Modulation of Cereal Biochemistry via Solid-State Fermentation: A Fruitful Way for Nutritional Improvement
Abstract
:1. Introduction
2. The Effect of Solid-State Fermentation (SSF) on the Nutritional Profile of Cereals
2.1. The Effect of SSF on Wheat Grain
2.2. The Effect of SSF on Barley Grain
2.3. The Effect of SSF on Oat Grain
2.4. The Effect of SSF on Millet Grain
2.5. The Effect of SSF on Rice Grain
2.6. The Effect of SSF on Maize Grain
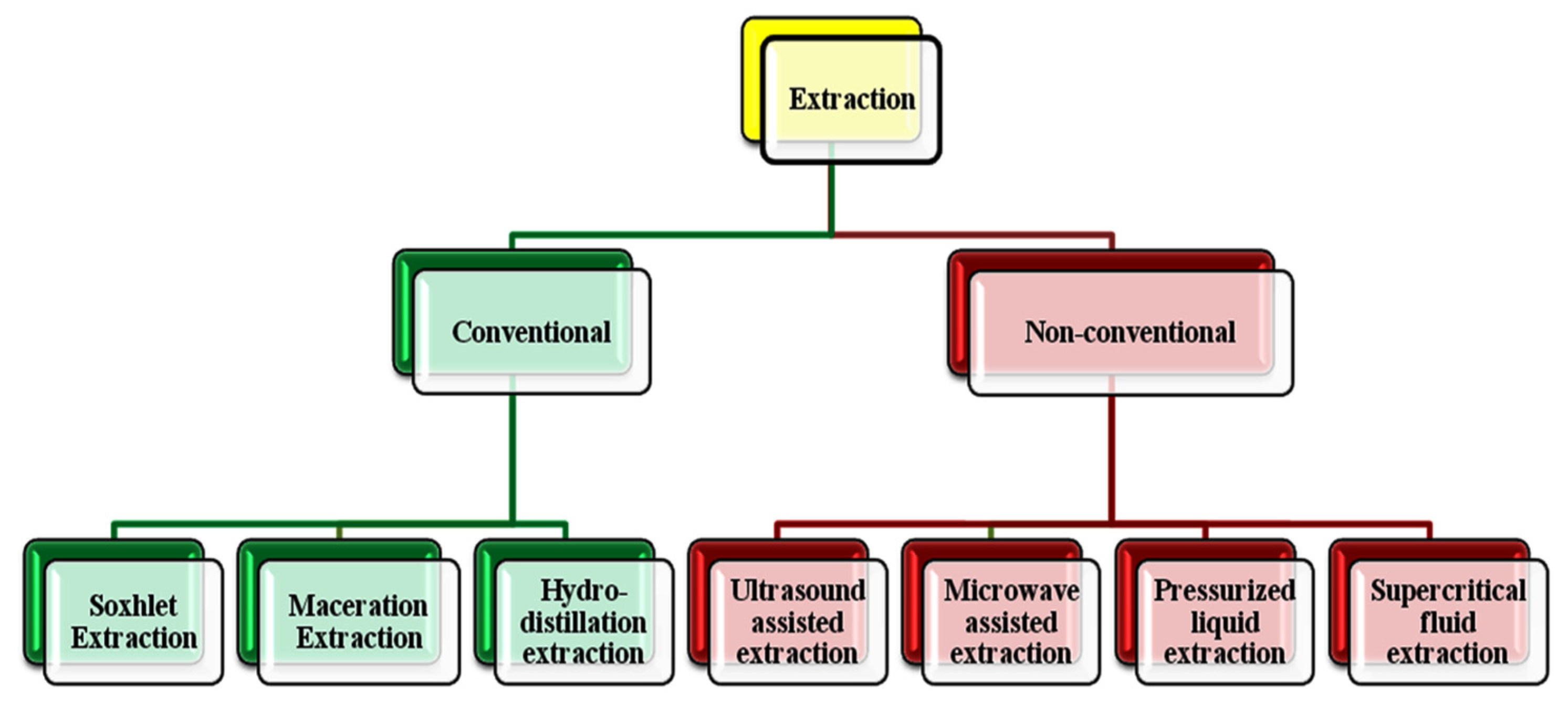
3. Extraction Medium for Cereal Phenolics
3.1. Conventional Extraction
3.2. Non-Conventional Extraction Methods
3.2.1. Ultrasound-Assisted Extraction
3.2.2. Microwave-Assisted Extraction
3.2.3. Pressurized Liquid Extraction (PLE)
3.2.4. Supercritical Fluid Extraction
4. Cereal-Based Food Products
4.1. Fermented Drinks and Products
4.2. Tarhana
4.3. Shalgam Juice and Hardaliye
4.4. Sordough Bread
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Kaur, M.; Sogi, D.S.; Purewal, S.S. A comparative study of phytochemicals, antioxidant potential and in-vitro DNA damage protection activity of different oat (Avena sativa) cultivars from India. J. Food Meas. Charact. 2019, 13, 347–356. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S. Phenolic content, antioxidant potential and DNA damage protection of pearl millet (Pennisetum glaucum) cultivars of North Indian region. J. Food Meas. Charact. 2017, 11, 126–133. [Google Scholar] [CrossRef]
- Kaur, P.; Sandhu, K.S.; Purewal, S.S.; Kaur, M.; Singh, S.K. Rye: A wonder crop with industrially important macromolecules and health benefits. Food Res. Int. 2021, 150, 110769. [Google Scholar] [CrossRef] [PubMed]
- Punia, S.; Sandhu, K.S.; Dhull, S.B.; Siroha, A.K.; Purewal, S.S.; Kaur, M.; Kidwai, M.K. Oat starch: Physico-chemical, morphological, rheological characteristics and its application-A review. Int. J. Biol. Macromol. 2020, 154, 493–498. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Kaur, P.; Siroha, A.K.; Purewal, S.S. Phytochemicals and Antioxidant Properties in Pearl Millet: A Cereal Grain with Potential. In Pearl Millet: Properties, Functionality and Its Applications; CRC Press: Raton, FL, USA; Taylor & Francis Group: Raton, FL, USA, 2020; pp. 33–50. ISBN 9780429331732. [Google Scholar]
- Carvalho, D.O.; Guido, L.F. A review on the fate of phenolic compounds during malting and brewing: Technological strategies and beer styles. Food Chem. 2022, 372, 131093. [Google Scholar] [CrossRef]
- Suprayogi, W.P.S.; Ratriyanto, A.; Akhirini, N.; Hadi, R.F.; Setyono, W.; Irawan, A. Changes in nutritional and antinutritional aspects of soybean meals by mechanical and solid-state fermentation treatments with Bacillus subtilis and Aspergillus oryzae. Bioresour. Technol. Rep. 2022, 17, 100925. [Google Scholar] [CrossRef]
- Sruthi, N.U.; Premjit, Y.; Pandiselvam, R.; Kothakota, A.; Ramesh, S.V. An overview of conventional and emerging techniques of roasting: Effect on food bioactive signatures. Food Chem. 2021, 348, 129088. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Fermented pearl millet (Pennisetum glaucum) with in vitro DNA damage protection activity, bioactive compounds and antioxidant potential. Food Res. Int. 2017, 100, 204–210. [Google Scholar] [CrossRef]
- Gallegos-Infante, J.A.; Rocha-Guzman, N.E.; Gonzalez-Laredo, R.F.; Pulido-Alonso, J. Effect of processing on the antioxidant properties of extracts from Mexican barley (Hordeum vulgare) cultivar. Food Chem. 2010, 119, 903–906. [Google Scholar] [CrossRef]
- Omotoke-Azeez, S.; Chinma, C.E.; Bassey, S.O.; Eze, U.R.; Makinde, A.F.; Sakariyah, A.A.; Okubanjo, S.S.; Danbaba, N.; Adebo, O.A. Impact of germination alone or in combination with solid-state fermentation on the physicochemical, antioxidant, in vitro digestibility, functional and thermal properties of brown finger millet flours. LWT 2022, 154, 112734. [Google Scholar] [CrossRef]
- Wu, H.; Liu, H.N.; Ma, A.; Zhou, J.Z.; Xia, X.D. Synergetic effects of Lactobacillus plantarum and Rhizopus oryzae on physicochemical, nutritional and antioxidant properties of whole-grain oats (Avena sativa L.) during solid-state fermentation. LWT 2022, 154, 112687. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Mimi-Sakinah, A.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef] [PubMed]
- Purewal, S.S.; Sandhu, K.S.; Salar, R.K.; Kaur, P. Fermented pearl millet: A product with enhanced bioactive compounds and DNA damage protection activity. J. Food Meas. Charact. 2019, 13, 1479–1488. [Google Scholar] [CrossRef]
- Bhanja, T.; Kumari, A.; Banerjee, R. Enrichment of phenolics and free radical scavenging property of wheat koji prepared with two filamentous fungi. Bioresour. Technol. 2009, 100, 2861–2866. [Google Scholar] [CrossRef]
