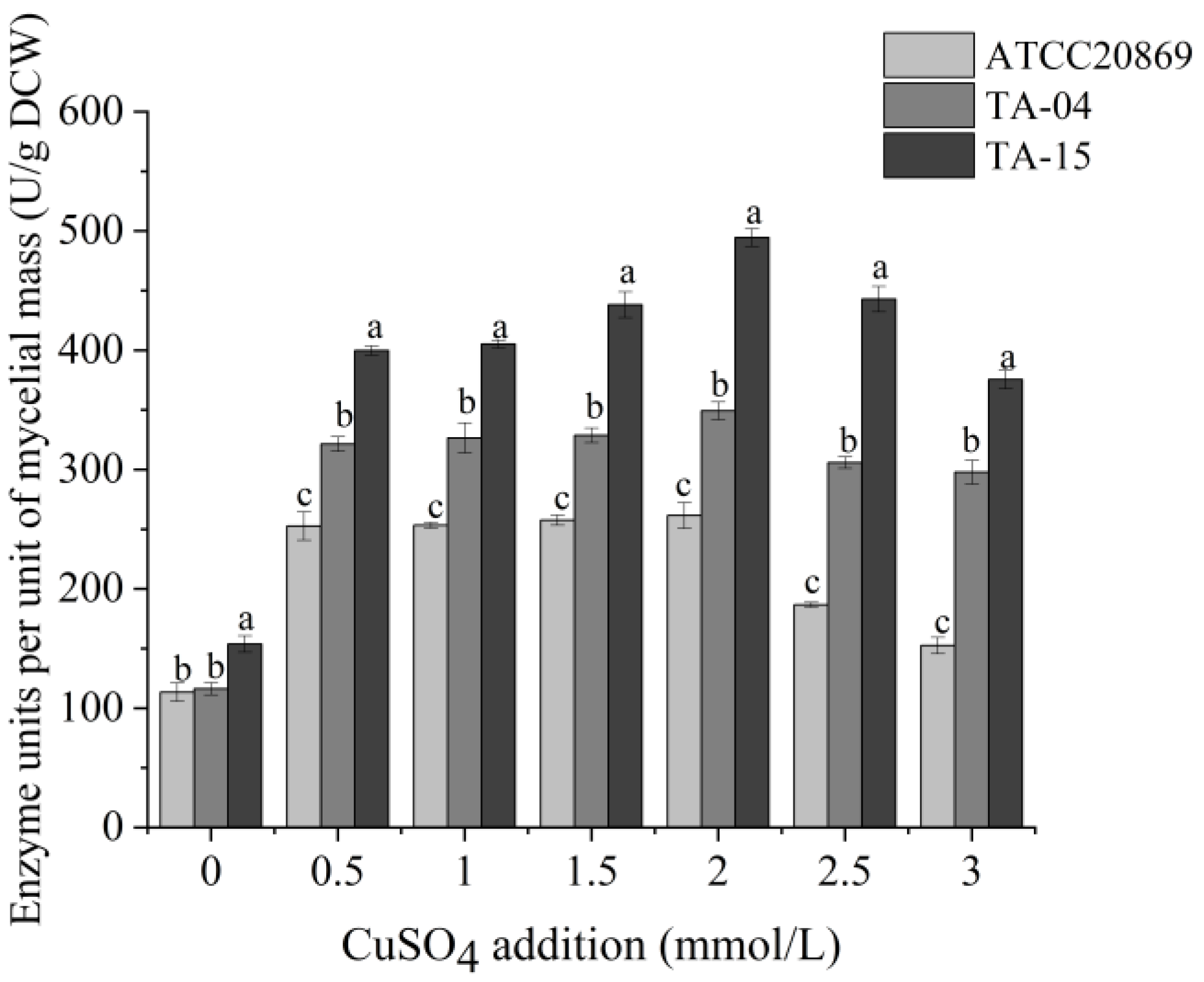
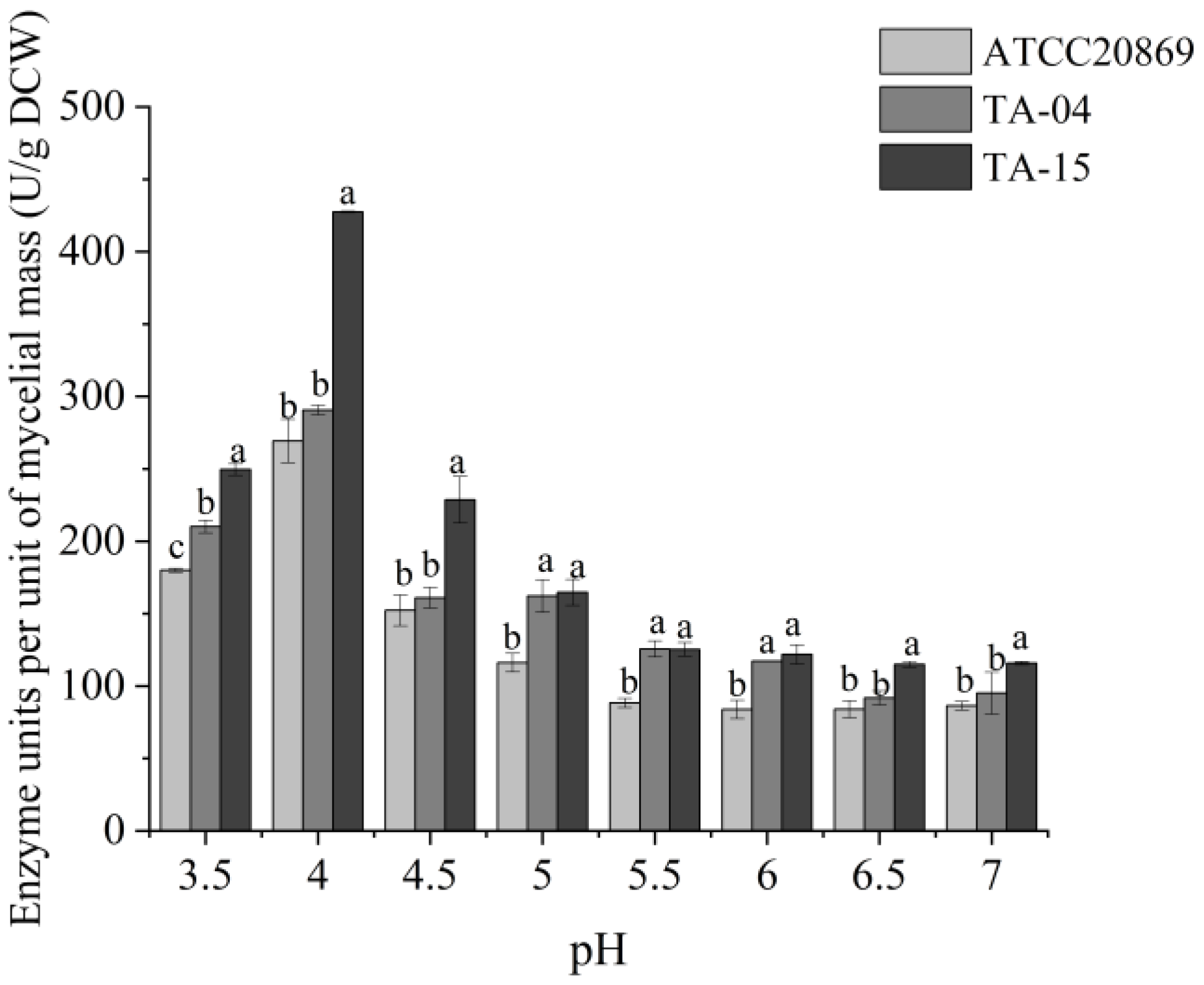
3.3. Optimization of Laccase Production Conditions through One-Factor Analysis
3.4. Experimental Findings Based on the Box–Behnken Design
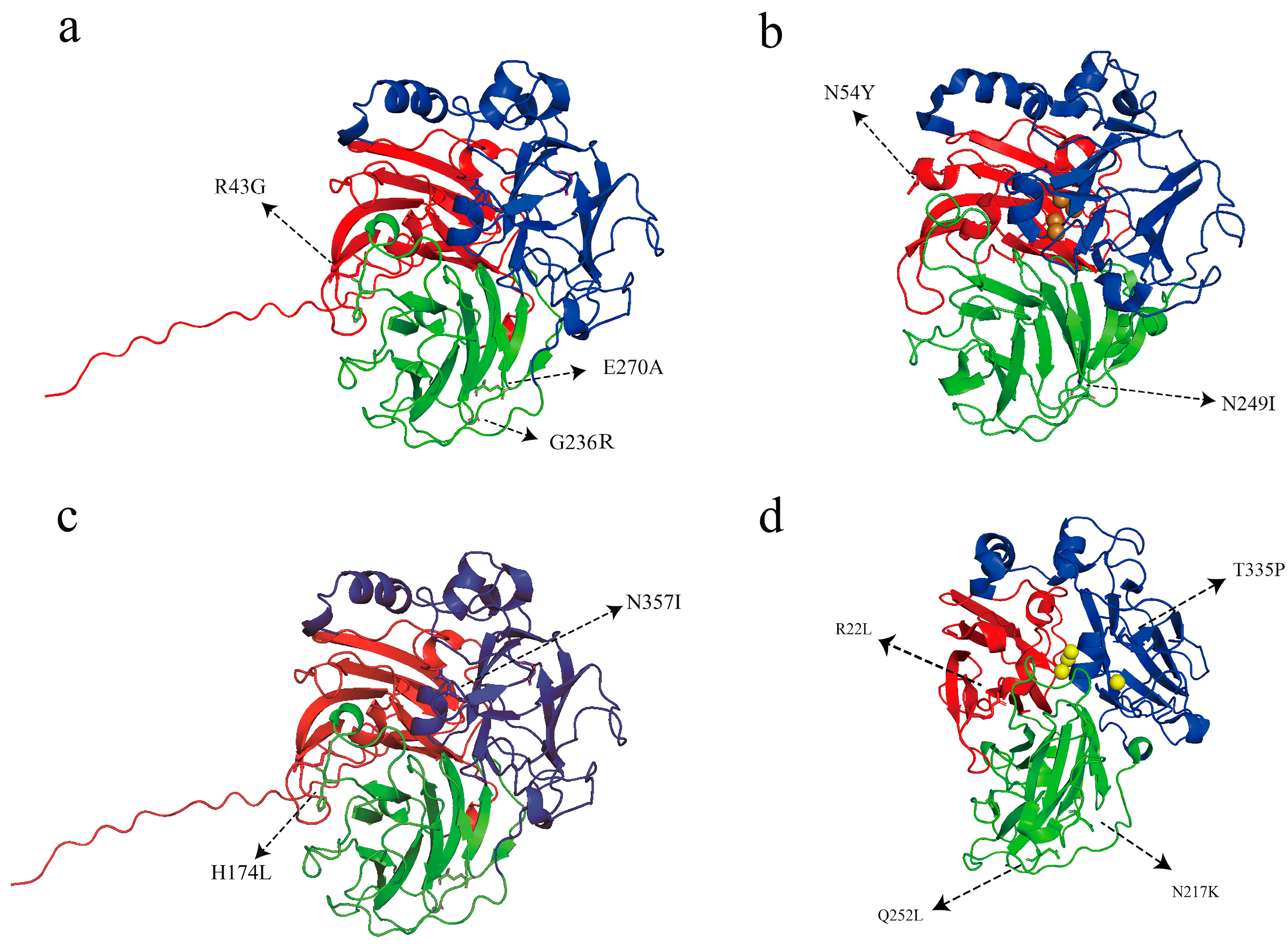
3.5. Analysis of Sequencing and Homology Modeling for Mutant Laccases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of lignocellulose using ligninolytic enzymes from white-rot fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef] [PubMed]
- Zerva, A.; Simic, S.; Topakas, E.; Nikodinovic-Runic, J. Applications of Microbial Laccases: Patent Review of the Past Decade (2009–2019). Catalysts 2019, 9, 1023. [Google Scholar] [CrossRef]
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various types and applications. Biotechnol. Appl. Biochem. 2022, 69, 2658–2672. [Google Scholar] [CrossRef] [PubMed]
- Marina, T.; Polona, Ž.-P.; Gordana, Š.; Ivana, T.; Marcela, Š.; Ana, B.-K.; Mirela, P. Trametes versicolor in lignocellulose-based bioeconomy: State of the art, challenges and opportunities. Bioresour. Technol. 2021, 330, 124997. [Google Scholar] [CrossRef]
- Takao, K.; Ayumi, H.; Hiroshi, T.; Daisuke, U. Effect of pH on the Dehydrogenative Polymerization of Monolignols by Laccases from Trametes versicolor and Rhus vernicifera. ACS Omega 2022, 7, 9846–9852. [Google Scholar] [CrossRef]
- Yang, J.; Ng, T.B.; Lin, J.; Ye, X.Y. A novel laccase from basidiomycete Cerrena sp.: Cloning, heterologous expression, and characterization. Int. J. Biol. Macromol. 2015, 77, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.J.; Zheng, F.; Long, L.K.; Wang, J.; Ding, S.J. Engineering the Expression and Characterization of Two Novel Laccase Isoenzymes from Coprinus comatus in Pichia pastoris by Fusing an Additional Ten Amino Acids Tag at N-Terminus. PLoS ONE 2014, 9, e93912. [Google Scholar] [CrossRef]
