Valorization of Fermented Food Wastes and Byproducts: Bioactive and Valuable Compounds, Bioproduct Synthesis, and Applications
Abstract
:1. Introduction
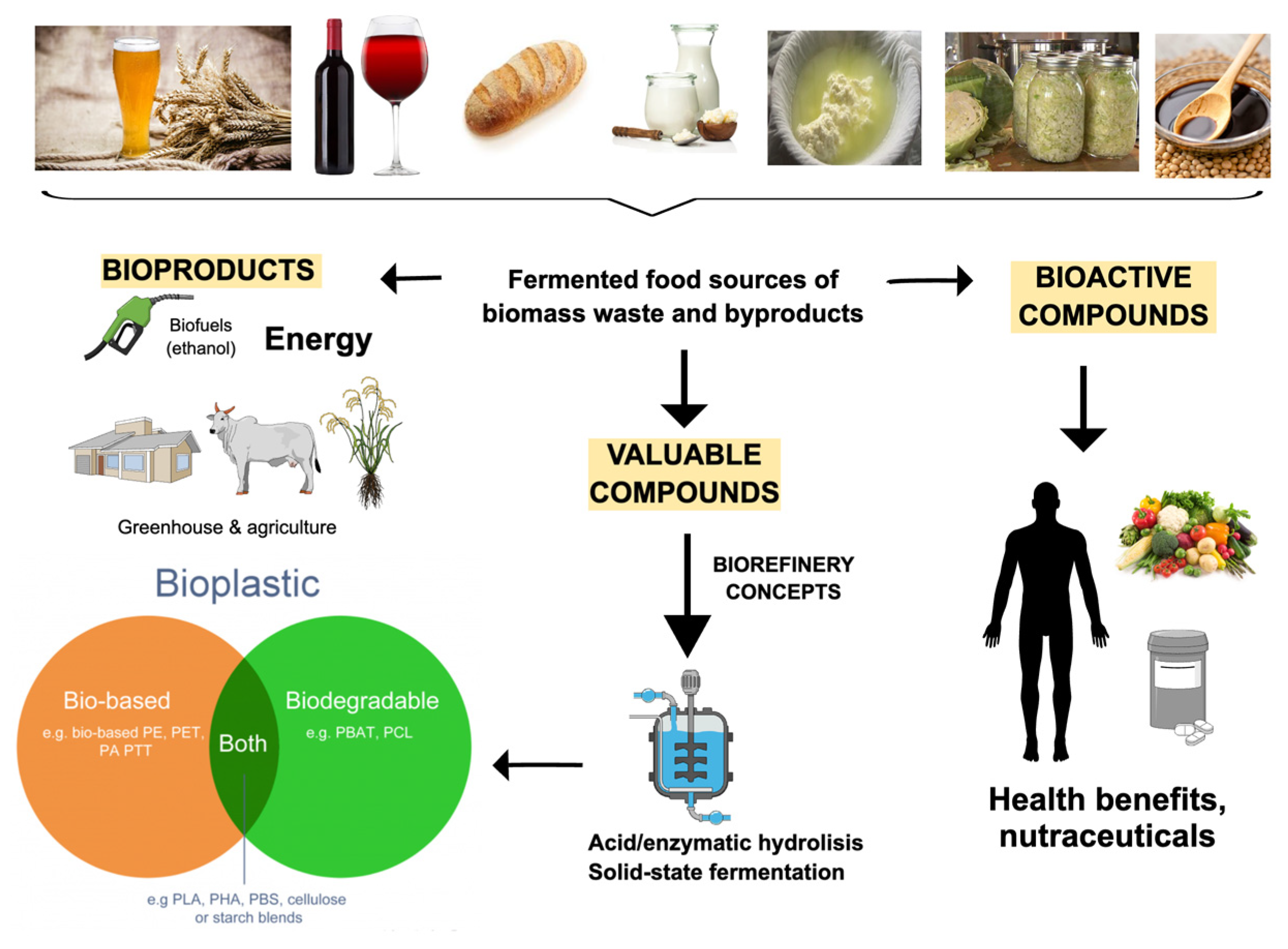
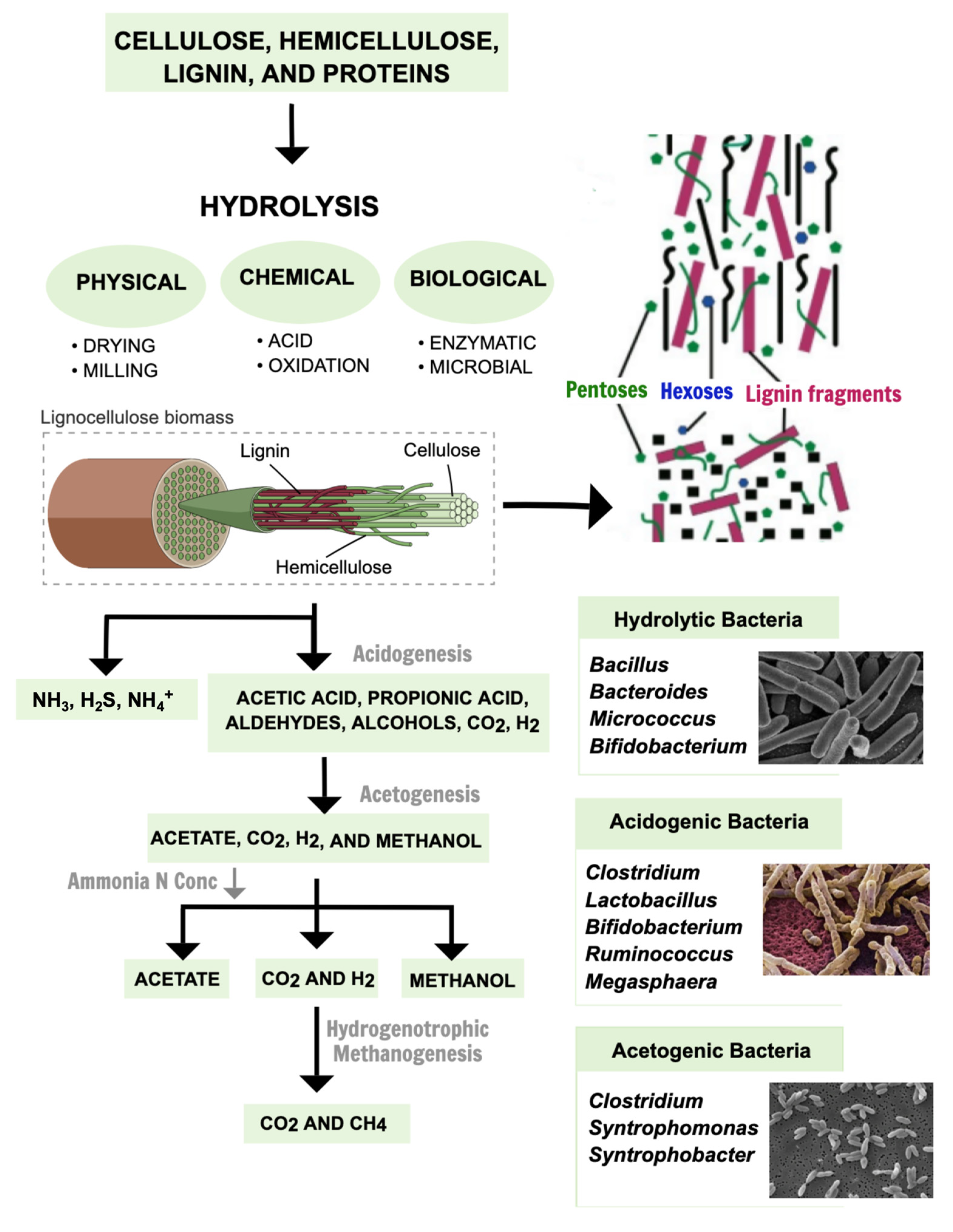
2. From Industrial Waste to Value-Added Bioproducts
3. Bioactive and Valuable Compounds
Biological Activities, Functional Foods, and Health Benefits
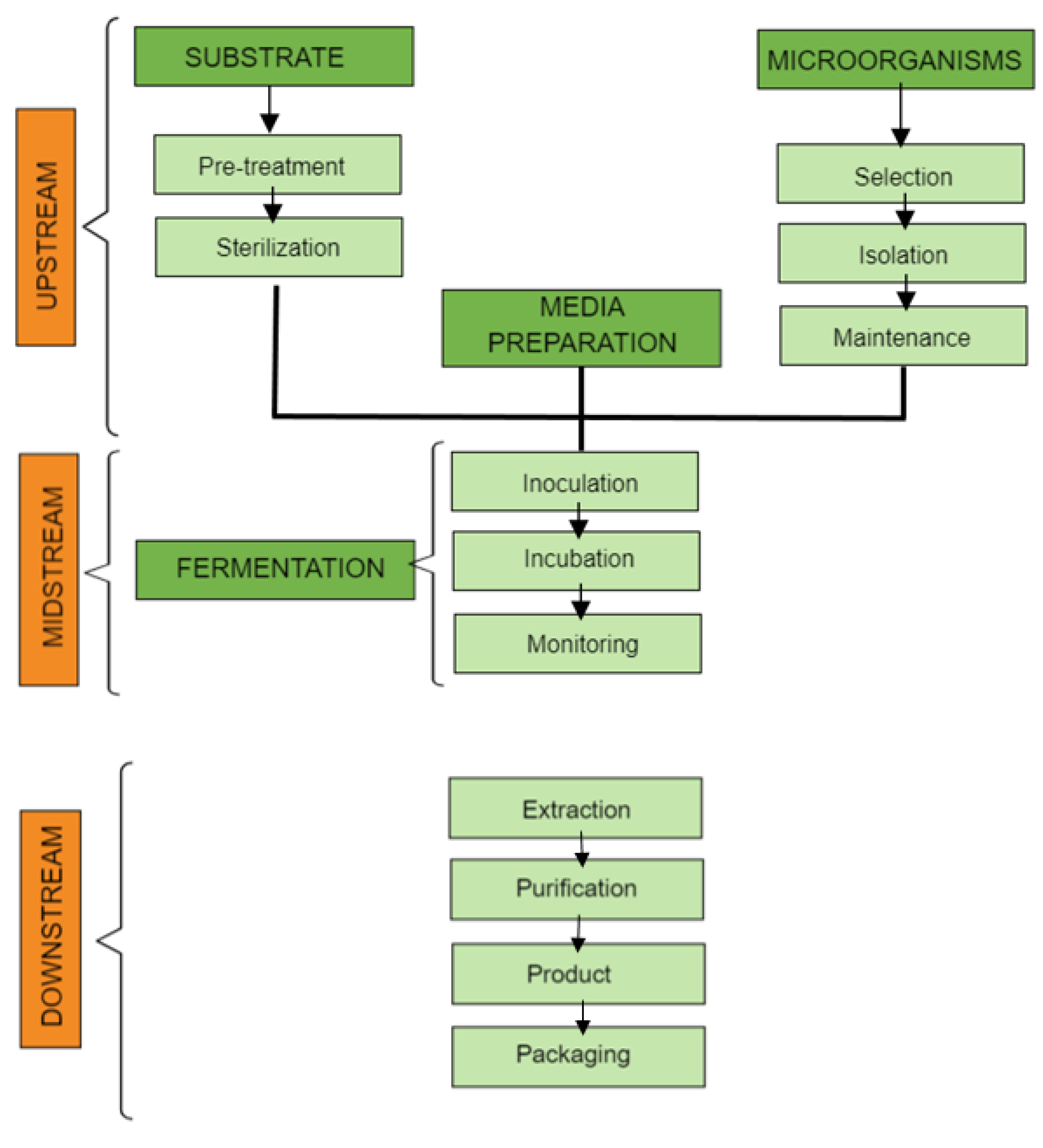
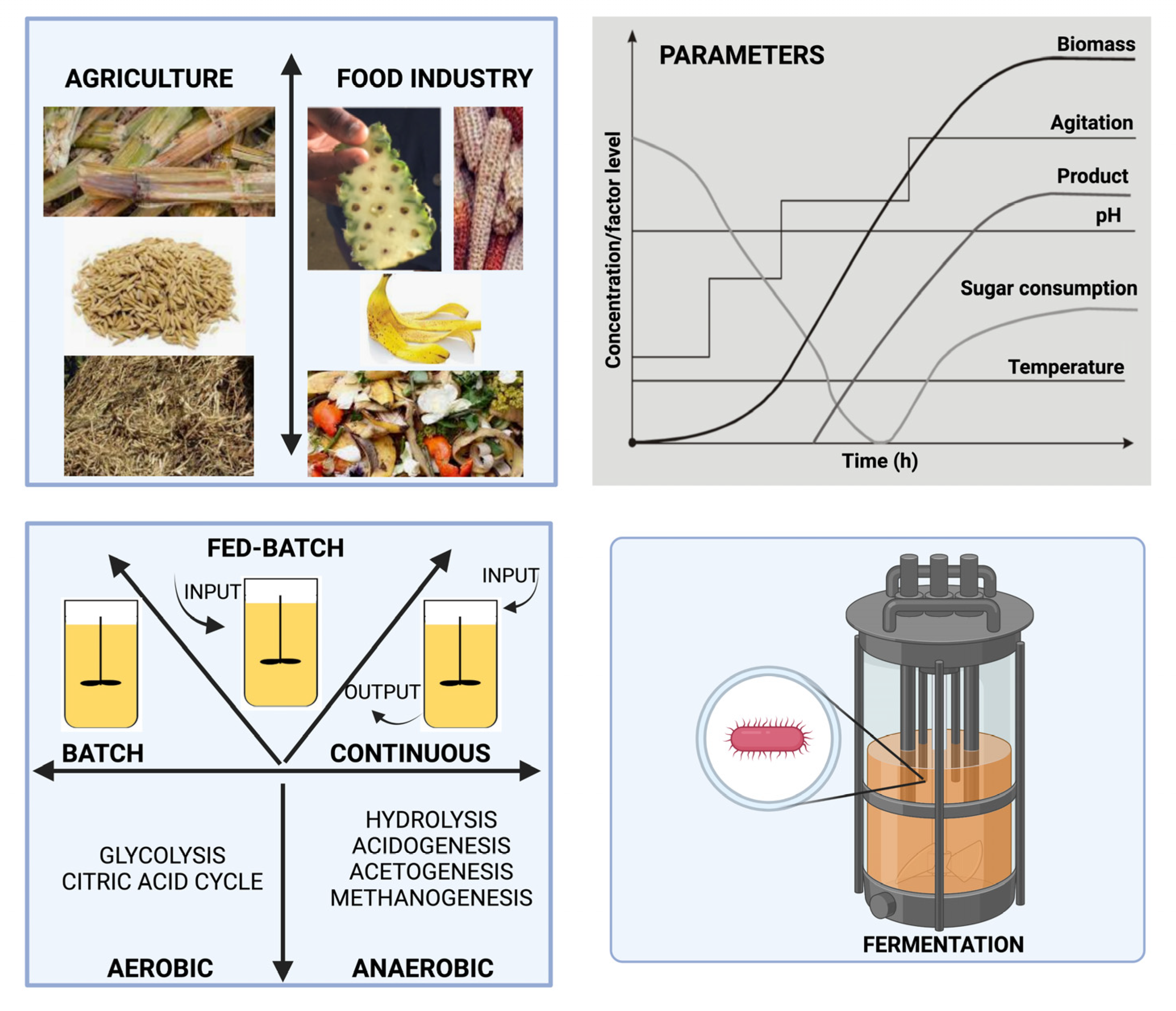
4. Methods of Obtaining Products
Waste Fermentation: Steps and Optimization Factors
5. Applications of Valuable Compounds from Fermented Residues
5.1. Animal Feeding
5.2. Biofuels
5.3. Biopolymers and Bioplastics
5.4. Nanomaterials
5.5. Other Applications Using Fermentative Residues
6. Challenges and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- THE WORLD BANK Solid Waste Management. Available online: https://www.worldbank.org/en/topic/urbandevelopment/brief/solid-waste-management (accessed on 23 July 2023).
- STATISTA Global Generation of Municipal Solid Waste by Country. Available online: https://www.statista.com/statistics/916749/global-generation-of-municipal-solid-waste-by-country/ (accessed on 23 July 2023).
- Kumar, J.A.; Sathish, S.; Prabu, D.; Renita, A.A.; Saravanan, A.; Deivayanai, V.C.; Anish, M.; Jayaprabakar, J.; Baigenzhenov, O.; Hosseini-Bandegharaei, A. Agricultural Waste Biomass for Sustainable Bioenergy Production: Feedstock, Characterization and Pre-Treatment Methodologies. Chemosphere 2023, 331, 138680. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Z.; Davis, S.; Ciais, P. Monitoring Global Carbon Emissions in 2022. Nat. Rev. Earth Environ. 2023, 4, 205–206. [Google Scholar] [CrossRef] [PubMed]
- Usmani, Z.; Sharma, M.; Awasthi, A.K.; Sivakumar, N.; Lukk, T.; Pecoraro, L.; Thakur, V.K.; Roberts, D.; Newbold, J.; Gupta, V.K. Bioprocessing of Waste Biomass for Sustainable Product Development and Minimizing Environmental Impact. Bioresour. Technol. 2021, 322, 124548. [Google Scholar] [CrossRef] [PubMed]