- Kaur, P.; Purewal, S.S.; Sandhu, K.S.; Kaur, M. DNA damage protection: An excellent application of bioactive compounds. Bioresour. Bioprocess. 2019, 6, 2. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Relationships between DNA damage protection activity, total phenolic content, condensed tannin content and antioxidant potential among Indian barley cultivars. Biocatal. Agric. Biotechnol. 2017, 11, 201–206. [Google Scholar] [CrossRef]
- Dhull, S.B.; Kaur, P.; Purewal, S.S. Phytochemical analysis, phenolic compounds, condensed tannin content and antioxidant potential in Marwa (Origanum majorana) seed extracts. Resour. Effic. Technol. 2016, 2, 168–174. [Google Scholar] [CrossRef]
- Purewal, S.S.; Sandhu, K.S. Debittering of citrus juice by different processing methods: A novel approach for food industry and agro-industrial sector. Sci. Hortic. 2021, 276, 109750. [Google Scholar] [CrossRef]
- Salar, R.K.; Certik, M.; Brezova, V. Modulation of Phenolic Content and antioxidant activity of maize by solid state fermentation with Thamnidium elegans CCF 1456. Biotechnol. Bioprocess Eng. 2012, 17, 109–116. [Google Scholar] [CrossRef]
- Abd El Aty, A.A.; Saleh, S.A.A.; Eid, B.M.; Ibrahim, N.A.; Mostafa, F.A. Thermodynamics characterization and potential textile applications of Trichoderma longibrachiatum KT693225 xylanase. Biocatal. Agric. Biotechnol. 2018, 14, 129–137. [Google Scholar] [CrossRef]
- Marques, N.P.; de Cassia Pereira, J.; Gomes, E.; da Silva, R.; Araújo, A.R.; Ferreira, H.; Rodrigues, A.; Dussan, K.J.; Bocchini, D.A. Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind. Crop. Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, D.E.; Rodríguez-León, J.A.; de Carvalho, J.C.; Sturm, W.; Soccol, C.R. The behavior of kinetic parameters in production of pectinase and xylanase by solid-state fermentation. Bioresour. Technol. 2011, 102, 10657–10662. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; Tokuioshi, K.; da Silva Martins, E.; Da Silva, R.; Gomes, E. Production of pectinase by solid-state fermentation with Penicillium viridicatum RFC3. Process Biochem. 2005, 40, 2885–2889. [Google Scholar] [CrossRef]
- Purewal, S.S.; Salar, R.K.; Bhatti, M.S.; Sandhu, K.S.; Singh, S.K.; Kaur, P. Solid-state fermentation of pearl millet with Aspergillus oryzae and Rhizopus azygosporus: Effects on bioactive profile and DNA damage protection activity. J. Food Meas. Charact. 2020, 14, 150–162. [Google Scholar] [CrossRef]
- Hasbay, I.; Galanakis, C.M. Recovery technologies and encapsulation techniques. In Polyphenols: Properties, Recovery, and Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 233–264. [Google Scholar]
- Salar, R.K.; Purewal, S.S. Improvement of DNA damage protection and antioxidant activity of biotransformed pearl millet (Pennisetum glaucum) cultivar PUSA-415 using Aspergillus oryzae MTCC 3107. Biocatal. Agric. Biotechnol. 2016, 8, 221–227. [Google Scholar] [CrossRef]
- Skrypnik, L.; Novikova, A. Response surface modeling and optimization of polyphenols extraction from apple pomace based on nonionic emulsifiers. Agronomy 2020, 10, 92. [Google Scholar] [CrossRef]
- Salar, R.K.; Purewal, S.S.; Bhatti, M.S. Optimization of extraction condition and enhancement of phenolic content and antioxidant activity of pearl millet fermented with Aspergillus awamori MTCC-548. Resour. Effic. Technol. 2016, 2, 148–157. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martinez-Avila, G.; Montanez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef]
- Hajji, T.; Mansouri, S.; Vecino-Bello, X.; Cruz-Freire, J.M.; Rezgui, S.; Ferchichi, A. Identification and characterization of phenolic compounds extracted from barley husks by LC-MS and antioxidant activity in vitro. J. Cereal Sci. 2012, 81, 83–90. [Google Scholar] [CrossRef]
- Chew, K.K.; Khoo, M.Z.; Ng, S.Y.; Thoo, Y.Y.; Wan Aida, W.M.; Ho, C.W. Effect of ethanol concentration, extraction time and extraction temperature on the recovery of phenolic compounds and antioxidant capacity of Orthosiphon stamineus extracts. Int. Food Res. J. 2011, 18, 571–578. [Google Scholar]
- Bharathi, B.K.; Shivakumar, H.B.; Soumya, N.S. Depleted antioxidant vitamins and enhanced oxidative stress in urolithiasis. Int. J. Pharm. Biosci. 2011, 3, 71–75. [Google Scholar]
- Rajaei, A.; Barzegar, M.; Hamidi, Z.; Sahari, M.A. Optimization of Extraction Conditions of Phenolic Compounds from Pistachio (Pistachia vera) Green Hull through Response Surface Method. J. Agric. Sci. Technol. 2010, 12, 605–615. [Google Scholar]
- Uma, D.B.; Ho, C.W.; Wan-Aida, W.M. Optimization of Extraction Parameters of Total Phenolic Compounds from Henna (Lawsoniainermis) Leaves. Sains Malays. 2010, 39, 119–128. [Google Scholar]
- Prasad, D.C.G.; Vidyalakshmi, R.; Baskaran, N.; Tito, A.M. Influence of Pichia myanmarensis in fermentation to produce quinoa based non-alcoholic beer with enhanced antioxidant activity. J. Cereal Sci. 2011, 103, 103390. [Google Scholar]
