Impact of Incorporating the Aqueous Extract of Hawthorn (C. oxyanatha) Leaves on Yogurt Properties and Its Therapeutic Effects against Oxidative Stress Induced by Carbon Tetrachloride in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hawthorn Leaves Aqueous Extract
2.3. Yogurt Fortified with Aqueous Hawthorn Leaves Extract Manufacture
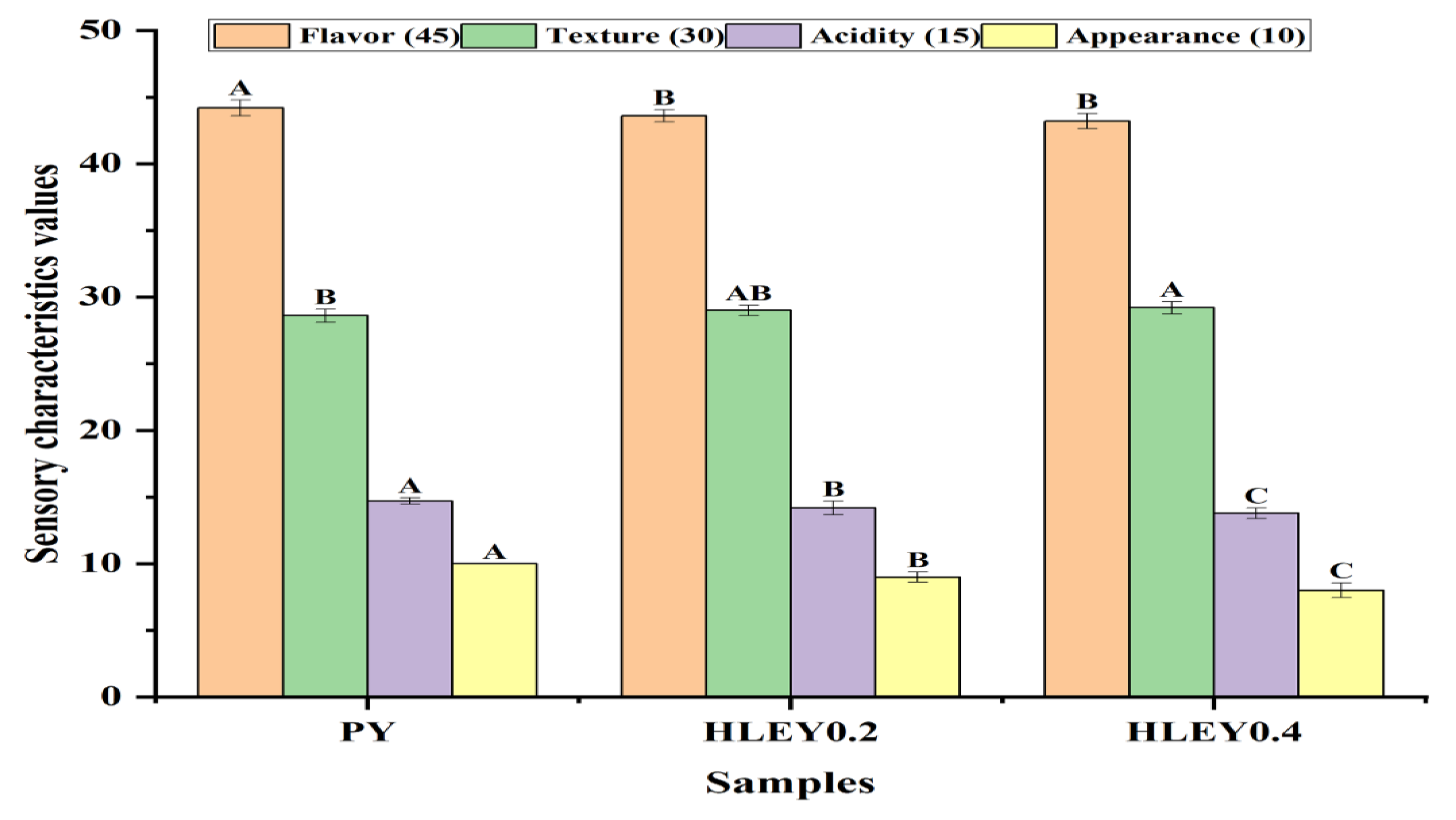
2.4. Chemical Compositions, Physicochemical Analysis, and Sensory Evaluation of Yogurt Fortified with Aqueous Hawthorn Leaves Extract
2.5. Animal Experimental Design
2.6. Biochemical Analysis
2.7. Histopathological Examination