- Stikane, A.; Dace, E.; Stalidzans, E. Closing the Loop in Bioproduction: Spent Microbial Biomass as a Resource within Circular Bioeconomy. N. Biotechnol. 2022, 70, 109–115. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Chamorro, F.; Carpena, M.; Fraga-Corral, M.; Echave, J.; Riaz Rajoka, M.S.; Barba, F.J.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Valorization of Kiwi Agricultural Waste and Industry By-Products by Recovering Bioactive Compounds and Applications as Food Additives: A Circular Economy Model. Food Chem. 2022, 370, 131315. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.; Xie, D.; Wang, M.; Zhang, Y.; Zhang, H.; Du, Q.; Jin, P. Enhanced Biotransformation of Bioactive Components and Volatile Compounds of Bamboo (Phyllostachys glauca McClure) Leaf Juice Fermented by Probiotic Streptococcus Thermophiles. LWT 2023, 173, 114363. [Google Scholar] [CrossRef]
- Diez-Ozaeta, I.; Astiazaran, O.J. Fermented Foods: An Update on Evidence-Based Health Benefits and Future Perspectives. Food Res. Int. 2022, 156, 111133. [Google Scholar] [CrossRef]
- de Oliveira, S.D.; Araújo, C.M.; Borges, G.d.S.C.; Lima, M.d.S.; Viera, V.B.; Garcia, E.F.; de Souza, E.L.; de Oliveira, M.E.G. Improvement in Physicochemical Characteristics, Bioactive Compounds and Antioxidant Activity of Acerola (Malpighia emarginata D.C.) and Guava (Psidium guajava L.) Fruit by-Products Fermented with Potentially Probiotic Lactobacilli. LWT 2020, 134, 110200. [Google Scholar] [CrossRef]
- Camargo, D.A.; Pereira, M.S.; dos Santos, A.G.; Fleuri, L.F. Isolated and Fermented Orange and Grape Wastes: Bromatological Characterization and Phytase, Lipase and Protease Source. Innov. Food Sci. Emerg. Technol. 2022, 77, 102978. [Google Scholar] [CrossRef]
- Gautam, K.; Vishvakarma, R.; Sharma, P.; Singh, A.; Kumar Gaur, V.; Varjani, S.; Kumar Srivastava, J. Production of Biopolymers from Food Waste: Constrains and Perspectives. Bioresour. Technol. 2022, 361, 127650. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, S.; Zhou, X.; Li, M.; Zhang, Y.; Ye, H. The Anti-Obesogenic Effects and Underpinning Mechanisms of Fermented Plant-Based Foods: A Review. Trends Food Sci. Technol. 2023, 136, 1–10. [Google Scholar] [CrossRef]
- IEA—International Energy Agency. World Energy Outlook 2022; International Energy Agency: Paris, Italy, 2022.