- Dordevic, T.M.; Marinkovic, S.; Slavica, S.; Brankovic, D.; Suzana, I. Effect of fermentation on antioxidant properties of some cereals and pseudo cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; Faveri, D.M.D. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Lou, H.; Yang, C.; Gong, Y.; Li, Y.; Li, Y.; Tian, S.; Zhao, Y.; Zhao, R. Edible fungi efficiently degrade aflatoxin B1 in cereals and improve their nutritional composition by solid-state fermentation. J. Hazard. Mater. 2023, 451, 131139. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.Y.; Zhang, X.J.; Huang, T.; Zhing, X.Z.; Chai, L.J.; Lu, Z.M.; Shi, J.S.; Xu, Z.H. Komagataeibacter europaeus improves community stability and function in solid-state cereal vinegar fermentation ecosystem: Non-abundant species plays important role. Food Res. Int. 2021, 150, 110815. [Google Scholar] [CrossRef]
- Wu, J.; Ren, L.; Zhao, N.; Wu, T.; Liu, R.; Sui, W.; Zhang, M. Solid-state fermentation by Rhizopus oryzae improves flavor of wheat bran for application in food. J. Cereal Sci. 2022, 107, 103536. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, Y.; Tan, B. Comparative study of solid-state fermentation with different microbial strains on the bioactive compounds and microstructure of brown rice. Food Chem. 2022, 397, 133735. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, B.; Song, D. Co-microbiological regulation of phenolic release through solid-state fermentation of corn kernels (Zea mays L.) to improve their antioxidant activity. LWT 2021, 142, 111003. [Google Scholar] [CrossRef]
- Naik, B.; Goyal, S.K.; Tripathi, A.D.; Kumar, V. Optimization of pullulanase production by Aspergillus flavus under solid-state fermentation. Bioresour. Technol. Rep. 2022, 17, 100963. [Google Scholar] [CrossRef]
- Namboodiri, M.M.T.; Paul, T.; Medisetti, R.M.N.; Pakshirajan, K.; Narayanasamy, S.; Pugazhenthi, G. Solid state fermentation of rice straw using Penicillium citrinum for chitosan production and application as nanobiosorbent. Bioresour. Technol. Rep. 2022, 18, 101005. [Google Scholar] [CrossRef]
- Zanellati, A.; Spina, F.; Poli, A.; Rolle, L.; Varese, G.C.; Dinuccio, E. Fungal pretreatment of non-sterile maize silage and solid digestate with a Cephalotrichumstemonitis strain selected from agricultural biogas plants to enhance anaerobic digestion. Biomass Bioener. 2021, 144, 105934. [Google Scholar] [CrossRef]
- Sala, A.; Artola, A.; Sanchez, A.; Barrena, R. Rice husk as a source for fungal biopesticide production by solid-state fermentation using B. bassiana and T. harzianum. Bioresour. Technol. 2020, 296, 122322. [Google Scholar] [CrossRef] [PubMed]
- da-Cunha, M.C.; Silva, L.C.; Sato, H.H.; de-Castro, R.J.S. Using response surface methodology to improve the L-asparaginase production by Aspergillus niger under solid-state fermentation. Biocatal. Agric. Biotechnol. 2018, 16, 31–36. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Villela-Castrejon, J.; Perez-Carrillo, E.; Gomez-Sanchez, C.E.; Gutierrez-Uribe, J.A. Effects of solid-state fungi fermentation on phenolic content, antioxidant properties and fiber composition of lime cooked maize by-product (nejayote). J. Cereal Sci. 2019, 90, 102837. [Google Scholar] [CrossRef]
- Ito, K.; Kawase, T.; Sammoto, H.; Gomi, K.; Kariyama, M.; Miyake, T. Uniform culture in solid-state fermentation with fungi and its efficient enzyme production. J. Biosci. Bioeng. 2011, 111, 300–305. [Google Scholar] [CrossRef]
- Bei, Q.; Chen, G.; Lu, F.; Wu, S.; Wu, Z. Enzymatic action mechanism of phenolic mobilization in oats (Avena sativa L.) during solid-state fermentation with Monascusanka. Food Chem. 2018, 245, 297–304. [Google Scholar] [CrossRef]
- Yang, F.C.; Yang, Y.H.; Lu, H.C. Enhanced antioxidant and antitumor activities of Antrodiacinnamomea cultured with cereal substrates in solid state fermentation. Biochem. Eng. J. 2013, 78, 108–113. [Google Scholar] [CrossRef]
- Xiao, Y.; Rui, X.; Xing, G.; Wu, H.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Solid state fermentation with Cordyceps militaris SN-18 enhanced antioxidant capacity and DNA damage protective effect of oats (Avena sativa L.). J. Funct. Food 2015, 16, 58–73. [Google Scholar] [CrossRef]
- Sharma, R.K.; Arora, D.S. Production of lignocellulolytic enzymes and enhancement of in vitro digestibility during solid state fermentation of wheat straw by Phlebiafloridensis. Bioresour. Technol. 2010, 101, 9248–9253. [Google Scholar] [CrossRef]
- Farawahida, A.H.; Palmer, J.; Flint, S. Monascus spp. and citrinin: Identification, selection of Monascus spp. isolates, occurrence, detection and reduction of citrinin during the fermentation of red fermented rice. Int. J. Food Microbiol. 2022, 379, 109829. [Google Scholar] [CrossRef] [PubMed]
- Ritthibut, N.; Oh, S.J.; Lim, S.T. Enhancement of bioactivity of rice bran by solid-state fermentation with Aspergillus strains. LWT 2021, 135, 110273. [Google Scholar] [CrossRef]
