Abstract
The dynamic changes in phenolic composition and antioxidant activity, and the potential effect on foam cell formation and cholesterol efflux during noni (Morinda citrifolia Linn.) fruit juice fermentation were investigated in this study. The composition of phenolic compounds was significantly different at various fermentation times. Rutin, quercetin, and isoquercitrin were the major phenolics in fermented noni fruit juice based on a quantitative analysis of representative phenolics. The contents of caffeic acid, 2,4-dihydroxybenzoic acid, p-coumaric acid, rutin, and quercetin tended to increase, while those of isoquercitrin decreased during the fermentation process. Fermented noni juice extracts showed high antioxidant activities against 2,2-diphenyl-1-picrylhydrazyl and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), hydroxyl radical scavenging activity, and ferric reducing antioxidant power. Notably, the highest antioxidant activity was observed after 28 days of fermentation. Furthermore, the treatment of fermented noni juice extracts was shown to reduce foam cell formation, intracellular cholesterol level, and the cholesterol esterification ratio. A correlation analysis indicated a strong positive relationship between the phenolic composition, antioxidant activity, and the ratio of cholesterol ester and total cholesterol. This study may provide a theoretical basis for the quality improvement and standardized production of fermented noni fruit juice, thus promoting the development of the noni food industry.
1. Introduction
Polyphenols are a class of widely distributed, natural polyhydroxylated phenolic compounds which are characterized by their strong antioxidant properties [1]. Based on their chemical structure, polyphenols can be categorized into flavonoids, phenolic acids, and lignans. Numerous phenolic compounds exhibit significant biological activities, such as anti-inflammatory [2], antitumor [3], and antidepressant [4,5] effects, as well as cardiovascular protection [6]. Epidemiological and clinical studies have shown that the consumption of foods and beverages rich in phenolic compounds correlates with a reduced risk of several chronic diseases, such as obesity [7], type 2 diabetes [8], cardiovascular diseases [9], and neurodegenerative disorders [10]. The main sources of phenolic compounds are fruits and vegetables [11]. In the past two decades, various pharmacological effects and health benefits of dietary polyphenols have been revealed [12]. In addition, previous studies have shown that the fermentation process may have a significant effect on the compositions and bioactivities of phenolic compounds. For instance, the gentisic acid content of curly kale significantly increased after fermentation [13]. Gong X et al. [14] reported that the composition of phenolics of Dendrobium candidum substantially changed after fermentation, and the content of syringic acid, 4-hydroxybenzoic acid, and p-hydroxycinnamic acid increased significantly.
Morinda citrifolia Linn. (Noni) is an evergreen, fruit-bearing plant which is widely grown in tropical and subtropical areas. Noni has been used to treat headaches, arthritis, and diabetes in Polynesia for more than a thousand years [15]. This plant contains many valuable chemical and biological compounds, such as phenolic compounds, polysaccharides, organic acids, vitamins, and minerals [16]. Phenolic compounds play a key role in the plant’s therapeutic properties; for example, rutin, b-sitosterol, asperuloside, and andursolic acid are important biologically active compounds present in noni [17]. The flavonoids isolated from noni fruit, such as kaempferol, quercetin, catechin, and epicatechin, have shown antidepressant and antioxidant activities [18]. With ongoing investigations into the health-promoting properties of noni, noni-related products, especially fermented noni juice, have become remarkably popular worldwide. Fermented noni juice is a beverage with high nutritional value [19]. Traditionally, this juice is produced by fermenting noni fruits in sealed jars or barrels for approximately 2 months or longer and then recovering the juice through drip extraction and/or mechanical pressure [20,21]. It is generally produced by natural fermentation processes, i.e., it is fermented by its intrinsic enzymes and natural mutualistic microorganisms. Deng et al. [22] compared the phytochemical fingerprints of 13 commercial noni fermented juices in the global market and found that they all contained scopolamine, rutin, and quercetin.
Most commercial fermented noni juice is currently produced through a natural fermentation process. Due to the lack of scientific evaluation methods and unified quality standards, the marketplace is full of various forms of fermented noni juice, with differences in quality and price [23]. Available data on the content of phenolic compounds during noni fermentation are limited, and the composition and activity of phenolic compounds remain unclear. Therefore, the content and composition of phenolic extracts were analyzed in this study using ultraperformance liquid chromatography-quadrupole-time-of-flight tandem mass (UPLC–Q–TOF–MS/MS). Meanwhile, their antioxidant activities and inhibition potential of macrophage foam cell formation were also evaluated. This study may provide basic data for product quality improvement and standardized production of fermented noni juice.
2. Materials and Methods
2.1. Chemical and Reagents
Phenolic acid and flavonoid standards (caffeic acid, 2,4-dihydroxybenzoic acid, p-coumaric acid, rutin, quercetin, and isoquercitrin) were purchased from Sigma-Aldrich (Beijing, China). 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,4,6-tripyridyl-s-triazine (TPTZ), and 2,2′-azino-bis [3-ethylbenzothiazoline-6-sulfonic acid] (ABTS) were purchased from Solarbio Technology Co., Ltd. (Beijing, China). Formic acid and acetonitrile was LC/MS grade and obtained from Fisher Scientific (Hampton, NH, USA). Ethyl acetate, ethanol, K2S2O8, methanol, o-phenanthroline, FeSO4, FeCl3, formaldehyde and isopropanol of analytical grade were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). DMEM (Dulbecco’s modified Eagle’s medium) was bought from Hyclone Co. (Shanghai, China). The cholesterol assay kit was purchased from Pulilai Gene Technology Co., Ltd. (Beijing, China). Oil Red O were purchased from Sigma-Aldrich (Louis, MO, USA).