- Stevens, J.C.; Rodgers, D.W.; Dumon, C.; Shi, J. Characterization and Enzyme Engineering of a Hyperthermophilic Laccase Toward Improving Its Activity in Ionic Liquid. Front. Energy Res. 2020, 8, 158. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; Lai, C.; Huang, J.H.; Pang, Y.; Luo, K.; Liao, X.S. Activities of laccase produced by a strains Penicillium simplicissimum induced by chemical agentia and UV radiation. J. Cent. South Univ. 2017, 24, 1953–1958. [Google Scholar] [CrossRef]
- Wang, Q.; Qian, Y.Y.; Ma, Y.H.; Zhu, C.W. A Preliminary Study on the Newly Isolated High Laccase-Producing Fungi: Screening, Strain Characteristics and Induction of Laccase Production. Open Life Sci. 2018, 13, 463–469. [Google Scholar] [CrossRef]
- Ma, X.J.; Zhang, H.M.; Lu, X.F.; Han, J.; Zhu, H.X.; Wang, H.; Yao, R.S. Mutant breeding of Starmerella bombicola by atmospheric and room-temperature plasma (ARTP) for improved production of specific or total sophorolipids. Bioprocess Biosyst. Eng. 2020, 43, 1869–1883. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Wang, L.Y.; Zhang, X.; Su, N.; Li, H.P.; Oda, Y.; Xing, X.H. Quantitative evaluation of DNA damage caused by atmospheric and room-temperature plasma (ARTP) and other mutagenesis methods using a rapid umu-microplate test protocol for microbial mutation breeding. Chin. J. Chem. Eng. 2021, 39, 205–210. [Google Scholar] [CrossRef]
- Zhu, C.; Bao, G.; Huang, S. Optimization of laccase production in the white-rot fungus Pleurotus ostreatus (ACCC 52857) induced through yeast extract and copper. Biotechnol. Biotechnol. Equip. 2016, 30, 270–276. [Google Scholar] [CrossRef]
- Zhao, L.H.; Ma, Q.Q.; Nie, F.; Chen, W.; Sun, H.J. Increasing laccase activity of white rot fungi by mutagenesis and treating papermaking wastewater. IOP Conf. Ser. Earth Environ. Sci. 2018, 191, 012053. [Google Scholar] [CrossRef]