- Molaverdi, M.; Mirmohamadsadeghi, S.; Karimi, K.; Aghbashlo, M.; Tabatabaei, M. Efficient ethanol production from rice straw through cellulose restructuring and high solids loading fermentation by Mucor indicus. J. Clean.Prod. 2022, 339, 130702. [Google Scholar] [CrossRef]
- Razali, S.A.; Rasit, N.; Ooi, C.K. Statistical analysis of xylanase production from solid state fermentation of rice husk associated fungus Aspergillus niger. Mater. Today Proc. 2021, 39, 1082–1087. [Google Scholar] [CrossRef]
- Shetty, A.V.K.; Dave, N.; Murugesan, G.; Pai, S.; Pagazhendhi, A.; Varadavenkatesan, T.; Vinayagam, R.; Selvaraj, R. Production and extraction of red pigment by solid-state fermentation of broken rice using Monascussanguineus NFCCI 2453. Biocatal. Agric. Biotechnol. 2021, 33, 101964. [Google Scholar] [CrossRef]
- Janarny, G.; Gunathilake, K.D.P.P. Changes in rice bran bioactives, their bioactivity, bioaccessibility and bioavailability with solid-state fermentation by Rhizopus oryzae. Biocatal. Agric. Biotechnol. 2020, 23, 101510. [Google Scholar] [CrossRef]
- Shin, H.Y.; Kim, S.M.; Lee, J.H.; Lim, S.T. Solid-state fermentation of black rice bran with Aspergillus awamori and Aspergillus oryzae: Effects on phenolic acid composition and antioxidant activity of bran extracts. Food Chem. 2019, 272, 235–241. [Google Scholar] [CrossRef]
- Ravichandra, K.; Balaji, R.; Devarapalli, K.; Cheemalamarri, C.; Poda, S.; Banoth, L.; Pinnamaneni, S.R.; Prakasham, R.S. Impact of structure and composition of different sorghum xylans as substrates on production of xylanase enzyme by Aspergillus fumigatus RSP-8. Indus. Crop. Prod. 2022, 188, 115660. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Punia, S.; Kaur, M. Effect of duration of solid state fermentation by Aspergillus awamorinakazawa on antioxidant properties of wheat cultivars. LWT 2016, 71, 323–328. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Punia, S. Enhancement of bioactive compounds in barley cultivars by solid substrate fermentation, J. Food Meas. Charact. 2017, 11, 1355–1361. [Google Scholar] [CrossRef]
- Demir, H.; Tari, C. Valorization of wheat bran for the production of polygalacturonase in SSF of Aspergillus sojae. Indus. Crop Prod. 2014, 54, 302–309. [Google Scholar] [CrossRef]
- Dey, T.H.; Kahad, R.C. Upgrading the antioxidant potential of cereals by their fungal fermentation under solid-state. Lett. Appl. Microbiol. 2014, 59, 493–499. [Google Scholar]
- Zhao, H.M.; Guo, X.N.; Zhu, K.X. Impact of solid state fermentation on nutritional, physical and flavor properties of wheat bran. Food Chem. 2017, 217, 28–36. [Google Scholar] [CrossRef]
- Nelofer, R.; Nadeem, M.; Irfan, M.; Syed, Q. Nutritional enhancement of barley in solid state fermentation by Rhizopus oligosporus ML-10. Nutr. Food Sci. Int. J. 2018, 1–7. [Google Scholar] [CrossRef]
- Bartkiene, E.; Mozuriene, E.; Lele, V.; Zokaityte, E.; Gruzauskas, R.; Jakobsone, I.; Juodeikiene, G.; Ruibys, R.; Bartkevics, V. Changes of bioactive compounds in barley industry by-products during submerged and solid state fermentation with antimicrobial Pediococcusacidilactici strain LUHS29. Food Sci. Nutr. 2019, 8, 340–350. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ra, C.H. Comparison of liquid and solid-state fermentation processes for the production of enzymes and beta-glucan from hulled barley. J. Microbiol. Biotechnol. 2022, 32, 317–323. [Google Scholar] [CrossRef]
- Zhai, X.T.; Wang, H.Q.; Han, L.; Wang, F.Y.; Zhang, Y.H.; Li, J.; He, C.A.; Tan, B.; Wang, M. Impact of solid-state fermentation by Aspergillus niger 4Q and Cyberlindnerafabianii J2 on antioxidant and flavor properties of whole grain barley. Cereal Chem. 2023, 100, 914–926. [Google Scholar] [CrossRef]
- Cavazos, A.; de-Mejia, E.G. Identification of bioactive peptides from cereal storage proteins and their potential role in prevention of chronic diseases. Compr. Rev. Food Sci. Food Saf. 2013, 12, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Calinoiu, L.F.; Catoi, A.F.; Vodnar, D.C. Solid-state yeast fermented wheat and oat bran as a route for delivery of antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef] [PubMed]
- Salar, R.K.; Purewal, S.S.; Sandhu, K.S. Bioactive profile, free-radical scavenging potential, DNA damage protection activity, and mycochemicals in Aspergillus awamori (MTCC 548) extracts: A novel report on filamentous fungi. 3 Biotech 2017, 7, 164. [Google Scholar] [CrossRef]
- Pampangouda, P.; Munishamanna, K.B.; Gurumurthy, H. Effect of Saccharomyces boulardii and Lactobacillus acidophilus fermentation on little millet (Panicum sumatrense). J. Appl. Nat. Sci. 2015, 7, 260–264. [Google Scholar] [CrossRef]
- Purwar, S.; Gupta, E.; Zaki, S. Effect of solid state fermentation on nutritive values of rice by Monascus spp. Bioved 2016, 27, 185–189. [Google Scholar]