2.2. Sample Preparation
Mature yellow or white Noni fruits (cultivars “Kuke”) were provided by the Hainan Wanning Noni Industrial Base (Hainan, China). The noni fruits were cleaned with running water, soaked with 60 mg/L chlorine dioxide for 30 min, and then rinsed with sterile water. A total of 15 sterile fermenters with each containing about 5 kg noni fruits were fermented at room temperature (25–28 °C) and kept from light. The fermented juice was collected on days 0, 7, 28, and 63 and stored at −80 °C for use.
2.3. Extraction of Phenolic Compounds
Fresh or fermented noni juice was centrifuged at 8000× g for 10 min (4 °C). The supernatants were extracted four times with ethyl acetate (1:1, v/v). The ethyl acetate fractions were combined and evaporated to dryness at 40 °C and redissolved in 10 mL 50% ethanol. The phenolic extracts were filtered through a Nylon filter (0.22 µm, 25 mm) and stored at −80 °C until further analysis. The phenolic extracts were marked as 0-FJP (from fresh noni juice), 7-FJP (after 7-day fermentation), 28-FJP (after 28-day fermentation), and 63-FJP (after 63-day fermentation).
2.4. Phenolic Composition Analysis by UPLC–Q-TOF–MS/MS
The phenolic compounds were analyzed on an Agilent 1290 UPLC coupled to a 6540 Q-TOF MS system with a dual ESI source (Agilent Technologies, Santa Clara, CA, USA). The column used was an Agilent ZORBAX Eclipse Plus C18 (3.0 × 100 mm, 1.8 μm). Elution was undertaken at a flow rate of 0.5 mL/min with solvent A (acetonitrile) and solvent B (0.15% formic acid) in a linear gradient manner: 10–20% A (0–6 min), 20–25% A (6–10 min), 60–95% A (15–20 min), and 95% A (20–25 min). DAD detector was used between 200–400 nm. The injection volume was 20 μL, and the temperature was 30 °C. MS spectrometry testing conditions were as follows: ionization mode, electrospray ionization, and the accurate mass data correction using electrospray ionization-L low concentration tuning mix (G1969-85000). MS operation conditions were as follows: drying gas temperature, 300 °C; drying gas flow rate, 8 mL/min; nebulizer pressure, 45 psi; sheath gas temperature, 400 °C; sheath gas flow, 12 L/min; capillary voltages, 3.5 kV; nozzle voltage, 175 V; and the mass range was scanned as m/z 100–1100. The LC-MS data were collected and analyzed using Mass Hunter B.03.01 (Agilent Technologies). Negative ion analysis and MRE scan modes were also adopted.
2.5. Quantitative Analysis by UPLC
The quantification of targeted phenolic compounds was performed in accordance with the method proposed by Wang, R.M. et al. [24] and conducted on an Agilent 1290 series UPLC (Agilent Technologies, Santa Clara, CA, USA) equipped with a photodiode array detector. The same column and conditions were described as above.
2.6. Antioxidant Activity Assay
2.6.1. DPPH Radical Scavenging Activity Assay
The DPPH radical scavenging activity was measured in accordance with the previously reported method [25]. The sample (1.0 mL) was mixed with a freshly prepared methanol solution of DPPH (1.0 mL, 80 µg/mL). The absorbance was measured at 517 nm after the mixture was incubated for 30 min at 25 °C. The radical scavenging capability of DPPH was calculated using the following equation:
where Acontrol is the absorbance of the control and Asample is the absorbance of the extract.
2.6.2. ABTS+ Radical Scavenging Activity Assay
The ABTS+ radical cation decolorization assay was performed using the previously reported method with slight modifications [26]. Briefly, 5 mL of 7 mM ABTS+ and 140 mM K2S2O8 aqueous solutions were mixed in equal volumes and reacted for 12–16 h in the dark. The stock solution was diluted with methanol to an absorbance value of 0.7 ± 0.02 at 734 nm to prepare an ABTS+ solution. Then, 0.4 mL of FJP was mixed with 2.6 mL of ABTS+ solution and reacted for 6 min in the dark. The absorbance at 734 nm was measured using an ultraviolet spectrophotometer with absolute ethyl alcohol as a blank control. The radical scavenging activity was calculated using the following equation:
where Acontrol is the absorbance of the control and Asample is the absorbance of the extract.
2.6.3. OH∙(Hydroxyl) Radical Scavenging Activity Assay
The OH radical scavenging activity was determined in accordance with the method proposed by Ren et al. [27] with minor modifications. The reaction mixture contained 1.0 mL of phosphate buffer saline (PBS, 0.02 mol/L, pH 7.4), 0.5 mL o-phenanthroline (2.5 mmol/L), 0.5 mL FeSO4 (2.5 mmol/L), 0.5 mL H2O2 (2.0 mmol/L), and 0.5 mL sample. The reaction mixture was centrifuged at 4000× g for 10 min after reaction for 1 h in a water bath at 37 °C. Optical density (OD) at 536 nm of the supernatant was measured as A1. OD 536 nm of the blank, in which the sample was replaced with distilled water, was measured as A0. Distilled water was used to replace H2O2 as the control group to measure OD 536 nm, which was recorded as A2.
where A0 is the absorbance of the control, A1 is the absorbance of the extract and A2 is the absorbance of the reagent blank without sodium salicylate.
2.6.4. Ferric Reducing Antioxidant Power (FRAP) Assay
The FRAP was determined in accordance with the method proposed in a previous report [27]. The FRAP dye was prepared by mixing sodium acetate solution (300 mM), TPTZ solution (10 mM), and FeCl3 solution (20 mM) in a 10:1:1 (v/v) ratio, respectively. A total of 200 µL of extract (50-fold dilution) or standard was added to 2800 µL of prepared FRAP dye solution. The mixture reacted for 30 min under darkness for 10 min (37 °C). The absorbance was measured at 593 nm. The antioxidant capacity was expressed in µg Trolox equivalents per 100 mL of extract (µmol TE/100 mL). The standard curve, which is plotted at different concentrations of standard, ranges from 0–500 µg/mL.