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Alzate, C.A.C. Food Waste Valorization Applying the Biorefinery Concept in the Colombian Context: Pre-Feasibility Analysis of the Organic Kitchen Food Waste Processing. Biochem. Eng. J. 2023, 194, 108864. [Google Scholar] [CrossRef]
- De Guidi, I.; Legras, J.L.; Galeote, V.; Sicard, D. Yeast Domestication in Fermented Food and Beverages: Past Research and New Avenues. Curr. Opin. Food Sci. 2023, 51, 101032. [Google Scholar] [CrossRef]
- Ranganathan, S.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Utilization of Food Waste Streams for the Production of Biopolymers. Heliyon 2020, 6, e04891. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Xia, C.; Alqahtani, A.; Sharma, A.; Pugazhendhi, A. A Review on Optimistic Biorefinery Products: Biofuel and Bioproducts from Algae Biomass. Fuel 2023, 338, 127378. [Google Scholar] [CrossRef]
- Gautério, G.V.; da Silva, R.M.; Karraz, F.C.; Coelho, M.A.Z.; Ribeiro, B.D.; Lemes, A.C. Cell Disruption and Permeabilization Methods for Obtaining Yeast Bioproducts. Clean. Chem. Eng. 2023, 6, 100112. [Google Scholar] [CrossRef]
- Tavares, C.S.; Martins, A.; Miguel, M.G.; Carvalheiro, F.; Duarte, L.C.; Gameiro, J.A.; Figueiredo, A.C.; Roseiro, L.B. Bioproducts from Forest Biomass II. Bioactive Compounds from the Steam-Distillation by-Products of Cupressus Lusitanica Mill. and Cistus Ladanifer L. Wastes. Ind. Crops Prod. 2020, 158, 112991. [Google Scholar] [CrossRef]
- Sruthi, P.; Naidu, M.M. Cashew Nut (Anacardium occidentale L.) Testa as a Potential Source of Bioactive Compounds: A Review on Its Functional Properties and Valorization. Food Chem. Adv. 2023, 3, 100390. [Google Scholar] [CrossRef]
- Cerro, D.; Rojas, A.; Torres, A.; Villegas, C.; Galotto, M.J.; Guarda, A.; Romero, J. Nanoencapsulation of Food-Grade Bioactive Compounds Using a Supercritical Fluid Extraction of Emulsions Process: Effect of Operational Variables on the Properties of Nanocapsules and New Perspectives. LWT 2023, 115115. [Google Scholar] [CrossRef]
- Guan, C.; Tan, Z.; Li, S.; Wang, Y.; Yamamoto, N.; Zhang, C.; Wang, S.; Chen, J.; Xing, X. Transcriptomic Analysis of Andrias davidianus Meat and Experimental Validation for Exploring Its Bioactive Components as Functional Foods. Food Sci. Hum. Wellness 2024, 13, 166–172. [Google Scholar] [CrossRef]
- Saravanan, A.; Karishma, S.; Senthil Kumar, P.; Rangasamy, G. A Review on Regeneration of Biowaste into Bio-Products and Bioenergy: Life Cycle Assessment and Circular Economy. Fuel 2023, 338, 127221. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Venkatkarthick, R.; SenthilKannan, K.; Kuppam, C.; Mary Stephy, G.; Kamyab, H.; Chen, W.-H.; Thomas, J.; Ngamcharussrivichai, C. Advanced Technologies on the Sustainable Approaches for Conversion of Organic Waste to Valuable Bioproducts: Emerging Circular Bioeconomy Perspective. Fuel 2022, 324, 124313. [Google Scholar] [CrossRef]
- Kee, P.E.; Cheng, Y.-S.; Chang, J.-S.; Yim, H.S.; Tan, J.C.Y.; Lam, S.S.; Lan, J.C.-W.; Ng, H.S.; Khoo, K.S. Insect Biorefinery: A Circular Economy Concept for Biowaste Conversion to Value-Added Products. Environ. Res. 2023, 221, 115284. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Umesh, M.; Selvaraj, M.; Al-Shehri, B.M.; Chakraborty, P.; Duhan, L.; Sharma, S.; Pasrija, R.; Awasthi, M.K.; et al. Emerging Challenges for the Agro-Industrial Food Waste Utilization: A Review on Food Waste Biorefinery. Bioresour. Technol. 2022, 362, 127790. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.C.; de Carvalho, A.P.A.; da Silva, B.D.; Torres Neto, L.; de Figueiredo, M.R.d.S.; Chaves, P.H.T.; de Almeida, A.E.C.C.; Conte-Junior, C.A. Green Ultrasound-Assisted Extraction of Bioactive Compounds of Babassu (Attalea speciosa) Mesocarp: Effects of Solid-Liquid Ratio Extraction, Antioxidant Capacity, and Antimicrobial Activity. Appl. Food Res. 2023, 3, 100331. [Google Scholar] [CrossRef]
- de Carvalho, A.P.A.; Conte-Junior, C.A. Food-Derived Biopolymer Kefiran Composites, Nanocomposites and Nanofibers: Emerging Alternatives to Food Packaging and Potentials in Nanomedicine. Trends Food Sci. Technol. 2021, 116, 370–386. [Google Scholar] [CrossRef]
- Lima, R.C.; de Carvalho, A.P.A.; Vieira, C.P.; Moreira, R.V.; Conte-Junior, C.A. Green and Healthier Alternatives to Chemical Additives as Cheese Preservative: Natural Antimicrobials in Active Nanopackaging/Coatings. Polymers 2021, 13, 2675. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de de Carvalho, A.P.A.; Conte-Junior, C.A. Recent Advances in Biobased and Biodegradable Polymer Nanocomposites, Nanoparticles, and Natural Antioxidants for Antibacterial and Antioxidant Food Packaging Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3673–3716. [Google Scholar] [CrossRef]
- de Carvalho, A.P.A.; Conte Junior, C.A. Green Strategies for Active Food Packagings: A Systematic Review on Active Properties of Graphene-Based Nanomaterials and Biodegradable Polymers. Trends Food Sci. Technol. 2020, 103, 130–143. [Google Scholar] [CrossRef]
- Soares Mateus, A.R.; Pena, A.; Sendón, R.; Almeida, C.; Nieto, G.A.; Khwaldia, K.; Sanches Silva, A. By-Products of Dates, Cherries, Plums and Artichokes: A Source of Valuable Bioactive Compounds. Trends Food Sci. Technol. 2023, 131, 220–243. [Google Scholar] [CrossRef]
- de Carvalho, A.P.A.; Conte-Junior, C.A. Health Benefits of Phytochemicals from Brazilian Native Foods and Plants: Antioxidant, Antimicrobial, Anti-Cancer, and Risk Factors of Metabolic/Endocrine Disorders Control. Trends Food Sci. Technol. 2021, 111, 534–548. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; Conte-Junior, C.A. Nano-Delivery Systems for Food Bioactive Compounds in Cancer: Prevention, Therapy, and Clinical Applications. Crit. Rev. Food Sci. Nutr. 2022, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.R.S.; Tessaro, L.; Lima, A.K.O.; Velloso, I.P.S.; Conte-Junior, C.A. Recent Progress in Nanotechnology Improving the Therapeutic Potential of Polyphenols for Cancer. Nutrients 2023, 15, 3136. [Google Scholar] [CrossRef] [PubMed]
- Tossavainen, M.; Edelmann, M.; Lahti-Leikas, K.; Kivimäki, S.; Kymäläinen, M.; Piironen, V.; Lampi, A.-M.; Ojala, A.; Romantschuk, M. Chemical Composition and Biomethane Production Potential of Euglena Gracilis Biomass and Extraction Residue from Supercritical CO2 Extraction. Bioresour. Technol. Rep. 2022, 19, 101140. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Martí-Quijal, F.J.; Huertas-Alonso, A.J.; Sánchez-Verdú, M.P.; Cravotto, G.; Moreno, A.; Barba, F.J. Sequential Extraction of Almond Hull Biomass with Pulsed Electric Fields (PEF) and Supercritical CO2 for the Recovery of Lipids, Carbohydrates and Antioxidants. Food Bioprod. Process. 2023, 139, 216–226. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, H.; Li, B.; Pan, P.; Xu, G.; Zhao, Q.; Jiang, X. A Novel System Integrating Water Electrolysis and Supercritical CO2 Cycle for Biomass to Methanol. Appl. Therm. Eng. 2023, 225, 120234. [Google Scholar] [CrossRef]
- Hogan, P.; Otero, P.; Murray, P.; Saha, S.K. Effect of Biomass Pre-Treatment on Supercritical CO2 Extraction of Lipids from Marine Diatom Amphora sp. and Its Biomass Evaluation as Bioethanol Feedstock. Heliyon 2021, 7, e05995. [Google Scholar] [CrossRef]
- Swetha, T.A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Review on Biodegradable Polylactic Acid (PLA) Production from Fermentative Food Waste—Its Applications and Degradation. Int. J. Biol. Macromol. 2023, 234, 123703. [Google Scholar] [CrossRef]
- Woraprayote, W.; Janyaphisan, T.; Adunphatcharaphon, S.; Sonhom, N.; Showpanish, K.; Rumjuankiat, K.; Visessanguan, W.; Elliott, C.T.; Petchkongkaew, A. Bacteriocinogenic Lactic Acid Bacteria from Thai Fermented Foods: Potential Food Applications. Food Biosci. 2023, 52, 102385. [Google Scholar] [CrossRef]
- Ruiz Rodríguez, L.G.; Zamora Gasga, V.M.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sánchez Burgos, J.A. Fruits and Fruit By-Products as Sources of Bioactive Compounds. Benefits and Trends of Lactic Acid Fermentation in the Development of Novel Fruit-Based Functional Beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef]
- Nunui, K.; Boonsawang, P.; Chaiprapat, S.; Charnnok, B. Using Organosolv Pretreatment with Acid Wastewater for Enhanced Fermentable Sugar and Ethanol Production from Rubberwood Waste. Renew. Energy 2022, 198, 723–732. [Google Scholar] [CrossRef]
- Torres-León, C.; Chávez-González, M.L.; Hernández-Almanza, A.; Martínez-Medina, G.A.; Ramírez-Guzmán, N.; Londoño-Hernández, L.; Aguilar, C.N. Recent Advances on the Microbiological and Enzymatic Processing for Conversion of Food Wastes to Valuable Bioproducts. Curr. Opin. Food Sci. 2021, 38, 40–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, J.; Xu, G.; Han, R.; Zhou, J.; Ni, Y. Efficient Production of Hyaluronic Acid by Streptococcus Zooepidemicus Using Two-Stage Semi-Continuous Fermentation. Bioresour. Technol. 2023, 377, 128896. [Google Scholar] [CrossRef] [PubMed]
- Pfleger, B.F.; Takors, R. Recent Progress in the Synthesis of Advanced Biofuel and Bioproducts. Curr. Opin. Biotechnol. 2023, 80, 102913. [Google Scholar] [CrossRef]
- Mandaokar, A. Market Research Report.Polaris Market Research. 2021, pp. 1–117. Available online: https://www.marketresearchfuture.com/reports/bio-based-chemicals-market/toc (accessed on 20 July 2023).