- Lin, Y.L.; Wang, T.H.; Lee, M.H.; Su, N.W. Biologically active components and nutraceuticals in the Monascus-fermented rice: A review. Appl. Microbiol. Biotechnol. 2008, 77, 965–973. [Google Scholar] [CrossRef]
- Miyake, T.; Mori, A.; Kii, T.; Okuno, T.; Usui, Y.; Fumihiro, S.; Sammoto, H.; Watanabe, A.; Kariyama, M. Light effects on cell development and secondary metabolism in Monascus. J. Indus. Microbiol. Biotechnol. 2005, 32, 103–108. [Google Scholar] [CrossRef]
- Omarini, A.B.; Labuckas, D.; Zunino, M.P.; Pizzolitto, R.; Lahore, M.F.; Barrionuevo, D.; Zygadlo, J.A. Upgrading the nutritional value of rice bran by solid-state fermentation with Pleurotus sapidus. Fermentation 2019, 5, 44. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Graça, C.S.; Chiattoni, L.M.; Massarolo, K.C.; Duarte, F.A.; Mellado, M.L.S.; Soares, L.A.S. Fermentation process in the availability of nutrients in rice bran. Res. Rev. J. Microbiol. Biotechnol. 2017, 6, 45–52. [Google Scholar]
- Punia, S.; Sandhu, K.S.; Purewal, S.S.; Kaur, P.; Siroha, A.K.; Komal; Kumar, M.; Kumar, M. Fermented barley bran: An improvement in phenolic compounds and antioxidant properties. J. Food Process. Pres. 2021, 46, e15543. [Google Scholar]
- Postemsky, P.A.; Bidegain, M.A.; Matute, R.G.; Figlas, N.D.; Cubitto, M.A. Pilot-scale bioconversion of rice and sunflower agro-residues into medicinal mushrooms and laccase enzymes through solid-state fermentation with Ganoderma lucidum. Bioresour. Technol. 2017, 231, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Sawangwan, T.; Saman, P. Prebiotic synthesis from rice using Aspergillus oryzae with solid state fermentation. Agric. Nat. Resour. 2016, 50, 227–231. [Google Scholar] [CrossRef]
- Michel-Michel, M.R.; Aguilar-Zárate, P.; Lopez-Badillo, C.M.; Chávez-González, M.L.; Flores-Gallegos, A.C.; Espinoza-Velázquez, J.; Aguilar, C.N.; Rodríguez-Herrera, R. Mineral and fatty acid contents of maize kernels with different levels of polyembryony. Cereal Chem. 2020, 97, 723–732. [Google Scholar] [CrossRef]
- Chaves-López, C.; Rossi, C.; Maggio, F.; Paparella, A.; Serio, A. Changes occurring in spontaneous maize fermentation: An overview. Fermentation 2020, 6, 36. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kuban, V. Determination of phenolic compounds and their antioxidant activity in fruits and cereals. Talanta 2007, 71, 1741–1751. [Google Scholar] [CrossRef]
- Charalampopoulos, D.; Wang, R.; Pandiella, S.S.; Webb, C. Application of cereals and cereal components in functional foods: A review. Int. J. Food Microbiol. 2002, 79, 131–141. [Google Scholar] [CrossRef]
- Terefe, Z.K.; Omwamba, M.N.; Nduko, J.M. Effect of solid state fermentation on proximate composition, antinutritional factors and in vitro protein digestibility of maize flour. Food Sci. Nutr. 2021, 9, 6343–6352. [Google Scholar] [CrossRef]
- Luo, D.; Li, X.; Zhao, L.; Chen, G. Regulation of phenolic release in corn seeds (Zea mays L.) for improving their antioxidant activity by mix-culture fermentation with Monascusanka, Saccharomyces cerevisiae and Bacillus subtilis. J. Biotechnol. 2021, 325, 334–340. [Google Scholar] [CrossRef]
- Darwish Galila, A.M.A.; Bakr, A.A.; Abdallah, M.M.F. Nutritional value upgrading of maize stalk by using Pleurotusostreatus and Saccharomyces cerevisiae in solid state fermentation. Annal. Agric. Sci. 2012, 57, 47–51. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, Z.; Ding, H.; Li, M.; Shi, D.; Zhu, M.; Xia, L. Enhanced degradation of lignin in corn stalk by combined method of Aspergillus oryzae solid state fermentation and H2O2 treatment. Biomass Bioener. 2015, 81, 224–233. [Google Scholar] [CrossRef]
- Aliyah, A.; Alamsyah, G.; Ramadhani, R.; Hermansyah, H. Production of α-Amylase and β-Glucosidase from Aspergillus niger by solid state fermentation method on biomass waste substrates from rice husk, bagasse and corn cob. Energy Procedia 2017, 136, 418–423. [Google Scholar] [CrossRef]
- Velmurugan, P.; Hur, H.; Balachandar, V.; Kannan, S.K.; Lee, K.J.; Lee, S.M.; Chae, J.C.; Shea, P.J.; Oh, B.J. Monascus pigment production by solid-state fermentation with corn cob substrate. J. Biosci. Eng. 2011, 112, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Kaith, B.S.; Bhatti, M.S. Fabrication of biodegradable superabsorbent using RSM design for controlled release of KNO3. J. Polym. Environ. 2018, 266, 518–531. [Google Scholar] [CrossRef]
- Bhatti, M.S.; Thukral, A.K.; Reddy, A.S.; Kalia, R.K. RSM and ANN-GA experimental design optimization for electrocoagulation removal of chromium. Trend. Asian Water Environ. Sci. Technol. 2017, 1, 3–21. [Google Scholar]
- Bhatia, J.K.; Kaith, B.S.; Singla, R.; Mehta, P.; Yadav, V.; Dhiman, J.; Bhatti, M.S. RSM optimized soy protein fibre as a sorbent material for treatment of water contaminated with petroleum products. Desalin. Water Treat. 2016, 57, 4245–4254. [Google Scholar] [CrossRef]