2.7. Cell Experiments
2.7.1. Cell Culture and Treatment
The cell culture was performed following the method proposed by Mueller et al. [28] with slight modifications. RAW264.7 macrophages in the logarithmic growth phase were digested into a single-cell suspension, inoculated into 96-well plates (2 × 105 cells per well), and cultured in DMEM at 37 °C in a humidified atmosphere with 5% CO2. The medium was replaced with serum-free DMEM for 12 h starving culture after cells were adherent.
Experimental samples were divided into five groups: control group (DMEM), oxidized low-density lipoprotein (ox-LDL) group (100 μg/mL ox-LDL), and sample groups (0-FJP, 7-FJP, 28-FJP, and 63-FJ) were all diluted with DMEM solution under the necessary concentrations: 10, 50, and 100 ng/mL, respectively. The cells were further incubated for 24 h.
2.7.2. Oil Red O Staining and Determination of the Intracellular lipid
Intracellular lipid droplets were determined by oil red O staining. The cells were washed three times in ice-cold PBS (pH7.4) after incubation, followed by 10% formaldehyde fixation for 60 min at room temperature (25 °C). Neutral lipids were stained using 0.5% oil red O in isopropanol for 30 min, and cell morphology was observed using microscopy (Olympus IX81, Tokyo, Japan). The plate was washed three times with PBS, and 100 μL cell lysis solution (ethanol: acetic acid = 1:1 v/v) was added into each well. The absorbance was acquired at 520 nm after complete lysis.
2.7.3. Determination of the Cholesterol Content
Total cholesterol (TC) and free cholesterol (FC) contents were quantified using the cholesterol assay kit (Pulilai Gene Technology Co., Ltd. Beijing, China) according to the manufacturer’s instructions.The esterified cholesterol (CE) content was determined by subtracting FC from TC. CE/TC ratio levels were calculated as follows: CE/TC ratio = (TC − FC)/TC.
2.8. Statistical Analysis
All experiments were performed in triplicate with independent replicates. The data were reported as the mean ± standard deviation (SD) of three replicates. The results were compared by one-way analysis of variance (ANOVA), and Tukey’s test was used to identify significant differences. Differences of p < 0.05 were considered significant. The data and correlations were analyzed using Excel and SPSS software (version 18.0, SPSS, Inc. (Chicago, IL, USA)), respectively.
3. Results
3.1. Identification of Phenolic Compositions
Table 1 lists the detected phenolic compounds with retention time, observed m/z, generated molecular formula, and proposed compound detected in the extracts of fermented noni juice. A total of 21 compounds, including 12 phenolic acids, 7 flavonoid compounds, and 2 other compounds, were tentatively identified in the current study. The phenolic compounds changed in composition and quantity during the entire fermentation. Ten phenolic compounds were detected in the fresh noni juice (0-FJP), while 15, 15, and 14 phenolics were found in 7-FJP, 28-FJP, and 63-FJP, respectively.

Table 1.
UPLC–ESI–QTOF–MS/MS characterization of phenolic compounds in different fermentation times.
The parent ion at m/z 151.03 [M−H]− indicated that compound 1 was likely to be 2-hydroxy-2-phenylacetic acid with the molecular formula C8H8O3, which has also been tentatively characterized in palm fruits [29]. Compounds 2, 3, and 4 were hydroxycoumarins derivatives and were respectively identified as esculin [30], 4-hydroxycoumarin [31], and scopoletin [32] based on the parent ion at m/z 339.07 [M−H]−, m/z 161.02 [M−H]−, m/z 191.03 [M−H]−, and the related MS2 data. Three hydroxybenzoic acids (compounds 5, 6, and 7) were characterized. Among them, compound 5 with [M−H]− ion at m/z 153.01 was identified as protocatechuic acid consistent with previous literature [29], which was recognized in all the samples. Compound 6 with [M−H]− at m/z 137.02 was tentatively characterized as 3,4-dihydroxybenzaldehyde, while compound 7 with [M−H]− at m/z 135.04 was tentatively identified as 2-dydroxy-4-methylbenzaldehyde. Only one hydroxyphenylpropanoic acid (compound 8) was detected in 0-FJP, 7-FJP, and 28-FJP. Compound 8 showed [M−H]− at m/z 165.05, and the molecular formula C9H10O3 was tentatively characterized as 3-hydroxyphenylpropionic acid [33]. Three kinds of hydroxycinnamic acid were also characterized (compounds 9, 10, and 11). Compound 9, which exhibited the parent ion at m/z 147.04 [M−H]−, was tentatively identified as cinnamic acid; this compound is found in a variety of dietary plant materials, such as fruits, tea, vegetables, and cereals [34,35]. Compound 10 was identified as caffeic acid based on the parent ion at m/z 179.04 [M−H]− and the similar fragmentation pattern reported by Li et al. [31]. p-coumaric acid (compound 11 with [M−H]− ion at m/z 163.03) present in 0-FJP and 28-FJP was easily identified and confirmed by MS2 data and previous literature [36]. Compound 12 was detected in all fermented noni juice phenolic extracts (7-FJP, 28-FJP, and 63-FJP) with [M−H]− ion at m/z 212.06 tentatively identified as 2,3-dihydroxy-1-guaiacylpropanone. Three flavones (compounds 13, 14, and 15) were also identified during the entire fermentation process. Compound 13 was tentatively characterized as quercetin 3-O-glucosyl-xyloside, because it was detected in negative ionization modes with [M−H]− at m/z 595.13 and yielded aglycone at m/z 300, which is coherent with that derived from a cross-ring cleavage of its sugar moiety (quercetin moiety) [37]. In addition, compound 14, found in all samples, demonstrated a molecular ion at m/z 609.14 [M−H]− and fragment ions at m/z 301 and 151, and was easily identified as rutin based on the characterization and literature data [38,39]. Compound 15 showed [M−H]− at m/z 593.15 with the molecular formula C27H30O5 and was temporarily regarded as luteolin 7-neohesperidoside. Regarding isoflavonoids (derivatives), four compounds (compounds 16, 17, 18, and 19) were tentatively detected and identified. Compound 16, determined by comparing with results from a previous study, was characterized in Dalbergia odorifera using LC-MS/MS and was tentatively identified as sativanone [40]. Compounds 17 and 18 with respective precursor ions at [M−H]− m/z 463.08 and [M−H]− m/z 301.03 were detected in all samples, tentatively representing isoquercitrin and quercetin, which were identified on the basis of their characterization and literature data [41]. Compound 19, which has a precursor ion [M−H]− m/z at 313.07, was tentatively characterized as 2′,7-dihydroxy-4′,5′-dimethoxyisoflavone and was present in 0-FJP, 7-FJP, and 63-FJP. Two other phenolic derivatives, including isoscutellarein 7-xyloside (compound 20, [M−H]− m/z 417.08) and mucronulatol (±) (compound 21, [M−H]− m/z 301.10), were also tentatively detected (compounds 20 and 21).