- Nakazawa, K.; Ohashi, T.; Saiki, S.; Kikuchi, S. Local oxynitriding of AZ31 magnesium alloy by atmospheric-pressure plasma treatment at room temperature. J. Magnes. Alloys 2022, 10, 1878–1886. [Google Scholar] [CrossRef]
- Nie, X.L.; Xing, Y.; Li, Q.F.; Gao, F.; Wang, S.Y.; Liu, P.; Li, X.Q.; Tan, Z.B.; Wang, P.X.; Shi, H. ARTP mutagenesis promotes selenium accumulation in Saccharomyces boulardii br. LWT-Food Sci. Technol. 2022, 168, 113916. [Google Scholar] [CrossRef]
- Zhao, Y.; Xiang, W.; Yan, S.; Huang, Y.; Fan, W. Laccase activity in Sphagnum-dominated peatland: A study based on a novel measurement of delay dynamics (MDD) for determining laccase activity. Soil Biol. Biochem. 2019, 133, 108–115. [Google Scholar] [CrossRef]
- Tian, Q.P.; Zhang, Y.; Meng, D.; Zhai, L.X.; Shen, Y.; You, C.P.; Guan, Z.B.; Liao, X.R. Simultaneous removal of tetracycline and sulfamethoxazole by laccase-mediated oxidation and ferrate(VI) oxidation: The impact of mediators and metal ions. Environ. Sci. Pollut. Res. 2023, 30, 15708–15721. [Google Scholar] [CrossRef]
- Angell, A.R.; Mata, L.; de Nys, R.; Paul, N.A. The protein content of seaweeds: A universal nitrogen-to-protein conversion factor of five. J. Appl. Phycol. 2016, 28, 511–524. [Google Scholar] [CrossRef]
- Bettin, F.; Cousseau, F.; Martins, K.; Zaccaria, S.; Girardi, V.; Silveira, M.M.d.; Dillon, A.J.P. Effects of pH, Temperature and Agitation on the Decolourisation of Dyes by Laccase-Containing Enzyme Preparation from Pleurotus sajor-caju. Braz. Arch. Biol. Technol. 2019, 62, e19180338. [Google Scholar] [CrossRef]
- Si, J.; Wu, Y.; Ma, H.F.; Cao, Y.J.; Sun, Y.F.; Cui, B.K. Selection of a pH- and temperature-stable laccase from Ganoderma australe and its application for bioremediation of textile dyes. J. Environ. Manag. 2021, 299, 113619. [Google Scholar] [CrossRef]