- Dureja, J.S.; Singh, R.; Bhatti, M.S. Optimizing flank wear and surface roughness during hard turning of AISI D3 steel by Taguchi and RSM methods. Prod. Manuf. Res. 2014, 2, 767–783. [Google Scholar] [CrossRef]
- Kaith, B.S.; Jindal, R.; Kumar, V.; Bhatti, M.S. Optimal response surface design of Gum tragacanth-based poly [(acrylic acid)-co-acrylamide] IPN hydrogel for the controlled release of the antihypertensive drug losartan potassium. RSC Adv. 2014, 4, 39822–39829. [Google Scholar]
- Bhatti, M.S.; Kapoor, D.; Kalia, R.K.; Reddy, A.S.; Thukral, A.K. RSM and ANN modeling for electrocoagulation of copper from simulated wastewater: Multi objective optimization using genetic algorithm approach. Desalination 2011, 274, 74–80. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Saniler, N.; Gokcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.L. Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countries: A neglected food group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Basinskiene, L.; Juodeikiene, G.; Vidmantiene, D.; Tenkanen, M.; Makaravicius, T.; Bartkiene, E. Non-alcoholic beverages from fermented cereals with increased oligosaccharide content. Food Technol. Biotechnol. 2016, 54, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ignat, M.V.; Salanta, L.C.; Pop, O.L.; Pop, C.R.; Tofana, M.; Mudura, E.; Coldea, T.E.; Borsa, E.; Pasqualone, A. Current functionality and potential improvements of non-alcoholic fermented cereal beverages. Foods 2020, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Altay, F.; Karbancioglu-Guler, F.; Daskaya-Dikmen, C.; Heperkan, D. A review on traditional Turkish fermented non-alcoholic beverages: Microbiota, fermentation process and quality characteristics. Int. J. Food Microbiol. 2013, 167, 44–56. [Google Scholar] [CrossRef]
- Osimani, A.; Garofalo, C.; Aquilanti, L.; Milanovic, V.; Clementi, F. Unpasteurised commercial boza as a source of microbial diversity. Int. J. Food Microbiol. 2015, 194, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Botes, A.; Todorov, S.V.; Von-Mollendroff, J.W.; Botha, A.; Dicks, L.M.T. Identification of lactic acid bacteria and yeast from boza. Process Biochem. 2007, 42, 267–270. [Google Scholar] [CrossRef]
- Heperkan, D.; Dikmen, C.D.; Bayram, B. Evaluation of lactic acid bacterial strains of boza for their exopolysaccharide and enzyme production as a potential adjunct culture. Process Biochem. 2014, 49, 157–1594. [Google Scholar] [CrossRef]
- Caputo, L.; Quintieri, L.; Baruzzi, F.; Borcakli, M.; Morea, M. Molecular and phenotypic characterization of Pichia fermentans strains found among Boza yeasts. Food Res. Int. 2012, 48, 755–762. [Google Scholar] [CrossRef]
- Yegin, S.; Uren, A. Biogenic amine content of boza: A traditional cereal-based, fermented Turkish beverage. Food Chem. 2008, 111, 983–987. [Google Scholar] [CrossRef]
- Kancabas, A.; Karakaya, S. Angiotensin-converting enzyme (ACE)-inhibitory activity of boza, a traditional fermented beverage. J. Sci. Food Agric. 2013, 93, 641–645. [Google Scholar] [CrossRef]
- Todorov, S.D. Diversity of bacteriocinogenic lactic acid bacteria isolated from boza, a cereal-based fermented beverage from Bulgaria. Food Control 2010, 21, 1011–1021. [Google Scholar] [CrossRef]
- Daglioglu, O. Tarhana as a traditional Turkish fermented cereal food, Its Recipe, Production and Composition. Nahrung 2000, 44, 85–88. [Google Scholar] [CrossRef]
- Ekinci, R.; Kadakal, C. Determination of Seven Water-Soluble Vitamins in Tarhana, A Traditional Turkish cereal food, by high-performance liquid chromatography. Acta Chromatogr. 2005, 15, 289–297. [Google Scholar]
- Degirmencioglu, N.; Gocmen, D.; Dagdelen, A.; Dagdelen, F. Influence of Tarhana Herb (Echinophora sibthorpiana) on fermentation of tarhana, Turkish traditional fermented food. Food Technol. Biotechnol. 2005, 43, 175–179. [Google Scholar]
- Ibanoglu, S.; Kaya, S.; Kaya, A. Evaluation of sorption properties of Turkish tarhana Powder. Nahrung 1999, 43, 122–125. [Google Scholar] [CrossRef]
- Erbas, M.; Ertugay, M.F.; Erbas, M.Ö.; Certel, M. The effect of fermentation and storage on free amino acids of tarhana. Int. J. Food Sci. Nutr. 2005, 56, 349–358. [Google Scholar] [CrossRef]
- Erkan, H.; Celik, S.; Bilgi, B.; Koksel, H. A new approach for the utilization of barley in food products: Barley Tarhana. Food Chem. 2006, 97, 12–18. [Google Scholar] [CrossRef]
- Ozdemir, S.; Gocmen, D.; Kumral, A.Y. A Traditional Turkish Fermented Cereal Food: Tarhana. Food Rev. Int. 2007, 23, 107–121. [Google Scholar] [CrossRef]
- Ulucan, E.; Coklar, H.; Akbulut, M. Application of ultrasound to extend the shelf life of shalgam juice: Changes in various physicochemical, nutritional, and microbiological properties. J. Food Process. Pres. 2022, 46, e16501. [Google Scholar] [CrossRef]
- Coskun, F. A Traditional Turkish Fermented Non-Alcoholic Beverage, “Shalgam”. Beverages 2017, 3, 49. [Google Scholar] [CrossRef]