Many phenolics in the fermented noni juice did not come directly from fresh noni fruit, because phenolics were mostly bound with sugars and other compounds in the form of glycosidic bonds. Acidity increased and the glycosidic bonds could be hydrolyzed as fermentation continued; thus, significant amounts of phenolics were released [42]. The phenolics composition changed in the Momordica charantia juice after fermentation, demonstrating a reduction in caffeic and p-coumaric acids and an increase in dihydrocaffeic and phloretic acids [43]. Li et al. [44] reported that the phenolics composition also changed during apple beverage fermentation with Lactobacillus plantarum ATCC14917, in which sugar moieties were removed and galloyl moieties of a variety of phenolic compounds hydrolyzed, resulting in changes in phenolic profiles [45].
3.2. Quantification of Predominant Individual Phenolic Compounds
Table 2 provides the contents of predominant individual phenolic compounds, including caffeic acid, 2, 4-dihydroxybenzoic acid, p-coumaric acid, rutin, quercetin, and isoquercitrin. The amounts of the individual phenolic compounds were between 0.15–26.69 mg/L. The contents of individual phenolic substantially changed in the fermentation process. The major phenolic compounds identified were rutin, quercetin, and isoquercitrin. The result was similar to previous studies, which reported that rutin and quercetin are characteristic compounds in commercial noni juice fermentation [22]. The content of caffeic and 2,4-dihydroxybenzoic acids gradually increased from 0 to 28 days of the fermentation process and then slightly decreased on day 63. A similar trend was observed on the content of rutin and quercetin, which reached the highest value on the 28th day. By contrast, isoquercitrin content showed a marked decrease over time. The isoquercitrin concentration dropped from 8.25 ± 0.34 mg/L to 4.47 ± 0.05 mg/L at the end of the 63-day fermentation.

Table 2.
Contents of predominant individual phenolic compounds of noni juice extracts during fermentation.
To date, over 200 compounds, especially phenolic compounds and alkaloids, have been identified in noni and related products [22]. Fermented noni juice contains high quantities of total phenolic compounds, flavonoids, condensed tannin, and scopoletin [46]. Previous studies have shown that fermentation generally changes the content of phenolics. Several studies have reported that fermentation influences the phenolic content during the fermentation of plant sources. Li et al. [47] reported that the levels of vanillic acid considerably increased during wampee leaf fermentation, reaching their highest values in the 6th day (4.68 mg/100 g), while 7-hydroxycoumarin almost remained stable. Liu et al. [48] also showed that the catechin content from the beginning (0 days) to the end (27 days) of fermentation ranged from 16.0 ± 4.2 mg/L to 478.5 ± 3.5 mg/L, while quercetin content showed a fluctuating change during fermentation and reached its highest values on day 12, which is consistent with the current results.
3.3. Changes in Antioxidant Activity during Fermentation
The antioxidation activity of noni juice during fermentation was monitored in this study. Table 3 shows that fermented noni juice had higher antioxidant activity compared with unfermented noni juice extracts. The highest antioxidant activity during fermentation was found in the 28-FJP considering DPPH and ABTS+ assay. The DPPH and ABTS+ radical scavenging activity in 28-FJP (68 ± 0.29% and 72.05 ± 1.67%, respectively) were significantly higher than that in 7-FJP (64.49 ± 0.22% and 67.59 ± 1.05%, respectively) and 63-FJP (63.92 ± 0.53% and 67.61 ± 1.06%, respectively). Compared with the 0-FJP, the DPPH and ABTS+ radical scavenging activity in 28-FJP increased by 13.13% and 7.99%, respectively. However, the DPPH and ABTS+ radical scavenging activity of 7-FJP was insignificantly different compared with 63-FJP. The same trend was also observed with OH∙ and FRAP assays, in which 28-FJP (57.41 ± 0.63% and 1144.01 ± 6.86 µmol TE/100 mL) exhibited significantly higher antioxidant activities than those in 63-FJP (49.71 ± 0.57% and 927.10 ± 51.14 µmol TE/100 mL) and 7-FJP (52.45 ± 0.95% and 1013.33 ± 11.98 µmol TE/100 mL).

Table 3.
Antioxidant activity of noni juice extracts during fermentation.
All four antioxidant indexes (Table 3) showed significant improvements after fermentation, probably due to their strong ability to hydrolyze the polyphenols present in the noni juice. Furthermore, the relatively high diversity and content of phenolic compounds in 7-FJP and 28-FJP may explain the relatively high antioxidant activity compared with 0-FJP. Therefore, an increase in antioxidant activities was associated with the release of phenolic compounds during fermentation. Similar to the current study, Yang et al. [46] reported that fermented noni juice exhibited high reductive activity and improved superoxide anion and H2O2 scavenging activities.