- Zhuo, R.; Yuan, P.; Yang, Y.; Zhang, S.; Ma, F.; Zhang, X. Induction of laccase by metal ions and aromatic compounds in Pleurotus ostreatus HAUCC 162 and decolorization of different synthetic dyes by the extracellular laccase. Biochem. Eng. J. 2017, 117, 62–72. [Google Scholar] [CrossRef]
- Li, S.Y.; Liu, Q.Z.; Liu, J.; Sun, K.; Yang, W.; Si, Y.B.; Li, Y.C.; Gao, Y.Z. Inhibition mechanisms of Fe2+/Fe3+ and Mn2+ on fungal laccase-enabled bisphenol a polyreaction. Chemosphere 2022, 307, 135685. [Google Scholar] [CrossRef] [PubMed]
- Epiphany, E.C.; Uchechukwu, N.U. Decolourization of synthetic dyes by laccase produced from Bacillus sp. NU2. Biotechnol. Biotechnol. Equip. 2022, 36, 95–106. [Google Scholar] [CrossRef]
- Filomena, S.; Elena, D.M.; Mariarosaria, A.; Domenico, P. A Novel Approach, Based on the Combined Action of Chitosan Hydrogel and Laccases, for the Removal of Dyes from Textile Industry Wastewaters. Gels 2023, 9, 41. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, J.; Cavaco-Paulo, A.; Su, J.; Wang, H.B. Encapsulated laccase in bimetallic Cu/Zn ZIFs as stable and reusable biocatalyst for decolorization of dye wastewater. Int. J. Biol. Macromol. 2023, 233, 123410. [Google Scholar] [CrossRef] [PubMed]
- Ameni, K.; Aida, K.; Ines, M.; Sofiane, B. Enhancement of Aeribacillus pallidus strain VP3 lipase catalytic activity through optimization of medium composition using Box-Behnken design and its application in detergent formulations. Environ. Sci. Pollut. Res. Int. 2020, 27, 12755–12766. [Google Scholar] [CrossRef]
- Li, H.; Driesche, S.v.d.; Bunge, F.; Yang, B.; Vellekoop, M.J. Optimization of on-chip bacterial culture conditions using the Box-Behnken design response surface methodology for faster drug susceptibility screening. Talanta 2019, 194, 627–633. [Google Scholar] [CrossRef]
- Kaur, C.N.; Divya, S.; Ribhav, S.; Sonica, S. Structure analysis and molecular docking studies of laccase from “Bacillus licheniformis NS2324”. Sustain. Chem. Environ. 2023, 1, 100004. [Google Scholar] [CrossRef]