- Canbas, A.; Fenercioglu, H.A. Research on shalgam juice. Gida 1984, 95, 279–286. (In Turkish) [Google Scholar]
- Arici, M.; Coskun, F. Hardaliye: Fermented grape juice as a traditional Turkish beverage. Food Microbiol. 2001, 18, 417–421. [Google Scholar] [CrossRef]
- Aksoy, A.S.; Arici, M.; Yaman, M. The effect of hardaliye on reducing the formation of malondialdehyde during in vitro gastrointestinal digestion of meat products. Food Biosci. 2022, 47, 101747. [Google Scholar] [CrossRef]
- Ilıkkan, O.K.; Doganlar, O.; Doganlar, Z.B.; Mimiroğlu, P.A.; Kirbas, A.S. Anticancer activity of the “Hardaliye” on HT-29 Cell Line and proliferative activity on CF-1 cell line: Apoptosis and Antioxidant pathway responsive gene expressions. Integr. Mol. Med. 2017, 4, 1–8. [Google Scholar]
- Pacularu-Burada, B.; Georgescu, L.A.; Bahrim, G.E. Current approaches in sourdough production with valuable characteristics for technological and functional applications. Ann. Univ. Dunarea de Jos Galati 2020, 44, 132–148. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Zoumpopolou, G.; Georgalaki, M.; Alexandraki, V.; Kazou, M.; Anastasiou, V.; Tsakalidou, E. Sourdough bread. In Innovations in Traditional Foods; Woodhead Publishing: Sawston, UK, 2019; pp. 127–158. [Google Scholar]
- Reese, A.T.; Madden, A.A.; Joossens, M.; Lacaze, G.; Dunn, R.R. Influences of ingredients and bakers on the bacteria and fungi in sourdough starters and bread. mSphere 2020, 5, e00950-19. [Google Scholar] [CrossRef]
- Olojede, A.O.; Ogunsakin, A.O.; Sanni, A.I.; Banwo, K. Rheological, textural and nutritional properties of gluten-free sourdough made with functionally important lactic acid bacteria and yeast from Nigerian sorghum. LWT 2020, 120, 108875. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Yang, Y.; Yi, H.; Zhang, L.; He, G. The influence of different lactic acid bacteria on sourdough flavor and a deep insight into sourdough fermentation through RNA sequencing. Food Chem. 2020, 307, 125529. [Google Scholar] [CrossRef]
- Saa, D.L.T.; Nissen, L.; Gianotti, A. Metabolomic approach to study the impact of flour type and fermentation process on volatile profile of bakery products. Food Res. Int. 2019, 119, 510–516. [Google Scholar] [CrossRef]
- Menezes, L.A.A.; Molognoni, L.; Ploencio, L.A.; Costa, F.B.M.; Daguer, H.; Lindner, J. Use of sourdough fermentation to reducing FODMAPs in breads. Eur. Food Res. Technol. 2019, 245, 1183–1195. [Google Scholar] [CrossRef]
- Ribet, L.; Dessalles, R.; Lesens, C.; Brusselaers, N.; Durand-Dubief, M. Nutritional benefits of sourdoughs: A systematic review. Adv. Nutr. 2023, 14, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S. Innovations in sourdough bread making. Fermentation 2021, 7, 29. [Google Scholar] [CrossRef]
- Canesin, M.R.; Cazarin, C.B.B. Nutritional quality and nutrient bioaccessibility in sourdough bread. Curr. Opin. Food Sci. 2021, 37, 81–86. [Google Scholar] [CrossRef]
| Substrate | Microbial Strain Used for Fermentation | Purpose | References |
|---|---|---|---|
| Oats | Rhizopus oryzae | To study synergetic effects of Lactobacillus plantarum and Rhizopus oryzae on physicochemical, nutritional and antioxidant properties of whole-grain oats (Avena sativa L.) during solid-state fermentation. | [12] |
| Corn | Ganoderma sinense | To screen edible fungi with laccase activity and determine their effects on the degradation of Aflatoxin B1. | [40] |
| Cereal vinegar | Komagataeibacter europaeus | To study the assembly and co-occurrence patterns for abundant and non-abundant bacterial sub-communities using Zhenjiang aromatic vinegar fermentation as a model system. | [41] |
| Wheat bran | Rhizopus oryzae | Study aimed at evaluating the effect of SSF on flavor and sensory properties of wheat bran containing cake. | [42] |
| Brown rice | Aspergillus oryzae | To study the effect of SSF on bioactive compounds of brown rice. | [43] |
| Corn kernel | Monascusanka | In this study, regulation of phenolic release and antioxidant activity in corn kernels by co-microbiological fermentation was investigated. | [44] |
| Wheat bran | Aspergillus flavus | To study the optimization of pullulanase production by Aspergillus flavus under solid-state fermentation. | [45] |
| Rice straw | Penicillium citrinum | Solid-state fermentation of rice straw using Penicillium citrinum for chitosan production and application as nanobiosorbent. | [46] |
| Maize silage | Cephalotrichum stemonitis | Fungal pretreatment of non-sterile maize silage and 581 solid digestate with a Cephalotrichum stemonitis strain selected from agricultural biogas plants to enhance anaerobic digestion. | [47] |
| Rice husk | Trichoderma harzianum | Rice husk as a source for fungal biopesticide production by solid-state fermentation using B. bassiana and T. harzianum. | [48] |