3.4. Oil Red O Staining and Analysis of Lipid Accumulation
Furthermore, oil red O staining was performed to evaluate the protective effect of FNJ against Ox-LDL-induced lipid accumulation in macrophages. Figure 1 shows that treatment with FJP of different fermentation times for 24 h lowered the Ox-LDL-induced cellular accumulation of lipid droplets in macrophages.
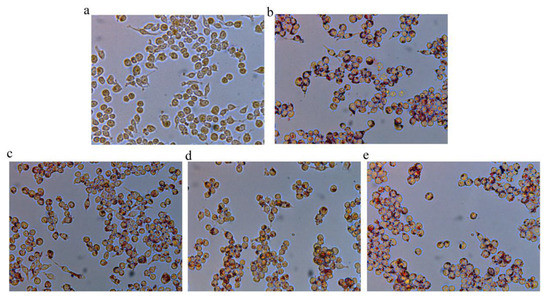
Figure 1.
Representative microscopy images of oil red O-stained lipid droplets at a magnification of ×400. (a) control group, (b) ox-LDL group, (c) 100 ng/mL 0-FJP + ox-LDL group, (d) 100 ng/mL 28-FJP + ox-LDL group, and (e) 100 ng/mL 63-FJP + ox-LDL group.
Figure 2 shows that the OD value in the ox-LDL group reached 0.24, which revealed a significant difference from the control group (0.12). The OD values of experimental groups were significantly different from that of the ox-LDL group (p < 0.05) under the concentration range from 10–100 ng/mL. 0-FJP and 28-FJP significantly inhibited lipid accumulation in macrophages (p < 0.05) and showed a dose-dependent manner relationship. The inhibition rates of 0-FJP and 28-FJP groups were 42.62% and 45.70%, respectively, under the 100 ng/mL concentration, showing the strongest inhibitory effect on macrophage lipids. Maximal inhibition was observed for group 63-FJP under the 10 ng/mL concentration, revealing an inhibition rate of 31.05% (p < 0.05). The 28-FJP exhibited the strongest inhibitory effect on lipid droplets compared with other groups under the concentration range of 10 to 100 ng/mL. These findings suggest that appropriate FJP concentrations can enhance the ability of macrophages to clear pathological lipids and inhibit lipid aggregation to a certain extent.
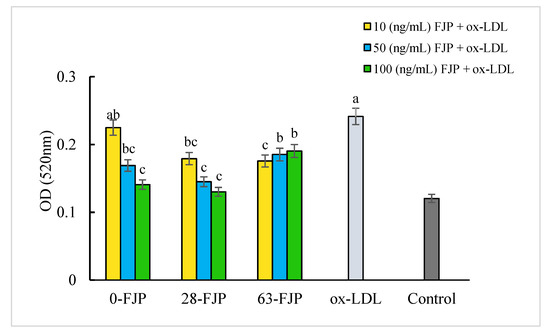
Figure 2.
Effect of FJP on lipid accumulation in macrophage foam cells. Data expressed as mean ± SD of three replicates; Different letters (a–c) indicate that the data are significantly different from the other data of the same column (p < 0.05). The significant difference was calculated through a one-way analysis of variance (ANOVA) and Tukey’s test.
3.5. Effect of FJP on Intracellular Cholesterol Content in Foam Cells
Additionally, the cholesterol levels were analyzed. Table 4 shows that the percentage of cholesterol ester was 54.79 ± 2.72% after treating with 80 ng/mL OX-LDL for 24 h, indicating the establishment of the foam cell model. Compared with the ox-LDL group, the total cholesterol and cholesterol ester content reduced in cells in the sample groups, and the foam phenomenon of cells demonstrated reduction ranges of 10–100 ng/mL. The 0-FJP and 28-FJP decreased the intracellular CE/TC ratio in a dose-dependent manner. The decrease in intracellular CE/TC value was the strongest and the CE/TC value was 39.8 ± 0.29% and 44.22 ± 0.28%, respectively (p < 0.01) when the concentration was 100 ng/mL in 0-FJP and 28-FJP. However, 63-FJP had a negative dose-dependent relationship when the extract concentration at 10 ng/mL the CE/TC ratio was 40.38 ± 0.07% (p < 0.01), showing the strongest effect on the decrease in intracellular CE/TC ratio. These findings confirm that 0-FJP, 28-FJP, and 63-FJP could inhibit ox-LDL-induced macrophage lipid deposition. Moreover, fermented noni juice regulated cholesterol metabolism in macrophages, increased the flow of cholesterol from the inside to the outside of macrophages, and reduced the accumulation of intracellular cholesterol.

Table 4.
Effects of noni juice extracts on cholesterol efflux from macrophages during fermentation.
The circulating level of ox-LDL is considered to be one of the most important biomarkers in the progression of atherosclerosis. A considerable amount of evidence shows that dietary antioxidants (e.g., phenolic and flavonoid) can potentially protect against LDL oxidation [49]. For example, olive (Olea europaea L.) extracts show anti-inflammatory and anti-atherogenic functions, such as the inhibition of LDL oxidation, platelet aggregation, and other factors involved in the development of atherosclerosis [50,51]. Grape seed phenolics can slow down the formation of foam cells by regulating key genes in cholesterol efflux and inflammation [52]. Zhang et al. [53] reported that procyanidin A2 and its metabolites significantly reduced the ox-LDL-induced intracellular CE/TC ratio in megalocytes.