- Li, T.; Huang, L.; Li, Y.; Xu, Z.; Ge, X.; Zhang, Y.; Wang, N.; Wang, S.; Yang, W.; Lu, F.; et al. The heterologous expression, characterization, and application of a novel laccase from Bacillus velezensis. Sci. Total Environ. 2020, 713, 136713. [Google Scholar] [CrossRef]
- Zhang, Q.; Miao, R.; Feng, R.; Yan, J.; Wang, T.; Gan, Y.; Zhao, J.; Lin, J.; Gan, B. Application of Atmospheric and Room-Temperature Plasma (ARTP) to Microbial Breeding. Curr. Issues Mol. Biol. 2023, 45, 6466–6484. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Jing, Y.L.; Zhang, Q.; Xiu, J.L.; Tian, M.Z.; Cui, Q.F.; Ma, Y.D.; Yi, L.A.; Han, L.; Qian, Y.C.; et al. Improving Rhamnolipids Biosynthesis in Pseudomonas sp. L01 through Atmospheric and Room-Temperature Plasma (ARTP) Mutagenesis. Microorganisms 2023, 11, 1182. [Google Scholar] [CrossRef] [PubMed]
- Aisha, U. Screening and evaluation of laccase produced by different Trichoderma species along with their phylogenetic relationship. Arch. Microbiol. 2021, 203, 4319–4327. [Google Scholar] [CrossRef]
- Asgher, M.; Noreen, S.; Bilal, M. Enhancing catalytic functionality of Trametes versicolor IBL-04 laccase by immobilization on chitosan microspheres. Chem. Eng. Res. Des. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Senthilvelan, T.; Kanagaraj, J.; Panda, R.C. Effective bioremoval of syntan using fungal laccase to reduce pollution from effluent. Int. J. Environ. Sci. Technol. 2018, 15, 1429–1440. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Okoh, A.I.; Nwodo, U.U. Aptitude of Oxidative Enzymes for Treatment of Wastewater Pollutants: A Laccase Perspective. Molecules 2019, 24, 2064. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Zhongchuan, L.; Ganggang, W. Structural Insight into the Allosteric Coupling of Cu1 Site and Trinuclear Cu Cluster in CotA Laccase. ChemBioChem 2018, 19, 1502–1506. [Google Scholar] [CrossRef]