- Santos, J.R.; Viana, G.C.C.; Barbosa, R.S.; Borges, M.d.S.; Rambo, M.K.D.; Bertuol, D.A.; Scapin, E. Effect of Different Pretreatments of Passiflora Edulis Peel Biomass on the Conversion Process into Bioproducts for Biorefineries. Sustain. Chem. Environ. 2023, 2, 100013. [Google Scholar] [CrossRef]
- Cheirsilp, B.; Billateh, A.; Intasit, R.; Upaichit, A.; Boonsawang, P.; Louhasakul, Y. Fungal Pretreatment and Acid Post-Treatment for Fractionation and Biovalorization of Palm Biomass Wastes into Fungal Oil, Bioethanol, and Lactic Acid. Ind. Crops Prod. 2023, 196, 116503. [Google Scholar] [CrossRef]
- Quintero, J.A.; Rincón, L.E.; Cardona, C.A. Production of Bioethanol from Agroindustrial Residues as Feedstocks. In Biofuels; Elsevier: Amsterdam, The Netherlands, 2011; pp. 251–285. [Google Scholar]
- Zaafouri, K.; Ziadi, M.; Ben Farah, R.; Farid, M.; Hamdi, M.; Regaya, I. Potential of Tunisian Alfa (Stipa tenassicima) Fibers for Energy Recovery to 2G Bioethanol: Study of Pretreatment, Enzymatic Saccharification and Fermentation. Biomass Bioenergy 2016, 94, 66–77. [Google Scholar] [CrossRef]
- Sharma, B.; Larroche, C.; Dussap, C.-G. Comprehensive Assessment of 2G Bioethanol Production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef]
- Joseph, A.M.; Tulasi, Y.; Shrivastava, D.; Kiran, B. Techno-Economic Feasibility and Exergy Analysis of Bioethanol Production from Waste. Energy Convers. Manag. X 2023, 18, 100358. [Google Scholar] [CrossRef]
- de Jesús González-Chavez, J.; Arenas-Grimaldo, C.; Amaya-Delgado, L.; Vázquez-Núñez, E.; Suarez-Vázquez, S.; Cruz-López, A.; Segovia-Hernández, J.G.; Pérez-Vega, S.; Salmerón, I.; Molina-Guerrero, C.E. Sotol Bagasse (Dasylirion sp.) as a Novel Feedstock to Produce Bioethanol 2G: Bioprocess Design and Biomass Characterization. Ind. Crops Prod. 2022, 178, 114571. [Google Scholar] [CrossRef]
- Elias, A.M.; Longati, A.A.; de Campos Giordano, R.; Furlan, F.F. Retro-Techno-Economic-Environmental Analysis Improves the Operation Efficiency of 1G-2G Bioethanol and Bioelectricity Facilities. Appl. Energy 2021, 282, 116133. [Google Scholar] [CrossRef]
- Balasundaram, G.; Banu, R.; Varjani, S.; Kazmi, A.A.; Tyagi, V.K. Recalcitrant Compounds Formation, Their Toxicity, and Mitigation: Key Issues in Biomass Pretreatment and Anaerobic Digestion. Chemosphere 2022, 291, 132930. [Google Scholar] [CrossRef] [PubMed]
- Sazuan, N.S.A.; Zubairi, S.I.; Mohd, N.H.; Daik, R. Synthesising Injectable Molecular Self-Curing Polymer from Monomer Derived from Lignocellulosic Oil Palm Empty Fruit Bunch Biomass: A Review on Treating Osteoarthritis. Arab. J. Chem. 2023, 16, 104500. [Google Scholar] [CrossRef]
- Blair, E.M.; Dickson, K.L.; O’Malley, M.A. Microbial Communities and Their Enzymes Facilitate Degradation of Recalcitrant Polymers in Anaerobic Digestion. Curr. Opin. Microbiol. 2021, 64, 100–108. [Google Scholar] [CrossRef]
- Vieira, E.; Brandão, T.; Ferreira, I.M.P.L.V.O. Evaluation of Brewer’s Spent Yeast To Produce Flavor Enhancer Nucleotides: Influence of Serial Repitching. J. Agric. Food. Chem. 2013, 61, 8724–8729. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y. Optimization of Extraction Parameters for Trehalose from Beer Waste Brewing Yeast Treated by High-Intensity Pulsed Electric Fields (PEF). Afr. J. Biotechnol. 2011, 10, 19144–19152. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, B.-R.; Heo, Y.; Bae, G.-S.; Chang, M.B.; Moon, B. Feasibility of Using Kimchi By-Products as a Source of Functional Ingredients. Appl. Biol. Chem. 2016, 59, 799–806. [Google Scholar] [CrossRef]
- Nagai, H.; Kobayashi, M.; Tsuji, Y.; Nakashimada, Y.; Kakizono, T.; Nishio, N. Biological and Chemical Treatment of Solid Waste from Soy Sauce Manufacture. Water Sci. Technol. 2002, 45, 335–338. [Google Scholar] [CrossRef]
- Ichikawa, N.; Ng, L.S.; Makino, S.; Goh, L.L.; Lim, Y.J.; Ferdinandus; Sasaki, H.; Shibata, S.; Lee, C.-L.K. Solid-State Fermented Okara with Aspergillus spp. Improves Lipid Metabolism and High-Fat Diet Induced Obesity. Metabolites 2022, 12, 198. [Google Scholar] [CrossRef]
- Zhou, R.; Ren, Z.; Ye, J.; Fan, Y.; Liu, X.; Yang, J.; Deng, Z.-Y.; Li, J. Fermented Soybean Dregs by Neurospora Crassa: A Traditional Prebiotic Food. Appl. Biochem. Biotechnol. 2019, 189, 608–625. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Veloza, A.; Zhong, Q.; Kim, W.-S.; D’Souza, D.; Krishnan, H.B.; Dia, V.P. Utilization of Tofu Processing Wastewater as a Source of the Bioactive Peptide Lunasin. Food Chem. 2021, 362, 130220. [Google Scholar] [CrossRef] [PubMed]
- Jansone, L.; Kruma, Z.; Straumite, E. Evaluation of Chemical and Sensory Characteristics of Sauerkraut Juice Powder and Its Application in Food. Foods 2022, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Bosco, F.; Carletto, R.A.; Marmo, L. An Integrated Cheese Whey Valorization Process. Chem. Eng. Trans. 2018, 64, 379–384. [Google Scholar] [CrossRef]
- Crowley, S.V.; Molitor, M.S.; Kalscheuer, R.; Lu, Y.; Kelly, A.L.; O’Mahony, J.A.; Lucey, J.A. Size Classification of Precipitated Calcium Phosphate Using Hydrocyclone Technology for the Recovery of Minerals from Deproteinised Acid Whey. Int. J. Dairy Technol. 2019, 72, 142–151. [Google Scholar] [CrossRef]
- Conway, V.; Couture, P.; Gauthier, S.; Pouliot, Y.; Lamarche, B. Effect of Buttermilk Consumption on Blood Pressure in Moderately Hypercholesterolemic Men and Women. Nutrition 2014, 30, 116–119. [Google Scholar] [CrossRef]
- Jenab, A.; Roghanian, R.; Emtiazi, G. Encapsulation of Platelet in Kefiran Polymer and Detection of Bioavailability of Immobilized Platelet in Probiotic Kefiran as a New Drug for Surface Bleeding. J. Med. Bacteriol. 2015, 4, 45–55. [Google Scholar]
- Piermaria, J.; Diosma, G.; Aquino, C.; Garrote, G.; Abraham, A. Edible Kefiran Films as Vehicle for Probiotic Microorganisms. Innov. Food Sci. Emerg. Technol. 2015, 32, 193–199. [Google Scholar] [CrossRef]
- Gagliarini, N.; Diosma, G.; Garrote, G.L.; Abraham, A.G.; Piermaria, J. Whey Protein-Kefiran Films as Driver of Probiotics to the Gut. LWT 2019, 105, 321–328. [Google Scholar] [CrossRef]
- Montoille, L.; Morales Vicencio, C.; Fontalba, D.; Ortiz, J.A.; Moreno-Serna, V.; Peponi, L.; Matiacevich, S.; Zapata, P.A. Study of the Effect of the Addition of Plasticizers on the Physical Properties of Biodegradable Films Based on Kefiran for Potential Application as Food Packaging. Food Chem. 2021, 360, 129966. [Google Scholar] [CrossRef]
- Adessi, A.; Venturi, M.; Candeliere, F.; Galli, V.; Granchi, L.; De Philippis, R. Bread Wastes to Energy: Sequential Lactic and Photo-Fermentation for Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 9569–9576. [Google Scholar] [CrossRef]
- Narisetty, V.; Nagarajan, S.; Gadkari, S.; Ranade, V.V.; Zhang, J.; Patchigolla, K.; Bhatnagar, A.; Kumar Awasthi, M.; Pandey, A.; Kumar, V. Process Optimization for Recycling of Bread Waste into Bioethanol and Biomethane: A Circular Economy Approach. Energy Convers. Manag. 2022, 266, 115784. [Google Scholar] [CrossRef]
- Maina, S.; Schneider, R.; Alexandri, M.; Papapostolou, H.; Nychas, G.-J.; Koutinas, A.; Venus, J. Volumetric Oxygen Transfer Coefficient as Fermentation Control Parameter to Manipulate the Production of Either Acetoin or D-2,3-Butanediol Using Bakery Waste. Bioresour. Technol. 2021, 335, 125155. [Google Scholar] [CrossRef] [PubMed]
- Narisetty, V.; Zhang, L.; Zhang, J.; Sze Ki Lin, C.; Wah Tong, Y.; Loke Show, P.; Kant Bhatia, S.; Misra, A.; Kumar, V. Fermentative Production of 2,3-Butanediol Using Bread Waste—A Green Approach for Sustainable Management of Food Waste. Bioresour. Technol. 2022, 358, 127381. [Google Scholar] [CrossRef]
- Jung, J.-M.; Kim, J.Y.; Kim, J.-H.; Kim, S.M.; Jung, S.; Song, H.; Kwon, E.E.; Choi, Y.-E. Zero-Waste Strategy by Means of Valorization of Bread Waste. J. Clean Prod. 2022, 365, 132795. [Google Scholar] [CrossRef]
- Dymchenko, A.; Geršl, M.; Gregor, T. The perspective of circular food waste management in the combined case of bakery and brewery. In Zero Waste Management and Circular Economy; Mendelova Univerzita v Brně: Brno, Czech Republic, 2021; pp. 129–135. [Google Scholar]
- Demirci, A.S.; Palabiyik, I.; Apaydın, D.; Mirik, M.; Gumus, T. Xanthan Gum Biosynthesis Using Xanthomonas Isolates from Waste Bread: Process Optimization and Fermentation Kinetics. LWT 2019, 101, 40–47. [Google Scholar] [CrossRef]
- Samray, M.N.; Masatcioglu, T.M.; Koksel, H. Bread Crumbs Extrudates: A New Approach for Reducing Bread Waste. J. Cereal. Sci. 2019, 85, 130–136. [Google Scholar] [CrossRef]
- Sadaf, A.; Kumar, S.; Nain, L.; Khare, S.K. Bread Waste to Lactic Acid: Applicability of Simultaneous Saccharification and Solid State Fermentation. Biocatal. Agric. Biotechnol. 2021, 32, 101934. [Google Scholar] [CrossRef]
- Suescun–Ospina, S.T.; Ávila–Stagno, J.; Vera-Aguilera, N.; Astudillo-Neira, R.; Trujillo-Mayol, I.; Alarcón-Enos, J. Effects of Drying Method on Bioactive Compounds Contents, Rumen Fermentation Parameters and in Vitro Methane Output of Waste Dried País Grape (Vitis vinifera L.) Marc. Food Biosci. 2023, 51, 102154. [Google Scholar] [CrossRef]