3.6. Correlation Analysis
A correlation study between the results obtained in different antioxidant activity assays, CE/TC ratios, and determined phenolic contents was performed (Table 5) to elucidate the influence of phenolics on the antioxidant activity and cholesterol efflux from macrophages. Among these findings, the contents of caffeic acid, 2,4-dihydroxybenzoic acid, and quercetin showed a highly significant positive correlation (p < 0.05) with antioxidant activity. The contents of p-coumaric acid with DPPH, OH·, and FRAP assay demonstrated a significant positive correlation (p < 0.05). The contents of rutin with ABTS+·, OH, and FRAP assay showed a significant positive correlation (p < 0.05), while the isoquercitrin revealed a negative correlation with DPPH·assay (p < 0.05). Moreover, the contents of caffeic and 2,4-dihydroxybenzoic acids showed a positive correlation with CE/TC ratio (p < 0.05). Many studies have demonstrated the correlations between bioactive compounds and antioxidant activities in numerous fruits and vegetables [54,55]. Zhu et al. [56] found the significant positive correlations of gallic acid, rutin, quercetin, and kaempferol-3-O-rutinoside contents with DPPH, ABTS+, OH, and FRAP values. These results are consistent with the present study.

Table 5.
Pearson correlation analysis between phenolics and antioxidant activities.
4. Conclusions
The characteristics and contents of FJP at different fermentation times and their corresponding in vitro biological activities (especially antioxidant and inhibited lipid deposition) were reported in this study. Twenty-one phenolic compounds, comprising 12 phenolic acids, 7 flavonoids, and 2 others, were tentatively identified. Rutin, quercetin, and isoquercitrin were the most abundant components in FJP. The 28-FJP showed the highest antioxidant activities of DPPH, ABTS+, OH∙, and FRAP, among other samples. FJP treatment significantly inhibited lipid deposition and reduced ox-LDL-induced intracellular CE/TC ratio. Furthermore, significant positive correlations between phenolics, flavonoids, antioxidant activities, and CE/TC ratio were observed by Pearson’s correlation coefficient analysis. The present study suggests that the optimal fermentation period to achieve maximal yields of phenolic compounds is around 28 days,. The obtained findings provide useful information for processing fermented noni juice, which is rich in phenolics with upgraded bioactivities.
Author Contributions
K.C.: Conceptualization, Methodology, Writing-original manuscript; R.D.: Investigation, Methodology; X.L., X.H. and Z.W.: Investigation, Data Curation; S.L.: Supervision; C.L.: Resources, Writing-review and editing, Funding acquisition; W.L.: Supervision, Writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [the National Natural Science Foundation of China] grant number [31760456], [the National Natural Science Foundation of China] grant number [32160559], [the Hainan Provincial Natural Science Foundation of China] grant number [321QN191] and [the Hainan Provincial Natural Science Foundation of China] grant number [320RC511]. The APC was funded by [the National Natural Science Foundation of China].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
This research was funded by the National Natural Science Foundation of China, grant number 31760456, the National Natural Science Foundation of China, grant number 32160559, the Hainan Provincial Natural Science Foundation of China, grant number 321QN191 and the Hainan Provincial Natural Science Foundation of China, grant number 320RC511.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Adani, F.; Pepe Sciarria, T.; Squillace, P.; Scarafoni, A.; Iametti, S.; Scaglia, B. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Raw and Fermented Tomato Pomace and Their Correlations with Aglycate-Polyphenols. Antioxidants 2020, 9, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, M.; Arnaez, E.; Moreira, I.; Quesada, S.; Azofeifa, G.; Wilhelm, K.; Vargas, F.; Chen, P. Polyphenolic Characterization, Antioxidant, and Cytotoxic Activities of Mangifera indica Cultivars from Costa Rica. Foods 2019, 8, 384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Li, W.; You, B.; Tang, W.; Gan, T.; Feng, C.; Li, C.; Yang, R. Serum Metabonomic Study on the Antidepressant-like Effects of Ellagic Acid in a Chronic Unpredictable Mild Stress-Induced Mouse Model. J. Agric. Food Chem. 2020, 68, 9546–9556. [Google Scholar] [CrossRef]
- Lira, S.M.; Dionisio, A.P.; Holanda, M.O.; Marques, C.G.; Silva, G.S.D.; Correa, L.C.; Santos, G.B.M.; de Abreu, F.A.P.; Magalhaes, F.E.A.; Reboucas, E.L.; et al. Metabolic profile of pitaya (Hylocereus polyrhizus (F.A.C. Weber) Britton & Rose) by UPLC-QTOF-MS(E) and assessment of its toxicity and anxiolytic-like effect in adult zebrafish. Food Res. Int. 2020, 127, 108701. [Google Scholar] [CrossRef]
- Adriouch, S.; Lampure, A.; Nechba, A.; Baudry, J.; Assmann, K.; Kesse-Guyot, E.; Hercberg, S.; Scalbert, A.; Touvier, M.; Fezeu, L.K. Prospective Association between Total and Specific Dietary Polyphenol Intakes and Cardiovascular Disease Risk in the Nutrinet-Sante French Cohort. Nutrients 2018, 10, 1587. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Tresserra-Rimbau, A.; Estruch, R.; Martinez-Gonzalez, M.A.; Medina-Remon, A.; Fito, M.; Corella, D.; Salas-Salvado, J.; Portillo, M.P.; Moreno, J.J.; et al. Polyphenol Levels Are Inversely Correlated with Body Weight and Obesity in an Elderly Population after 5 Years of Follow Up (The Randomised PREDIMED Study). Nutrients 2017, 9, 452. [Google Scholar] [CrossRef] [Green Version]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red Raspberries and Their Bioactive Polyphenols: Cardiometabolic and Neuronal Health Links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef] [Green Version]