- Buddhika, U.V.A.; Savocchia, S.; Steel, C.C. Copper induces transcription of BcLCC2 laccase gene in phytopathogenic fungus, Botrytis cinerea. Mycology 2021, 12, 48–57. [Google Scholar] [CrossRef]
- Klaus, P.; Matteo, A.; Thomas, C. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-A resolution containing a full complement of coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef]
- Niladevi, K.N.; Jacob, N.; Prema, P. Evidence for a halotolerant-alkaline laccase in Streptomyces psammoticus: Purification and characterization. Process Biochem. 2008, 43, 654–660. [Google Scholar] [CrossRef]
- Zheng, F.; Cui, B.K.; Wu, X.J.; Meng, G.; Liu, H.X.; Si, J. Immobilization of laccase onto chitosan beads to enhance its capability to degrade synthetic dyes. Int. Biodeterior. Biodegrad. 2016, 110, 69–78. [Google Scholar] [CrossRef]
- Polke, M.; Jacobsen, I.D. Quorum sensing by farnesol revisited. Curr. Genet. 2017, 63, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.F.; Sui, K.Y.; Guo, C.; Liu, C.Z. Quorum sensing molecule-farnesol increased the production and biological activities of extracellular polysaccharide from Trametes versicolor. Int. J. Biol. Macromol. 2017, 104, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Boone, C.H.T.; Gutzmann, D.J.; Kramer, J.J.; Atkin, A.L.; Nickerson, K.W. Quantitative assay for farnesol and the aromatic fusel alcohols from the fungus Candida albicans. Appl. Microbiol. Biotechnol. 2022, 106, 6759–6773. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Balda, S.; Gupta, N.; Capalash, N.; Sharma, P. Functional substitution of domain 3 (T1 copper center) of a novel laccase with Cu ions. Int. J. Biol. Macromol. 2019, 123, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Dhakar, K.; Pandey, A. Laccase production in a psychrotolerant Eutypella under varying temperature and pH conditions. Sydowia 2020, 72, 57–63. [Google Scholar] [CrossRef]