- Bassani, A.; Alberici, N.; Fiorentini, C.; Giuberti, G.; Dordoni, R.; Spigno, G. Hydrothermal Treatment of Grape Skins for Sugars, Antioxidants and Soluble Fibers Production. Chem. Eng. Trans. 2020, 79, 127–132. [Google Scholar] [CrossRef]
- Costabile, G.; Vitale, M.; Luongo, D.; Naviglio, D.; Vetrani, C.; Ciciola, P.; Tura, A.; Castello, F.; Mena, P.; Del Rio, D.; et al. Grape Pomace Polyphenols Improve Insulin Response to a Standard Meal in Healthy Individuals: A Pilot Study. Clin. Nutr. 2019, 38, 2727–2734. [Google Scholar] [CrossRef]
- Yu, J.; Bansode, R.R.; Smith, I.N.; Hurley, S.L. Impact of Grape Pomace Consumption on the Blood Lipid Profile and Liver Genes Associated with Lipid Metabolism of Young Rats. Food Funct. 2017, 8, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Ishimoto, E.Y.; Vicente, S.J.V.; Cruz, R.J.; da Silva Torres, E.A.F. Hypolipidemic and Antioxidant Effects of Grape Processing By-Products in High-Fat/Cholesterol Diet-Induced Hyperlipidemic Hamsters. Food Sci. Technol. 2020, 40, 558–567. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Martí, À.; Gil-Cardoso, K.; Blay, M.T.; Terra, X.; Pinent, M.; Ardévol, A. Acutely Administered Grape-Seed Proanthocyanidin Extract Acts as a Satiating Agent. Food Funct. 2016, 7, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Martí, À.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. Grape Seed Proanthocyanidins Influence Gut Microbiota and Enteroendocrine Secretions in Female Rats. Food Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Balea, Ş.S.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Andreicuț, A.; Pârvu, M. Phytochemical Profiling, Antioxidant and Cardioprotective Properties of Pinot Noir Cultivar Pomace Extracts. Farmacia 2018, 66, 432–441. [Google Scholar] [CrossRef]
- Balea, Ş.S.; Pârvu, A.E.; Pop, N.; Marín, F.Z.; Pârvu, M. Polyphenolic Compounds, Antioxidant, and Cardioprotective Effects of Pomace Extracts from Fetească Neagră Cultivar. Oxid Med. Cell Longev. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Chacar, S.; Hajal, J.; Saliba, Y.; Bois, P.; Louka, N.; Maroun, R.G.; Faivre, J.; Fares, N. Long-term Intake of Phenolic Compounds Attenuates Age-related Cardiac Remodeling. Aging Cell 2019, 18, e12894. [Google Scholar] [CrossRef]
- Pérez-Ortiz, J.M.; Alguacil, L.F.; Salas, E.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; González-Martín, C. Antiproliferative and Cytotoxic Effects of Grape Pomace and Grape Seed Extracts on Colorectal Cancer Cell Lines. Food Sci. Nutr. 2019, 7, 2948–2957. [Google Scholar] [CrossRef]
- Leone, A.; Longo, C.; Gerardi, C.; Trosko, J.E. Pro-Apoptotic Effect of Grape Seed Extract on MCF-7 Involves Transient Increase of Gap Junction Intercellular Communication and Cx43 Up-Regulation: A Mechanism of Chemoprevention. Int. J. Mol. Sci. 2019, 20, 3244. [Google Scholar] [CrossRef]
- Dimou, C.; Kopsahelis, N.; Papadaki, A.; Papanikolaou, S.; Kookos, I.K.; Mandala, I.; Koutinas, A.A. Wine Lees Valorization: Biorefinery Development Including Production of a Generic Fermentation Feedstock Employed for Poly(3-Hydroxybutyrate) Synthesis. Food Res. Int. 2015, 73, 81–87. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Pereira, J.O.; Ferreira, C.; Faustino, M.; Durão, J.; Pintado, M.E.; Carvalho, A.P. Peptide-Rich Extracts from Spent Yeast Waste Streams as a Source of Bioactive Compounds for the Nutraceutical Market. Innov. Food Sci. Emerg. Technol. 2022, 81, 103148. [Google Scholar] [CrossRef]
- Cejas, L.; Romano, N.; Moretti, A.; Mobili, P.; Golowczyc, M.; Gómez-Zavaglia, A. Malt Sprout, an Underused Beer by-Product with Promising Potential for the Growth and Dehydration of Lactobacilli Strains. J. Food Sci. Technol. 2017, 54, 4464–4472. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Characterization of a Potential Bioactive Food Ingredient from Inner Cellular Content of Brewer’s Spent Yeast. Waste Biomass Valorization 2019, 10, 3235–3242. [Google Scholar] [CrossRef]
- Bastos, R.; Coelho, E.; Coimbra, M.A. Modifications of Saccharomyces Pastorianus Cell Wall Polysaccharides with Brewing Process. Carbohydr. Polym. 2015, 124, 322–330. [Google Scholar] [CrossRef]
- Liepins, J.; Kovačova, E.; Shvirksts, K.; Grube, M.; Rapoport, A.; Kogan, G. Drying Enhances Immunoactivity of Spent Brewer’s Yeast Cell Wall β-d-Glucans. J. Biotechnol. 2015, 206, 12–16. [Google Scholar] [CrossRef]
- Tian, X.; Yang, P.; Jiang, W. Effect of Alkali Treatment Combined with High Pressure on Extraction Efficiency of β-d-Glucan from Spent Brewer’s Yeast. Waste Biomass Valorization 2019, 10, 1131–1140. [Google Scholar] [CrossRef]
- Mahalingam, R. Analysis of the Barley Malt Rootlet Proteome. Int. J. Mol. Sci. 2019, 21, 179. [Google Scholar] [CrossRef]
- Cheng, H.-J.; Zhang, L.-M.; Duan, H.-X.; Li, J.; Dai, Y.-J. Optimization of Extraction Technology of the Alkali-Soluble Components of Barley Malt Roots and Its Composition Analysis. In Medicine and Biopharmaceutical; World Scientific: Singapore, 2016; pp. 1497–1505. [Google Scholar]
- Budaraju, S.; Mallikarjunan, K.; Annor, G.; Schoenfuss, T.; Raun, R. Effect of Pre-Treatments on the Antioxidant Potential of Phenolic Extracts from Barley Malt Rootlets. Food Chem. 2018, 266, 31–37. [Google Scholar] [CrossRef]
- Gandolpho, B.; Almeida, A.; Gandolpho, G.; Freitas, D.; Gasparini, O.; Machado, M.; Barreto, P. Optimization of Brewing Waste’s (Trub) Phenolic Compounds Extraction by Ultrasound Assisted Using Response Surface Methodology. Quim. Nova 2020, 44, 478–483. [Google Scholar] [CrossRef]
- Senna Ferreira Costa, F.; Roquete Amparo, T.; Brandão Seibert, J.; Silveira, B.M.; Gomes da Silva, R.; Inocêncio Pereira, D.; Gontijo Garcia Barbosa, R.; dos Santos, O.D.H.; Brandão, G.C.; de Medeiros Teixeira, L.F.; et al. Reuse of Hot Trub as an Active Ingredient with Antioxidant and Antimicrobial Potential. Waste Biomass Valorization 2021, 12, 2037–2047. [Google Scholar] [CrossRef]
- Anioł, M.; Huszcza, E.; Bartmańska, A.; Żołnierczyk, A.; Mączka, W.; Wawrzeńczyk, C. Trace Analysis of Hop Essential Oils in Spent Hop. J. Am. Soc. Brew. Chem. 2007, 65, 214–218. [Google Scholar] [CrossRef]
- Anioł, M.; Żołnierczyk, A. Extraction of Spent Hops Using Organic Solvents. J. Am. Soc. Brew. Chem. 2008, 66, 208–214. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Increasing Value of Winery Residues through Integrated Biorefinery Processes: A Review. Molecules 2022, 27, 4709. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L.C.; Oliveira, A.L.S.; Carsanba, E.; Pintado, M.; Oliveira, C. Phenolic Compounds Modulation in β-Farnesene Fed-Batch Fermentation Using Sugarcane Syrup as Feedstock. Ind. Crops Prod. 2022, 188, 115721. [Google Scholar] [CrossRef]
- Leite, P.; Belo, I.; Salgado, J.M. Co-Management of Agro-Industrial Wastes by Solid-State Fermentation for the Production of Bioactive Compounds. Ind. Crops Prod. 2021, 172, 113990. [Google Scholar] [CrossRef]
- Melloul, S.; Zehioua, R.; Meniai, A.-H. Supercritical CO2 Extraction of Bioactive Compounds from Local Peganum Harmala Plant Seeds and Optimization of the Extraction Yield and the Antioxidant Activities. Sustain. Chem. Pharm. 2022, 28, 100729. [Google Scholar] [CrossRef]
- Cheng, F.; Brewer, C.E. Conversion of Protein-Rich Lignocellulosic Wastes to Bio-Energy: Review and Recommendations for Hydrolysis + Fermentation and Anaerobic Digestion. Renew. Sustain. Energy Rev. 2021, 146, 111167. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Anand Singh, T.; Joykumar Singh, N.; Prasad Shadangi, K.; Srivastava, R.K.; Singh, A.K.; Chandel, A.K.; Pareek, N.; Vivekanand, V. Sustainable Utilization of Pineapple Wastes for Production of Bioenergy, Biochemicals and Value-Added Products: A Review. Bioresour. Technol. 2022, 351, 127085. [Google Scholar] [CrossRef]
- Srimachai, T.; Nuithitikul, K.; O-thong, S.; Kongjan, P.; Panpong, K. Optimization and Kinetic Modeling of Ethanol Production from Oil Palm Frond Juice in Batch Fermentation. Energy Procedia 2015, 79, 111–118. [Google Scholar] [CrossRef]
- Qi, S.; Wang, Y.; Chu, X.; Wang, W.; Zhan, X.; Hu, Z.-H. Food Waste Fermentation for Carbon Source Production and Denitrification in Sequencing Batch Reactors. J. Clean. Prod. 2020, 253, 119934. [Google Scholar] [CrossRef]
- Zhao, L.; Wu, K.-K.; Chen, C.; Ren, H.-Y.; Wang, Z.-H.; Nan, J.; Yang, S.-S.; Cao, G.-L.; Ren, N.-Q. Role of Residue Cornstalk Derived Biochar for the Enhanced Bio-Hydrogen Production via Simultaneous Saccharification and Fermentation of Cornstalk. Bioresour. Technol. 2021, 330, 125006. [Google Scholar] [CrossRef]
- Shi, M.J.; Ma, Z.X.; Tian, Y.J.; Ma, C.; Li, Y.D.; Zhang, X.W. Effects of Corn Straw Treated with CaO on Rumen Degradation Characteristics and Fermentation Parameters and Their Correlation with Microbial Diversity in Rumen. Anim. Feed Sci. Technol. 2022, 292, 115403. [Google Scholar] [CrossRef]