| Wheat bran | Aspergillus niger | Using response surface methodology to improve the L-asparaginase production by Aspergillus niger under solid-state fermentation. | [49] |
| Lime-cooked maize | P. ostreatus Perla and Hericium erinaceus | To study the effects of solid-state fungi fermentation on phenolic content, antioxidant properties and fiber composition of lime-cooked maize by-product (nejayote). | [50] |
| Wheat bran | Aspergillus oryzae | Uniform culture in solid-state fermentation with fungi and its efficient enzyme production. | [51] |
| Oats | Monascus anka | To study enzymatic action mechanism of phenolic mobilization in oats (Avena sativa L.) during solid-state fermentation with Monascus anka. | [52] |
| Oats | Antrodia cinnamomea | Enhanced antioxidant and antitumor activities of Antrodia cinnamomea cultured with cereal substrates in solid-state fermentation. | [53] |
| Oats | Cordyceps militaris | To study the effect of solid-state fermentation on antioxidant capacity and DNA damage protective effect of oats (Avena sativa L.). | [54] |
| Wheat straw | Phlebia floridensis | Production of lignocellulolytic enzymes and enhancement of in vitro digestibility during solid-state fermentation of wheat straw by Phlebiafloridensis. | [55] |
| Red rice | Monascus spp. | To detect and reduce citrinin during the fermentation of red fermented rice. | [56] |
| Rice bran | Aspergillus sojae | Enhancement of bioactivity of rice bran by solid-state fermentation. | [57] |
| Rice straw | Mucor indicus | Efficient ethanol production from rice straw through cellulose restructuring and high-solids-loading fermentation by Mucor indicus. | [58] |
| Rice husk | Aspergillus niger | Xylanase production from solid-state fermentation of rice husk. | [59] |
| Broken rice | Monascus sanguineus | Production and extraction of red pigment by solid-state fermentation of broken rice. | [60] |
| Rice bran | Rhizopus oryzae | Changes in rice bran bioactives, their bioactivity, bioaccessibility and bioavailability with solid-state fermentation. | [61] |
| Black rice bran | Aspergillus awamori and Aspergillus oryzae | Effects of SSF on phenolic acid composition and antioxidant activity. | [62] |
| Sorghum | Aspergillus fumigatus | Sorghum xylans as substrates for the production of xylanase enzyme. | [63] |
| Substrate | Microbial Strain Used for Fermentation | Purpose | Modulation in TPC after SSF | References |
|---|---|---|---|---|
| Pearl millet | Aspergillus sojae | To enhance phenolic content and antioxidant properties. | 6.4–34.1 mg GAE/g | [9] |
| Finger millet | Yeast | To enhance phenolic content and antioxidant properties. | 122–155 mg GAE/100g | [11] |
| Pearl millet | Rhizopus azygosporus | To enhance phenolic content and antioxidant properties. | 6.58 to 21.78mg GAE/g | [14] |
| Wheat | Aspergillus oryzae | To enhance phenolic content and antioxidant properties. | 7.23–158.9 µmol GAE/g | [15] |
| Wheat | Aspergillus awamori | To enhance phenolic content and antioxidant properties. | 7.23–124.2 µmol GAE/g | [15] |
| Maize | Thamnidium elegans | To enhance phenolic content and antioxidant properties. | 327–409 GAE μmol/g | [20] |
| Pearl millet | Aspergillus oryzae | To enhance phenolic content and antioxidant properties. | 6.1–18.7 mg GAE/g | [27] |
| Oats | Cordyceps militaris | To enhance phenolic content and antioxidant properties. | 5.8–14.1 mg/g | [54] |
| Rice bran | Aspergillus sojae | To enhance phenolic content and antioxidant properties. | 10.6–36.5 mg GAE/g extract | [57] |
| Rice bran | Rhizopus oryzae | To enhance phenolic content and antioxidant properties. | 5.33–8.81 mg GAE/g | [61] |
| Black rice bran | Aspergillus oryzae | To enhance phenolic content and antioxidant properties. | 1028.2 to 1660.6 μg/g | [62] |
| Wheat | Aspergillus awamori | To enhance phenolic content and antioxidant properties. | 974–2056 µg GAE/g | [64] |
| Barley | Aspergillus awamori | To enhance phenolic content and antioxidant properties. | 3.25–4.59 mg GAE/g | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, A.; Purewal, S.S. Modulation of Cereal Biochemistry via Solid-State Fermentation: A Fruitful Way for Nutritional Improvement. Fermentation 2023, 9, 817. https://doi.org/10.3390/fermentation9090817
Kaur A, Purewal SS. Modulation of Cereal Biochemistry via Solid-State Fermentation: A Fruitful Way for Nutritional Improvement. Fermentation. 2023; 9(9):817. https://doi.org/10.3390/fermentation9090817
Chicago/Turabian StyleKaur, Avneet, and Sukhvinder Singh Purewal. 2023. "Modulation of Cereal Biochemistry via Solid-State Fermentation: A Fruitful Way for Nutritional Improvement" Fermentation 9, no. 9: 817. https://doi.org/10.3390/fermentation9090817
APA StyleKaur, A., & Purewal, S. S. (2023). Modulation of Cereal Biochemistry via Solid-State Fermentation: A Fruitful Way for Nutritional Improvement. Fermentation, 9(9), 817. https://doi.org/10.3390/fermentation9090817