- Du, W.; Sun, C.L.; Wang, J.; Wang, B.Q.; Yao, Z.G.; Qu, F.Z.; Xia, J.B.; Xie, W.J.; Sun, J.K.; Duan, D.X. Isolation, identification of a laccase-producing fungal strain and enzymatic properties of the laccase. 3 Biotech 2018, 8, 137. [Google Scholar] [CrossRef]
- Abdulrhman, A.M.; Lamia, A.; Rajeh, A.M.; Altayeb, H.N.; Samir, A.; Kamel, C. Whole genome sequencing analysis and Box-Behnken design for the optimization of the decolourization of mixture textile dyes by halotolerant microbial consortium. Microbiol. Res. 2023, 276, 109058. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.M.; Zhang, Q.; Cai, D.B.; Chen, S.W.; Wang, Y.H. Enhancement of alkaline protease production in recombinant Bacillus licheniformis by response surface methodology. Bioresour. Bioprocess. 2023, 10, 27. [Google Scholar] [CrossRef]


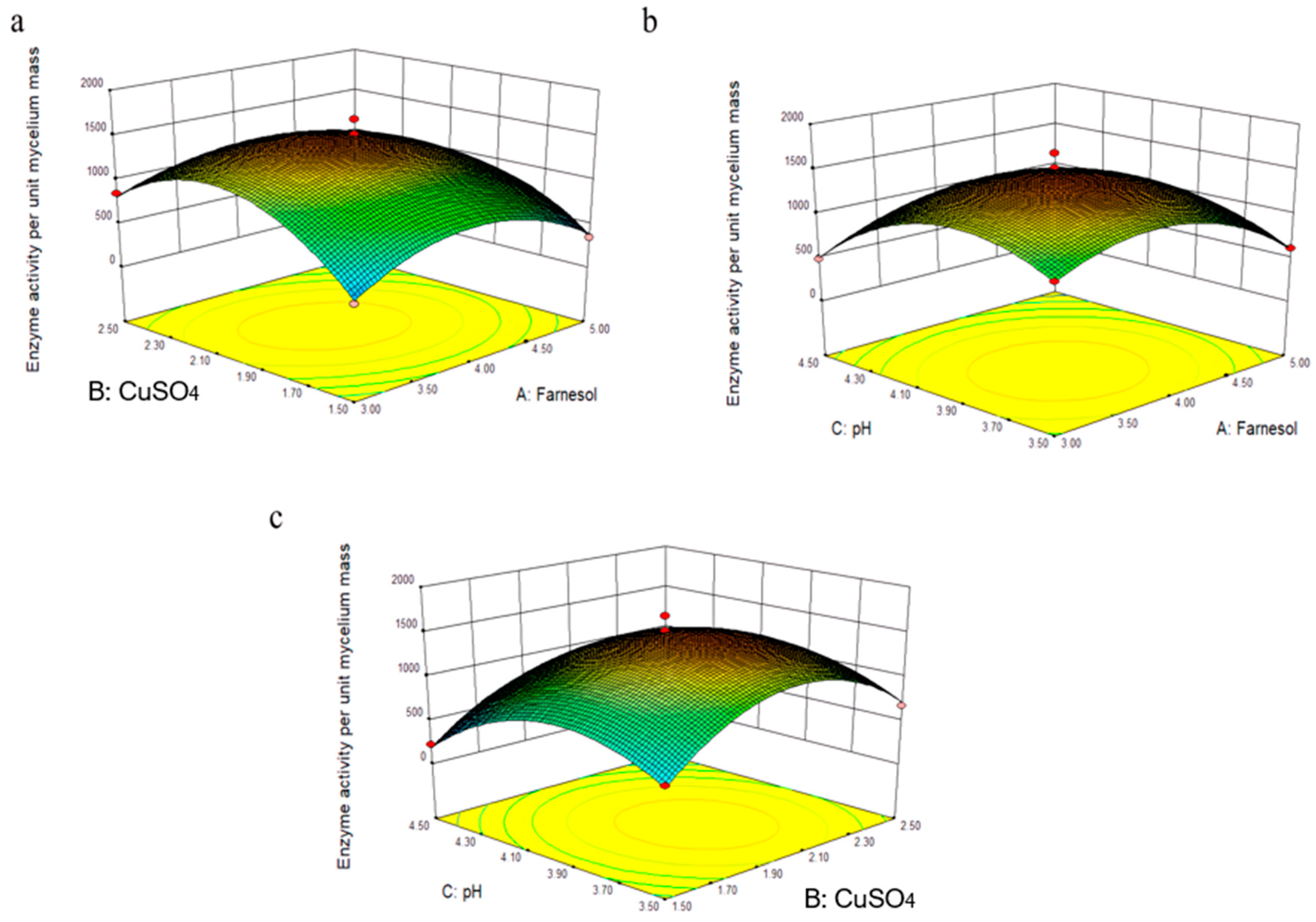
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 1.290 × 106 | 9 | 1.433 × 105 | 64.20 | <0.0001 | significant |
| A-Farnesol | 50,387.73 | 1 | 50,387.73 | 22.57 | 0.0021 | |
| B-CuSO4 | 70,855.31 | 1 | 70,855.31 | 31.74 | 0.0008 | |
| C-pH | 12,754.88 | 1 | 12,754.88 | 5.71 | 0.0481 | |
| AB | 54,779.69 | 1 | 54,779.69 | 24.54 | 0.0016 | |
| AC | 25,717.73 | 1 | 25,717.73 | 11.52 | 0.0115 | |
| BC | 1404.46 | 1 | 1404.46 | 0.63 | 0.4537 | |
| A2 | 3.916 × 105 | 1 | 3.916 × 105 | 175.41 | <0.0001 | |
| B2 | 2.895 × 105 | 1 | 2.895 × 105 | 129.67 | <0.0001 | |
| C2 | 2.806 × 105 | 1 | 2.806 × 105 | 125.71 | <0.0001 | |
| Residual | 15,626.53 | 7 | 2232.36 | |||
| Lack of Fit | 8755.18 | 3 | 2918.39 | 1.70 | 0.3040 | not significant |
| Pure Error | 6871.35 | 4 | 1717.84 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 4.358 × 106 | 9 | 4.842 × 105 | 60.96 | <0.0001 | significant |