- Mattera, R.; Benvenuto, M.; Giganti, M.G.; Tresoldi, I.; Pluchinotta, F.R.; Bergante, S.; Tettamanti, G.; Masuelli, L.; Manzari, V.; Modesti, A.; et al. Effects of Polyphenols on Oxidative Stress-Mediated Injury in Cardiomyocytes. Nutrients 2017, 9, 523. [Google Scholar] [CrossRef] [Green Version]
- Ravi, S.K.; Narasingappa, R.B.; Vincent, B. Neuro-nutrients as anti-alzheimer’s disease agents: A critical review. Crit. Rev. Food Sci. Nutr. 2019, 59, 2999–3018. [Google Scholar] [CrossRef]
- Nowak, D.; Goslinski, M.; Przygonski, K.; Wojtowicz, E. The antioxidant properties of exotic fruit juices from acai, maqui berry and noni berries. Eur. Food Res. Technol. 2018, 244, 1897–1905. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Szwajgier, D.; Paduch, R.; Kukula-Koch, W.; Wasko, A.; Polak-Berecka, M. Fermented curly kale as a new source of gentisic and salicylic acids with antitumor potential. J. Funct. Foods 2020, 67, 103866. [Google Scholar] [CrossRef]
- Gong, X.; Jiang, S.; Tian, H.; Xiang, D.; Zhang, J. Polyphenols in the Fermentation Liquid of Dendrobium candidum Relieve Intestinal Inflammation in Zebrafish Through the Intestinal Microbiome-Mediated Immune Response. Front. Immunol. 2020, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- Dixon, A.R.; McMillen, H.; Etkin, N.L. Ferment this: The transformation of Noni, a traditional Polynesian medicine (Morinda citrifolia, Rubiaceae). Econ. Bot. 1999, 53, 51–68. [Google Scholar] [CrossRef]
- Wall, M.M.; Miller, S.; Siderhurst, M.S. Volatile changes in Hawaiian noni fruit, Morinda citrifolia L., during ripening and fermentation. J. Sci. Food Agric. 2018, 98, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Pawlus, A.D.; Kinghorn, D.A. Review of the ethnobotany, chemistry, biological activity and safety of the botanical dietary supplement Morinda citrifolia (noni). J. Pharm. Pharmacol. 2007, 59, 1587–1609. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zin, Z.; Abdul Hamid, A.; Osman, A.; Saari, N.; Misran, A. Isolation and Identification of Antioxidative Compound from Fruit of Mengkudu (Morinda citrifolia L.). Int. J. Food Prop. 2007, 10, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Chan-Blanco, Y.; Vaillant, F.; Mercedes Perez, A.; Reynes, M.; Brillouet, J.-M.; Brat, P. The noni fruit (Morinda citrifolia L.): A review of agricultural research, nutritional and therapeutic properties. J. Food Compos. Anal. 2006, 19, 645–654. [Google Scholar] [CrossRef]
- Yang, J.; Paulino, R.; Janke-Stedronsky, S.; Abawi, F. Free-radical-scavenging activity and total phenols of noni (Morinda citrifolia L.) juice and powder in processing and storage. Food Chem. 2007, 102, 302–308. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Li, P.Z.; Hu, B.; Xu, L.; Liu, S.N.; Yu, H.; Guo, Y.H.; Xie, Y.F.; Yao, W.R.; Qian, H. Correlation analysis reveals the intensified fermentation via Lactobacillus plantarum improved the flavor of fermented noni juice. Food Biosci. 2021, 43, 101234. [Google Scholar] [CrossRef]
- Deng, S.X.; West, B.J.; Jensen, C.J. A quantitative comparison of phytochemical components in global noni fruits and their commercial products. Food Chem. 2010, 122, 267–270. [Google Scholar] [CrossRef]
- Motshakeri, M.; Ghazali, H.M. Nutritional, phytochemical and commercial quality of Noni fruit: A multi-beneficial gift from nature. Trends Food Sci. Technol. 2015, 45, 118–129. [Google Scholar] [CrossRef]
- Wang, R.M.; Yao, L.L.; Lin, X.; Hu, X.P.; Wang, L. Exploring the potential mechanism of Rhodomyrtus tomentosa (Ait.) Hassk fruit phenolic rich extract on ameliorating nonalcoholic fatty liver disease by integration of transcriptomics and metabolomics profiling. Food Res. Int. 2022, 151, 110824. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, Y.; Yang, N.; Jiang, T.; Xu, H.; Lei, H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem. 2022, 373, 131455. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ren, B.B.; Chen, C.; Li, C.; Fu, X.; You, L.; Liu, R.H. Optimization of microwave-assisted extraction of Sargassum thunbergii polysaccharides and its antioxidant and hypoglycemic activities. Carbohyd. Polym. 2017, 173, 192–201. [Google Scholar] [CrossRef]
- Mueller, M.; Hobiger, S.; Jungbauer, A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010, 122, 987–996. [Google Scholar] [CrossRef]
- Ma, C.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterization of Phenolic Compounds in Palm Fruits (Jelly and Fishtail Palm) and Their Potential Antioxidant Activities. Antioxidants 2019, 8, 483. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Patel, M.; Deshpande, S.; Alreshidi, M.; Siddiqui, A.J.; Reddy, M.N.; Emira, N.; De Feo, V. Effect of Adiantum philippense Extract on Biofilm Formation, Adhesion with Its Antibacterial Activities against Foodborne Pathogens, and Characterization of Bioactive Metabolites: An in vitro-in silico Approach. Front. Microbiol. 2020, 11, 823. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.L.; Ying, D.Y.; Yu, J.W.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlic-Maganja, D.; Bandelj, D.; Baruca Arbeiter, A.; Peeters, K.; Miklavcic Visnjevec, A.; Jenko Praznikar, Z. HPLC-DAD-ESI-QTOF-MS Determination of Bioactive Compounds and Antioxidant Activity Comparison of the Hydroalcoholic and Water Extracts from Two Helichrysum italicum Species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.F.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Characterization of Phenolic Compounds from Medicinal Plants (Hops and Juniper Berries) and Their Antioxidant Activity. Foods 2020, 9, 7. [Google Scholar] [CrossRef] [Green Version]