- Lal, N.; Seifan, M.; Berenjian, A. The Impact of Key Fermentation Parameters on the Production of the All-Trans Isomer of Menaquinone-7. Biocatal. Agric. Biotechnol. 2022, 46, 102548. [Google Scholar] [CrossRef]
- Farias, T.C.; Kawaguti, H.Y.; Bello Koblitz, M.G. Microbial Amylolytic Enzymes in Foods: Technological Importance of the Bacillus Genus. Biocatal. Agric. Biotechnol. 2021, 35, 102054. [Google Scholar] [CrossRef]
- Sahir, A.H.; Kumar, S.; Kumar, S. Modelling of a Packed Bed Solid-State Fermentation Bioreactor Using the N-Tanks in Series Approach. Biochem. Eng. J. 2007, 35, 20–28. [Google Scholar] [CrossRef]
- da Conceição Gomes, A.; Rodrigues, M.I.; de França Passos, D.; Machado de Castro, A.; Maria Mello Santa Anna, L.; Pereira, N. Acetone–Butanol–Ethanol Fermentation from Sugarcane Bagasse Hydrolysates: Utilization of C5 and C6 Sugars. Electron. J. Biotechnol. 2019, 42, 16–22. [Google Scholar] [CrossRef]
- de Morais, W.G.; Kamimura, E.S.; Ribeiro, E.J.; Pessela, B.C.; Cardoso, V.L.; de Resende, M.M. Optimization of the Production and Characterization of Lipase from Candida Rugosa and Geotrichum Candidum in Soybean Molasses by Submerged Fermentation. Protein Expr. Purif. 2016, 123, 26–34. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.Á. Application of Solid-State Fermentation to Food Industry—A Review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Pollice, A.; Laera, G.; Blonda, M. Biomass Growth and Activity in a Membrane Bioreactor with Complete Sludge Retention. Water Res. 2004, 38, 1799–1808. [Google Scholar] [CrossRef]
- Ullah, A.; Zhang, Y.; Liu, C.; Qiao, Q.; Shao, Q.; Shi, J. Process Intensification Strategies for Green Solvent Mediated Biomass Pretreatment. Bioresour. Technol. 2023, 369, 128394. [Google Scholar] [CrossRef]
- Bhushan, S.; Jayakrishnan, U.; Shree, B.; Bhatt, P.; Eshkabilov, S.; Simsek, H. Biological Pretreatment for Algal Biomass Feedstock for Biofuel Production. J. Environ. Chem. Eng. 2023, 11, 109870. [Google Scholar] [CrossRef]
- Tuan Hoang, A.; Phuong Nguyen, X.; Quang Duong, X.; Ağbulut, Ü.; Len, C.; Quy Phong Nguyen, P.; Kchaou, M.; Chen, W.-H. Steam Explosion as Sustainable Biomass Pretreatment Technique for Biofuel Production: Characteristics and Challenges. Bioresour. Technol. 2023, 385, 129398. [Google Scholar] [CrossRef]
- Li, P.; Cai, D.; Luo, Z.; Qin, P.; Chen, C.; Wang, Y.; Zhang, C.; Wang, Z.; Tan, T. Effect of Acid Pretreatment on Different Parts of Corn Stalk for Second Generation Ethanol Production. Bioresour. Technol. 2016, 206, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Malayil, S.; Surendran, A.N.; Kate, K.; Satyavolu, J. Impact of Acid Hydrolysis on Composition, Morphology and Xylose Recovery from Almond Biomass (Skin and Shell). Bioresour. Technol. Rep. 2022, 19, 101150. [Google Scholar] [CrossRef]
- Haldar, D.; Shabbirahmed, A.M.; Mahanty, B. Multivariate Regression and Artificial Neural Network Modelling of Sugar Yields from Acid Pretreatment and Enzymatic Hydrolysis of Lignocellulosic Biomass. Bioresour. Technol. 2023, 370, 128519. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Koo, B.; Lee, J.-W. Structural Changes in Biomass (Yellow Poplar and Empty Fruit Bunch) during Hydrothermal and Oxalic Acid Pretreatments and Their Effects on Enzymatic Hydrolysis Efficiency. Ind. Crops Prod. 2022, 178, 114569. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Jeong, K.; Li, S.; Leem, G.; Kim, K.H.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Investigation of a Lignin-Based Deep Eutectic Solvent Using p -Hydroxybenzoic Acid for Efficient Woody Biomass Conversion. ACS Sustain. Chem. Eng. 2020, 8, 12542–12553. [Google Scholar] [CrossRef]
- Ouyang, D.; Liu, T.; Astimar, A.A.; Lau, H.L.N.; Teh, S.S.; Nursyairah, J.; Liu, D.; Zhao, X. Model-Based Process Intensification of Dilute Acid Pre-Hydrolysis of Oil Palm Empty Fruit Bunch Biomass for Pretreatment and Furfural Production. Bioresour. Technol. 2023, 372, 128626. [Google Scholar] [CrossRef]
- Srivastava, K.; Mickan, B.S.; O’Connor, J.; Gurung, S.K.; Moheimani, N.R.; Jenkins, S.N. Development of a Controlled Release Fertilizer by Incorporating Lauric Acid into Microalgal Biomass: Dynamics on Soil Biological Processes for Efficient Utilisation of Waste Resources. J. Environ. Manag. 2023, 344, 118392. [Google Scholar] [CrossRef] [PubMed]
- Pendse, D.S.; Deshmukh, M.; Pande, A. Different Pre-Treatments and Kinetic Models for Bioethanol Production from Lignocellulosic Biomass: A Review. Heliyon 2023, 9, e16604. [Google Scholar] [CrossRef]
- Neis, U.; Cleary, R. The Use of Power Ultrasound for Wastewater and Biomass Treatment. In Power Ultrasonics; Elsevier: Amsterdam, The Netherlands, 2023; pp. 817–832. [Google Scholar]
- Zhang, J.; Wei, J.; Guo, C.; Tang, Q.; Guo, H. The Spatial Distribution Characteristics of the Biomass Residual Potential in China. J. Environ. Manag. 2023, 338, 117777. [Google Scholar] [CrossRef] [PubMed]
- Thamizhakaran Stanley, J.; Thanarasu, A.; Senthil Kumar, P.; Periyasamy, K.; Raghunandhakumar, S.; Periyaraman, P.; Devaraj, K.; Dhanasekaran, A.; Subramanian, S. Potential Pre-Treatment of Lignocellulosic Biomass for the Enhancement of Biomethane Production through Anaerobic Digestion—A Review. Fuel 2022, 318, 123593. [Google Scholar] [CrossRef]
- Lee, J.A.; Kim, H.U.; Na, J.-G.; Ko, Y.-S.; Cho, J.S.; Lee, S.Y. Factors Affecting the Competitiveness of Bacterial Fermentation. Trends Biotechnol. 2023, 41, 798–816. [Google Scholar] [CrossRef]
- Hazeena, S.H.; Shurpali, N.J.; Siljanen, H.; Lappalainen, R.; Anoop, P.; Adarsh, V.P.; Sindhu, R.; Pandey, A.; Binod, P. Bioprocess development of 2, 3-butanediol production using agro-industrial residues. Bioprocess. Biosyst. Eng. 2022, 45, 1527–1537. [Google Scholar] [CrossRef]
- Henstra, A.M.; Sipma, J.; Rinzema, A.; Stams, A.J. Microbiology of Synthesis Gas Fermentation for Biofuel Production. Curr. Opin. Biotechnol. 2007, 18, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Jugwanth, Y.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Valorization of Sugarcane Bagasse for Bioethanol Production through Simultaneous Saccharification and Fermentation: Optimization and Kinetic Studies. Fuel 2020, 262, 116552. [Google Scholar] [CrossRef]
- Sun, X.; Atiyeh, H.K.; Huhnke, R.L.; Tanner, R.S. Syngas Fermentation Process Development for Production of Biofuels and Chemicals: A Review. Bioresour. Technol. Rep. 2019, 7, 100279. [Google Scholar] [CrossRef]
- Yu, X.; Chen, G.; Widenmeyer, M.; Kinski, I.; Liu, X.; Kunz, U.; Schüpfer, D.; Molina-Luna, L.; Tu, X.; Homm, G.; et al. Catalytic Recycling of Medical Plastic Wastes over La0.6Ca0.4Co1–Fe O3− Pre-Catalysts for Co-Production of H2 and High-Value Added Carbon Nanomaterials. Appl. Catal. B 2023, 334, 122838. [Google Scholar] [CrossRef]
- Anniwaer, A.; Chaihad, N.; Choirun Az Zahra, A.; Kurnia, I.; Kasai, Y.; Kongparakul, S.; Samart, C.; Kusakabe, K.; Abudula, A.; Guan, G. Utilization of Fruit Waste for H2-Rich Syngas Production via Steam Co-Gasification with Brown Coal. Carbon Resour. Convers. 2023, 6, 315–325. [Google Scholar] [CrossRef]
- Mateus, S.; Carvalheira, M.; Cassidy, J.; Freitas, E.; Oehmen, A.; Reis, M.A.M. Two-Stage Anaerobic Digestion System Treating Different Seasonal Fruit Pulp Wastes: Impact on Biogas and Hydrogen Production and Total Energy Recovery Potential. Biomass Bioenergy 2020, 141, 105694. [Google Scholar] [CrossRef]
- Ishak, M.A.M.; Ani, A.Y.; Syed Ismail, S.N.A.; Ali, M.L.M.; Ahmad, R. Conversion of Biomass to Biofuels. In Value-Chain of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 49–67. [Google Scholar]
- Villanueva-Galindo, E.; Vital-Jácome, M.; Moreno-Andrade, I. Dark Fermentation for H2 Production from Food Waste and Novel Strategies for Its Enhancement. Int. J. Hydrogen Energy 2023, 48, 9957–9970. [Google Scholar] [CrossRef]
- Lu, C.; Wang, G.; Zhang, Q.; Yang, X.; Yu, J.; Liu, T.; Petracchini, F.; Zhang, Z.; Sun, Y.; Jiang, D.; et al. Comparison of Biorefinery Characteristics: Photo-Fermentation Biohydrogen, Dark Fermentation Biohydrogen, Biomethane, and Bioethanol Production. Appl. Energy 2023, 347, 121463. [Google Scholar] [CrossRef]
- Ahmad, A.; K, R.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen Production through Dark Fermentation: Recent Trends and Advances in Transition to a Circular Bioeconomy. Int. J. Hydrogen Energy 2023, in press. [CrossRef]
- Walker, T.R.; Fequet, L. Current Trends of Unsustainable Plastic Production and Micro(Nano)Plastic Pollution. TrAC Trends Anal. Chem. 2023, 160, 116984. [Google Scholar] [CrossRef]
- Plastics Europe. Plastic-the Facts 2022. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2022/ (accessed on 21 September 2023).
- Statista Research Department. Statista Annual Production of Plastics Worldwide from 1950 to 2021. 2022. Available online: https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/ (accessed on 21 September 2023).
- GRID Arendal. Global Plastic Production and Accumulation. 2021. Available online: https://www.grida.no/resources/1504.1 (accessed on 21 September 2023).