| A-Farnesol | 1.782 × 105 | 1 | 1.782 × 105 | 22.44 | 0.0021 | |
| B-CuSO4 | 35,744.91 | 1 | 35,744.91 | 4.50 | 0.0716 | |
| C-pH | 3.241 × 105 | 1 | 3.241 × 105 | 40.81 | 0.0004 | |
| AB | 63,791.16 | 1 | 63,791.16 | 8.03 | 0.0253 | |
| AC | 4074.30 | 1 | 4074.30 | 0.51 | 0.4970 | |
| BC | 4276.23 | 1 | 4276.23 | 0.54 | 0.4869 | |
| A2 | 8.165 × 105 | 1 | 8.165 × 105 | 102.80 | <0.0001 | |
| B2 | 1.524 × 106 | 1 | 1.524 × 106 | 191.91 | <0.0001 | |
| C2 | 1.021 × 106 | 1 | 1.021 × 106 | 128.53 | <0.0001 | |
| Residual | 55,595.16 | 7 | 7942.17 | |||
| Lack of Fit | 10,526.47 | 3 | 3508.82 | 0.31 | 0.8174 | not significant |
| Pure Error | 45,068.68 | 4 | 11,267.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yan, Z.; Li, X.; Wang, J.; Ren, X.; Liu, X. Comprehensive Analysis of Catalytic Characteristics and Molecular Mechanisms in Mutant Trametes versicolor Strains with Enhanced Laccase Activities. Fermentation 2023, 9, 995. https://doi.org/10.3390/fermentation9120995
Zhang C, Yan Z, Li X, Wang J, Ren X, Liu X. Comprehensive Analysis of Catalytic Characteristics and Molecular Mechanisms in Mutant Trametes versicolor Strains with Enhanced Laccase Activities. Fermentation. 2023; 9(12):995. https://doi.org/10.3390/fermentation9120995
Chicago/Turabian StyleZhang, Chi, Zhongjie Yan, Xiufang Li, Junming Wang, Xidong Ren, and Xinli Liu. 2023. "Comprehensive Analysis of Catalytic Characteristics and Molecular Mechanisms in Mutant Trametes versicolor Strains with Enhanced Laccase Activities" Fermentation 9, no. 12: 995. https://doi.org/10.3390/fermentation9120995
APA StyleZhang, C., Yan, Z., Li, X., Wang, J., Ren, X., & Liu, X. (2023). Comprehensive Analysis of Catalytic Characteristics and Molecular Mechanisms in Mutant Trametes versicolor Strains with Enhanced Laccase Activities. Fermentation, 9(12), 995. https://doi.org/10.3390/fermentation9120995