- Chou, O.; Ali, A.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential. Fermentation 2021, 7, 73. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Zhong, B.M.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterization of Seaweed Phenolics and Their Antioxidant Potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef]
- Castro-Lopez, C.; Bautista-Hernandez, I.; Gonzalez-Hernandez, M.D.; Martinez-Avila, G.C.G.; Rojas, R.; Gutierrez-Diez, A.; Medina-Herrera, N.; Aguirre-Arzola, V.E. Polyphenolic Profile and Antioxidant Activity of Leaf Purified Hydroalcoholic Extracts from Seven Mexican Persea americana Cultivars. Molecules 2019, 24, 173. [Google Scholar] [CrossRef] [Green Version]
- Pierson, J.T.; Monteith, G.R.; Roberts-Thomson, S.J.; Dietzgen, R.G.; Gidley, M.J.; Shaw, P.N. Phytochemical extraction, characterisation and comparative distribution across four mango (Mangifera indica L.) fruit varieties. Food Chem. 2014, 149, 253–263. [Google Scholar] [CrossRef]
- Li, S.J.; Wang, R.M.; Hu, X.P.; Li, C.F.; Wang, L. Bio-affinity ultra-filtration combined with HPLC-ESI-qTOF-MS/MS for screening potential alpha-glucosidase inhibitors from Cerasus humilis (Bge.) Sok. leaf-tea and in silico analysis. Food Chem. 2022, 373, 131528. [Google Scholar] [CrossRef]
- Zhao, X.S.; Zhang, S.H.; Liu, D.; Yang, M.H.; Wei, J.H. Analysis of Flavonoids in Dalbergia odorifera by Ultra-Performance Liquid Chromatography with Tandem Mass Spectrometry. Molecules 2020, 25, 389. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.J.; Zhang, D.; Yu, C.; Yao, L.; Chen, Z.; Tao, Y.D.; Cao, W.G. Phytochemical Analysis, Antioxidant and Analgesic Activities of Incarvillea compacta Maxim from the Tibetan Plateau. Molecules 2019, 24, 1692. [Google Scholar] [CrossRef] [Green Version]
- Huynh, N.T.; Van Camp, J.; Smagghe, G.; Raes, K. Improved Release and Metabolism of Flavonoids by Steered Fermentation Processes: A Review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wen, J.J.; Hu, J.L.; Nie, Q.X.; Chen, H.H.; Nie, S.P.; Xiong, T.; Xie, M.Y. Momordica charantia juice with Lactobacillus plantarum fermentation: Chemical composition, antioxidant properties and aroma profile. Food Biosci. 2019, 29, 62–72. [Google Scholar] [CrossRef]
- Li, Z.X.; Teng, J.; Lyu, Y.L.; Hu, X.Q.; Zhao, Y.L.; Wang, M.F. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus plantarum ATCC14917. Molecules 2019, 24, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dueñas, M.; Fernández, D.; Hernández, T.; Estrella, I.; Muñoz, R. Bioactive phenolic compounds of cowpeas (Vigna sinensis L). Modifications by fermentation with natural microflora and with Lactobacillus plantarum ATCC 14917. J. Sci. Food Agric. 2005, 85, 297–304. [Google Scholar] [CrossRef]
- Yang, S.C.; Chen, T.I.; Li, K.Y.; Tsai, T.C. Change in Phenolic Compound Content, Reductive Capacity and ACE Inhibitory Activity in Noni Juice during Traditional Fermentation. J. Food Drug Anal. 2007, 15, 290–298. [Google Scholar] [CrossRef]
- Li, Q.; Chang, X.; Guo, R.; Wang, Q.; Guo, X. Dynamic effects of fermentation on phytochemical composition and antioxidant properties of wampee (Clausena lansium (Lour.) Skeel) leaves. Food Sci. Nutr. 2019, 7, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Cheng, H.; Liu, H.; Ma, R.; Ma, J.; Fang, H. Fermentation by Multiple Bacterial Strains Improves the Production of Bioactive Compounds and Antioxidant Activity of Goji Juice. Molecules 2019, 24, 3519. [Google Scholar] [CrossRef] [Green Version]
- Aviram, M.; Fuhrman, B. Wine flavonoids protect against LDL oxidation and atherosclerosis. Ann. N.Y. Acad. Sci. 2002, 957, 146–161. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Bibiloni, M.D.; Tur, J.A.; Pons, A.; Sureda, A. Cardioprotective Effects of the Polyphenol Hydroxytyrosol from Olive Oil. Curr. Drug Targets 2017, 18, 1477–1486. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Benefits of Nut Consumption on Insulin Resistance and Cardiovascular Risk Factors: Multiple Potential Mechanisms of Actions. Nutrients 2017, 9, 1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terra, X.; Pallares, V.; Ardevol, A.; Blade, C.; Fernandez-Larrea, J.; Pujadas, G.; Salvado, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, X.L.; Li, T.Y.; Li, M.Y.; Huang, R.M.; Li, W.; Yang, R.L. 3-(4-Hydroxyphenyl)propionic acid, a major microbial metabolite of procyanidin A2, shows similar suppression of macrophage foam cell formation as its parent molecule. Rsc. Adv. 2018, 8, 6242–6250. [Google Scholar] [CrossRef] [Green Version]
- Belwal, T.; Pandey, A.; Bhatt, I.D.; Rawal, R.S.; Luo, Z. Trends of polyphenolics and anthocyanins accumulation along ripening stages of wild edible fruits of Indian Himalayan region. Sci. Rep. 2019, 9, 5894. [Google Scholar] [CrossRef]
- Afanas′ev, I.B.; Dorozhko, A.I.; Brodskii, A.V.; Kostyuk, V.A.; Potapovitch, A.I. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 1989, 38, 1763–1769. [Google Scholar] [CrossRef]
- Zhu, H.L.; Liu, S.X.; Yao, L.L.; Wang, L.; Li, C.F. Free and Bound Phenolics of Buckwheat Varieties: HPLC Characterization, Antioxidant Activity, and Inhibitory Potency towards alpha-Glucosidase with Molecular Docking Analysis. Antioxidants 2019, 8, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).