- Hathi, Z.J.; Haque, M.A.; Priya, A.; Qin, Z.; Huang, S.; Lam, C.H.; Ladakis, D.; Pateraki, C.; Mettu, S.; Koutinas, A.; et al. Fermentative Bioconversion of Food Waste into Biopolymer Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Using Cupriavidus Necator. Environ. Res. 2022, 215, 114323. [Google Scholar] [CrossRef] [PubMed]
- Hees, T.; Zhong, F.; Stürzel, M.; Mülhaupt, R. Tailoring Hydrocarbon Polymers and All-Hydrocarbon Composites for Circular Economy. Macromol. Rapid Commun. 2019, 40, 1800608. [Google Scholar] [CrossRef]
- Zhong, S.; Pearce, J.M. Tightening the Loop on the Circular Economy: Coupled Distributed Recycling and Manufacturing with Recyclebot and RepRap 3-D Printing. Resour. Conserv. Recycl. 2018, 128, 48–58. [Google Scholar] [CrossRef]
- Sohn, Y.J.; Kim, H.T.; Baritugo, K.; Jo, S.Y.; Song, H.M.; Park, S.Y.; Park, S.K.; Pyo, J.; Cha, H.G.; Kim, H.; et al. Recent Advances in Sustainable Plastic Upcycling and Biopolymers. Biotechnol. J. 2020, 15, 1900489. [Google Scholar] [CrossRef]
- Madhavan Nampoothiri, K.; Nair, N.R.; John, R.P. An Overview of the Recent Developments in Polylactide (PLA) Research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Otari, S.V.; Jeon, J.-M.; Gurav, R.; Choi, Y.-K.; Bhatia, R.K.; Pugazhendhi, A.; Kumar, V.; Rajesh Banu, J.; Yoon, J.-J.; et al. Biowaste-to-Bioplastic (Polyhydroxyalkanoates): Conversion Technologies, Strategies, Challenges, and Perspective. Bioresour. Technol. 2021, 326, 124733. [Google Scholar] [CrossRef]
- Oh, Y.-R.; Jang, Y.-A.; Song, J.K.; Eom, G.T. Efficient Enzymatic Depolymerization of Polycaprolactone into 6-Hydroxyhexanoic Acid by Optimizing Reaction Conditions and Microbial Conversion of 6-Hydroxyhexanoic Acid into Adipic Acid for Eco-Friendly Upcycling of Polycaprolactone. Biochem. Eng. J. 2022, 185, 108504. [Google Scholar] [CrossRef]
- Yagi, H.; Ninomiya, F.; Funabashi, M.; Kunioka, M. Anaerobic Biodegradation Tests of Poly(Lactic Acid) and Polycaprolactone Using New Evaluation System for Methane Fermentation in Anaerobic Sludge. Polym. Degrad. Stab. 2009, 94, 1397–1404. [Google Scholar] [CrossRef]
- Lanfranchi, A.; Tassinato, G.; Valentino, F.; Martinez, G.A.; Jones, E.; Gioia, C.; Bertin, L.; Cavinato, C. Hydrodynamic Cavitation Pre-Treatment of Urban Waste: Integration with Acidogenic Fermentation, PHAs Synthesis and Anaerobic Digestion Processes. Chemosphere 2022, 301, 134624. [Google Scholar] [CrossRef]
- Kerketta, A.; Vasanth, D. Madhuca Indica Flower Extract as Cheaper Carbon Source for Production of Poly (3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Using Ralstonia Eutropha. Process Biochem. 2019, 87, 1–9. [Google Scholar] [CrossRef]
- Barba, F.J. An Integrated Approach for the Valorization of Cheese Whey. Foods 2021, 10, 564. [Google Scholar] [CrossRef]
- Swetha, T.A.; Bora, A.; Mohanrasu, K.; Balaji, P.; Raja, R.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Comprehensive Review on Polylactic Acid (PLA)—Synthesis, Processing and Application in Food Packaging. Int. J. Biol. Macromol. 2023, 234, 123715. [Google Scholar] [CrossRef]
- Mehrali, F.; Ziyadi, H.; Hekmati, M.; Faridi-Majidi, R.; Qomi, M. Kefiran/Poly(Vinyl Alcohol)/Poly(Vinyl Pyrrolidone) Composite Nanofibers: Fabrication, Characterization and Consideration of Effective Parameters in Electrospinning. SN Appl. Sci. 2020, 2, 895. [Google Scholar] [CrossRef]
- Esnaashari, S.S.; Rezaei, S.; Mirzaei, E.; Afshari, H.; Rezayat, S.M.; Faridi-Majidi, R. Preparation and Characterization of Kefiran Electrospun Nanofibers. Int. J. Biol. Macromol. 2014, 70, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Jenab, A.; Roghanian, R.; Emtiazi, G.; Ghaedi, K. Manufacturing and Structural Analysis of Antimicrobial Kefiran/Polyethylene Oxide Nanofibers for Food Packaging. Iran. Polym. J. 2017, 26, 31–39. [Google Scholar] [CrossRef]
- Dadashi, S.; Boddohi, S.; Soleimani, N. Preparation, Characterization, and Antibacterial Effect of Doxycycline Loaded Kefiran Nanofibers. J. Drug Deliv. Sci. Technol. 2019, 52, 979–985. [Google Scholar] [CrossRef]
- Blandón, L.M.; Islan, G.A.; Castro, G.R.; Noseda, M.D.; Thomaz-Soccol, V.; Soccol, C.R. Kefiran-Alginate Gel Microspheres for Oral Delivery of Ciprofloxacin. Colloids Surf. B Biointerfaces 2016, 145, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, Q.; Zhang, R.; Zhang, Y.; Wang, F.; He, M.; Guo, X.; Yang, J.; Zhang, X.; Mu, J. Pyrolysis of Ca/Fe-Rich Antibiotic Fermentation Residues into Biochars for Efficient Phosphate Removal/Recovery from Wastewater: Turning Hazardous Waste to Phosphorous Fertilizer. Sci. Total Environ. 2023, 869, 161732. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Shi, W.; Zhao, M.; Huang, Z.; Liu, X.; Ruan, W. Preparation of Spiramycin Fermentation Residue Derived Biochar for Effective Adsorption of Spiramycin from Wastewater. Chemosphere 2022, 296, 133902. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, G.; Liu, H.; Dai, X. Application of Composted Lipstatin Fermentation Residue as Organic Fertilizer: Temporal Changes in Soil Characteristics and Bacterial Community. Chemosphere 2022, 306, 135637. [Google Scholar] [CrossRef]
- Pan, M.; Xin, Y.; Wang, Z.; Jia, W.; Lu, H.; Jiang, S.; Wu, Z.; Chen, X.; Wang, Q.; Du, H.; et al. Benign Treatment and Resource Utilization Characteristics of Doramectin Fermentation Residues. J. Clean. Prod. 2023, 401, 136777. [Google Scholar] [CrossRef]
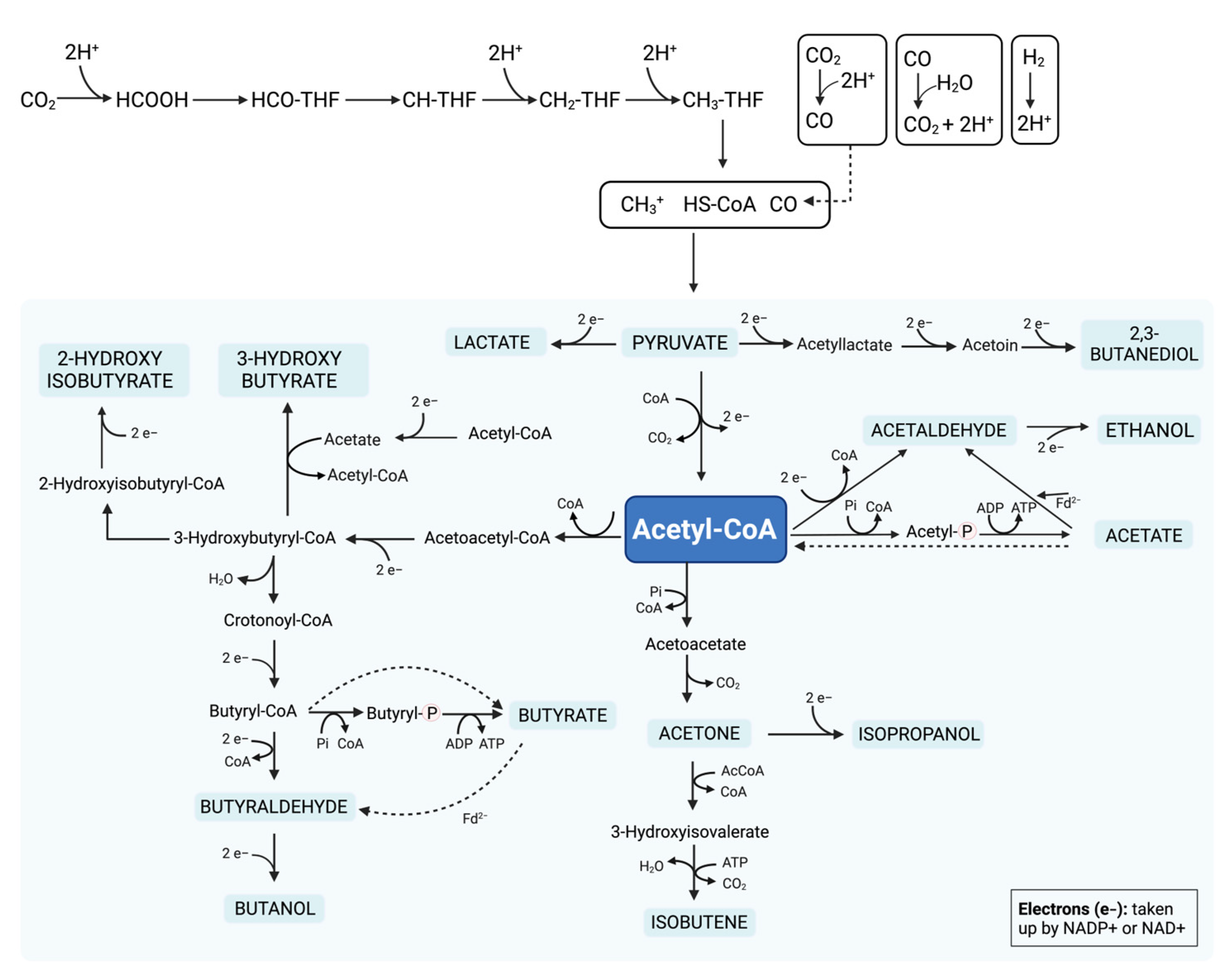
| Fermented Food | Biomass Resource (Natural, Byproduct, Waste) | Bioactive Compounds/Valuable Compounds | Obtaining Method | Potential Health Benefit/Application | Ref. |
|---|---|---|---|---|---|
| FERMENTED VEGETABLES | |||||
| Kimchi factory | Cabbage powder and radish powder | Dietary fibers | UAE (H2O:acetonitrile) | Functional foods | Lee et al., 2016 [63] |
| Soy sauce | Solid waste from soy sauce refuse | CH4, CO2 and VFAs | Anaerobic digestion | Clean energy (biomethane) | Nagai et al., 2022 [64] |
| Protein, insoluble fiber | NaOH treatment | Food seasonings and additives | |||
| Fermented soybean food products | Insoluble residue (okara) | Phenolic compounds and proteins | SSF by Aspergillus oryzae and Aspergillus sojae | Anti-obesity effects | Ichikawa et al., 2022 [65] |
| Fermented soybean food products | Soybean dregs (okara) | Oligosaccharides | Fermentation by Neurospora crassa | Prebiotic food | Zhou et al., 2019 [66] |
| Tofu | Wastewater (tofu whey) | Peptide lunasin | Ethanolic-isoelectric precipitation and gel filtration chromatography | Cancer prevention | Nieto-veloza et al., 2021 [67] |
| Fermented cabbage | Sauerkraut juice | Phenolic compounds, minerals, vitamins, sugars, and NaCl | Spray-drying to obtain sauerkraut juice powder | Salt alternatives in foods | Janosone et al., 2022 [68] |
| FERMENTED DAIRY PRODUCTS | |||||
| Cheese | Wastewater (cheese whey) | Lactose | Biological processes | Carbon sources for biomolecules | Bosco et al., 2018 [69] |
| Lipids and protein | Thermocalcic precipitation | Bioplastics: polyhydroxyalkanoates | |||
| Protein | Ultrafiltration | WPC for the food industry | |||
| Greek yogurt | Acid whey | Calcium phosphate | Liquid–solid hydrocyclone | Food ingredients, food additive | Crowley et al., 2019 [70] |
| Cultured butter | Buttermilk | MFGM proteins and lipids, minerals, lecithin, lactose | Fermentation by LAB | Functional foods; Antihypertensive effects | Conway et al., 2014 [71] |
| Milk kefir | Kefir grains | Polysaccharides, LAB, and yeasts | Milk fermentation: aqueous extraction, lyophilization | Biopolymer kefiran for food and medical applications | Jenab et al., 2015 [72]; Piermaria et al., 2015 [73] |
| Kefir grains | Bioactive polysaccharides and proteins; nisin | Immobilized platelet encapsulation on kefiran | Biomedical applications—drug delivery and tissue engineering | Jenab et al., 2015 [72] | |
| Kefir grains | Bioactive polysaccharides and proteins; lactobacilli | Film-forming dispersions | Probiotic delivery | Gagliani et al., 2019 [74] | |
| Kefir grains | Bioactive polysaccharides and proteins | Polymer solution-casting method | Biodegradable films for food packaging | Piermaria et al., 2015 [73]; Montoille et al., 2021 [75] | |
| BREAD WASTE | |||||
| Bread | Bread waste | Organic acids | Lactic fermentation (Lactobacillus amylovorus DSM 20532) | Energy recovery—biohydrogen production | Adessi et al., 2018 [76] |
| Organic acid from lactic fermentation | Biohydrogen (H2) | Photo-fermentation (Rhodopseudomonas palustris 42OL) | |||
| Bread | Out-of-date bread | Fermentable sugars—glucose (C₆H₁₂O₆) | Acid/enzymatic hydrolysis | Solid-state fermentation | Narisetty, Nagarajan, et al., 2022 [77] |
| Bread waste hydrolysate (glucose) | Bio-based ethanol (C₂H₆O) | SSF by Saccharomyces cerevisiae KL17 | Bioethanol production | ||
| Bio-based methane (CH4) | Solid residue of SSF in ethanol production | Biomethane production | |||
| Bread | Bread waste hydrolysate | D-2,3-Butanediol | Fermentation by Bacillus amyloliquefaciens | Butanediol production | Maina et al., 2021 [78] |
| Fermentation by Enterobacter ludwigii | Narisetty et al., 2022 [79] | ||||
| Bread | Bread waste | Fermentable sugars—glucose (C₆H₁₂O₆) | Enzymatic hydrolysis | Biosource for microalgae cultivation | Jung et al., 2022 [80] |
| Bread waste hydrolysate | Paramylon (β(1,3)-glucan) | Heterotrophic cultivation of microalga Euglena gracilis | Biosource for bioplastic | ||
| Bread | Remnants of bakery products | Sugars and proteins | Bread waste as malt substitute in brewing | Beer production | Dymchenko et al., 2021 [81] |
| Wheat Bread | Stale bread (unsold and returns from shops) | Fermentable sugars—glucose (C₆H₁₂O₆) | Enzymatic hydrolysis | Biosource for xanthan gum biosynthesis | Demirci et al., 2019 [82] |
| Bread waste hydrolysate | Xanthan gum (polysaccharide) | Fermentation by Xanthomonas spp. using glucose as a carbon source | Food additive | ||
| Breadcrumbs | Processing bread waste | Dietary fiber, starch | Bread crumble extrudates by an extrusion process | Wheat flour substitute in extrusion cooking | Samray et al., 2019 [83] |
| Bread | Indian’ bakery wastes | Reducing sugars | Simultaneous saccharification and SSF by LAB | Lactic acid production | Sadaf et al., 2021 [84] |
| FERMENTED BEVERAGES | |||||
| Winery | Grape pomace | Phenolic compounds, proanthocyanidins | In vitro rumen fermentation | Ruminant feeding with lower CH4 emission | Suescun-Ospina et al., 2023 [85] |
| Winery | Grape skins from winery waste | Soluble dietary fibers | High-pressure HTT (autohydrolysis) | Antioxidant effect | Bassani et al., 2020 [86] |
| Winery | Red grape pomace | Anthocyanins, flavan-3-ols, flavonols, procyanidins, galloyl glucose, and gallic acid | Aqueous extract drink of red grape pomace (RGPD) | Anti-hyperglicemic effect | Costabile et al., 2019 [87] |
| Winery—Noble Muscadine | Grape pomace | Anthocyanins, catechin, epicatechin | Drying, grounding, and sieving | Anti-hyperlipidemic effect | Yu et al., 2017 [88] |
| Winery—Cabernet Sauvignon (Vitis vinifera L.) | Grape pomaces (skin and seeds) | Catechin, epicathecin, rutin, quercetin, kaempeferol, transrerveratrol, cinnamic and benzoic acid derivatives. | Dehydration, freeze-drying, and milling to obtain WPF | Anti-hyperlipidemic and antioxidant effects | Ishimoto et al., 2020 [89] |
| Winery | Grape seed | Proanthocyanidins—monomer, dimer, trimers, oligomers | - | Satiating agent | Serrano et al., 2016 [90] |
| Gut health | Casanova-marti 2018 [91] | ||||
| Winery—Pinot Noir (Vitis vinifera L.) | Grape pomace | Proanthocyanidins, flavan-3-ol monomers, (+)-catechin, (−)-epicathecin, (−)-epicatechin-3-O-gallate, stillbenes | Water-ethanol extraction; freeze-drying; grinding | Cardioprotective effect | Ballea et al., 2018 [92] |
| Winery—Fetească Neagră (Vitis vinifera L.) | Grape pomace | Ballea et al., 2018 [93] | |||
| Winery—Cabernet Sauvignon, Marselan, Syrah | Grape pomace (skins, pulp, seeds, and stem) | Malvidin, delphinidin, rutin, quercetin, catechin, coumaric acid, kaempferol, trans-cinnamic acid | Solvent extract; spray-drying | Cardioprotective effect | Chacar et al., 2019 [94] |
| Winery | Red, white grape pomace/grape seed | Anthocyanins, flavan-3-ols, flavonols, stilbenes | Solvent extract; lyophilization | Cancer prevention | Pérez-Ortiz et al., 2019 [95] |
| Winery | Grape seed | Gallic acid, catechin, epicatechin gallate, and epicatechin | Solvent extract; lyophilization | Cancer prevention | Leone et al., 2019 [96] |
| Winery—Merlot, red wine | Wine less—liquid | Ethanol | Distillation | Biofuel production | Dimou et al., 2015 [97] |
| Alcohol-free nutrient-rich liquid | Solid-state fermentation | ||||
| Wine lees—solids | Phenolic compounds | sequential extraction with acetone:H2O (after distillation) | Antioxidants | ||
| Residual solids | Food additives | ||||
| Wine lees—Residual solids | Tartaric acid | Food additives | |||
| Remaining stream (yeast cells) | Enzymatic hydrolysis | Solid-state fermentation | |||
| Crude nutrient-rich hydrolysate + crude glycerol | Poly (3-hydroxybutyrate) | SSF by Aspergillus oryzae | Bioplastics | ||
| Brewing | Spent yeast | Peptides (48.3% protein; 86.4% essential amino acids) | Membrane filtration | Nutraceuticals | Oliveira et al., 2022 [98] |
| Brewing | Spent yeast | Proteins, lipids, and carbohydrates | Microwave-assisted extraction | Prebiotic/probiotic functional foods | Cejas et al., 2017 [99] |
| Brewing | Spent yeast | Trehalose | Pulsed electric field | Bioprotectant agent | Jin 2011 [62] |
| Brewing | Spent yeast | Nucleotides | CSLE | Flavor enhancer | Vieira et al., 2013 [61] |
| Brewing | Spent yeast | Proteins and phenolic compounds | - | Food ingredient | Vieira et al., 2019 [100] |
| Brewing | Spent yeast | β-glucan | CSLE | Immunomodulation | Bastos et al., 2015 [101]; Liepins et al., 2015 [102]; Tian et al., 2019 [103] |
| Brewing | Barley malt rootlets | Proteins | CSLE | Functional foods | Mahalingam, 2019 [104] |
| Brewing | Barley malt rootlets | Proteins and phenolic compounds | CSLE | Antioxidants (functional foods) | Cheng et al., 2016 [105]; Budaraju et al., 2018 [106] |
| Brewing | Spent hops | Phenolic compounds | UAE (ethanol) | Antioxidant and antimicrobial effects | Gandolpho et al., 2020; [107] Senna Ferreira Costa et al., 2021 [108] |
| Brewing | Spent hops | Essential oils;Xanthohumol | CSLE | Antioxidant and anticancer activities | Anioł et al., 2007 [109]; 2008 [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, D.J.; Carvalho, A.P.A.d.; Conte-Junior, C.A. Valorization of Fermented Food Wastes and Byproducts: Bioactive and Valuable Compounds, Bioproduct Synthesis, and Applications. Fermentation 2023, 9, 920. https://doi.org/10.3390/fermentation9100920
Faria DJ, Carvalho APAd, Conte-Junior CA. Valorization of Fermented Food Wastes and Byproducts: Bioactive and Valuable Compounds, Bioproduct Synthesis, and Applications. Fermentation. 2023; 9(10):920. https://doi.org/10.3390/fermentation9100920
Chicago/Turabian StyleFaria, Douglas José, Anna Paula Azevedo de Carvalho, and Carlos Adam Conte-Junior. 2023. "Valorization of Fermented Food Wastes and Byproducts: Bioactive and Valuable Compounds, Bioproduct Synthesis, and Applications" Fermentation 9, no. 10: 920. https://doi.org/10.3390/fermentation9100920
APA StyleFaria, D. J., Carvalho, A. P. A. d., & Conte-Junior, C. A. (2023). Valorization of Fermented Food Wastes and Byproducts: Bioactive and Valuable Compounds, Bioproduct Synthesis, and Applications. Fermentation, 9(10), 920. https://doi.org/10.3390/fermentation9100920