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content and Radical Scavenging Activity of Hawthorn Leaves Extract
3.2. Chemical Compositions, Physicochemical, and Sensory Characteristics of Yogurt Fortified with Aqueous Hawthorn Leaves Extract
3.3. Final Weight and Body Weight Gain in All Groups of Rats
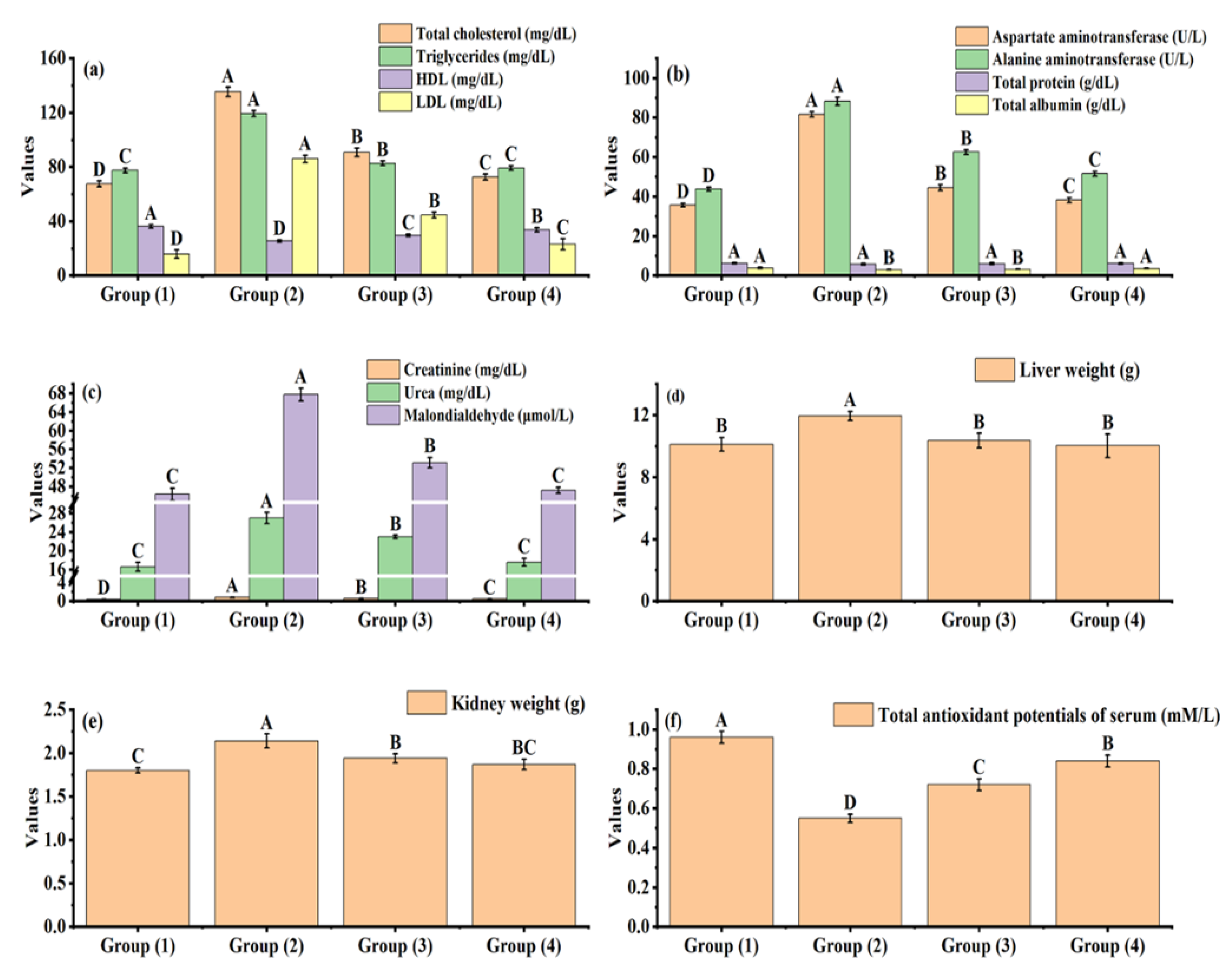
3.4. Effect of Yogurt Fortified with Hawthorn Leaves Extract on the Serum Lipid Profile, Liver Function Parameters, Kidney Function Parameters, Liver Weight, Kidney Weight, and Total Antioxidant Potentials of Serum of Oxidative Stress Rats
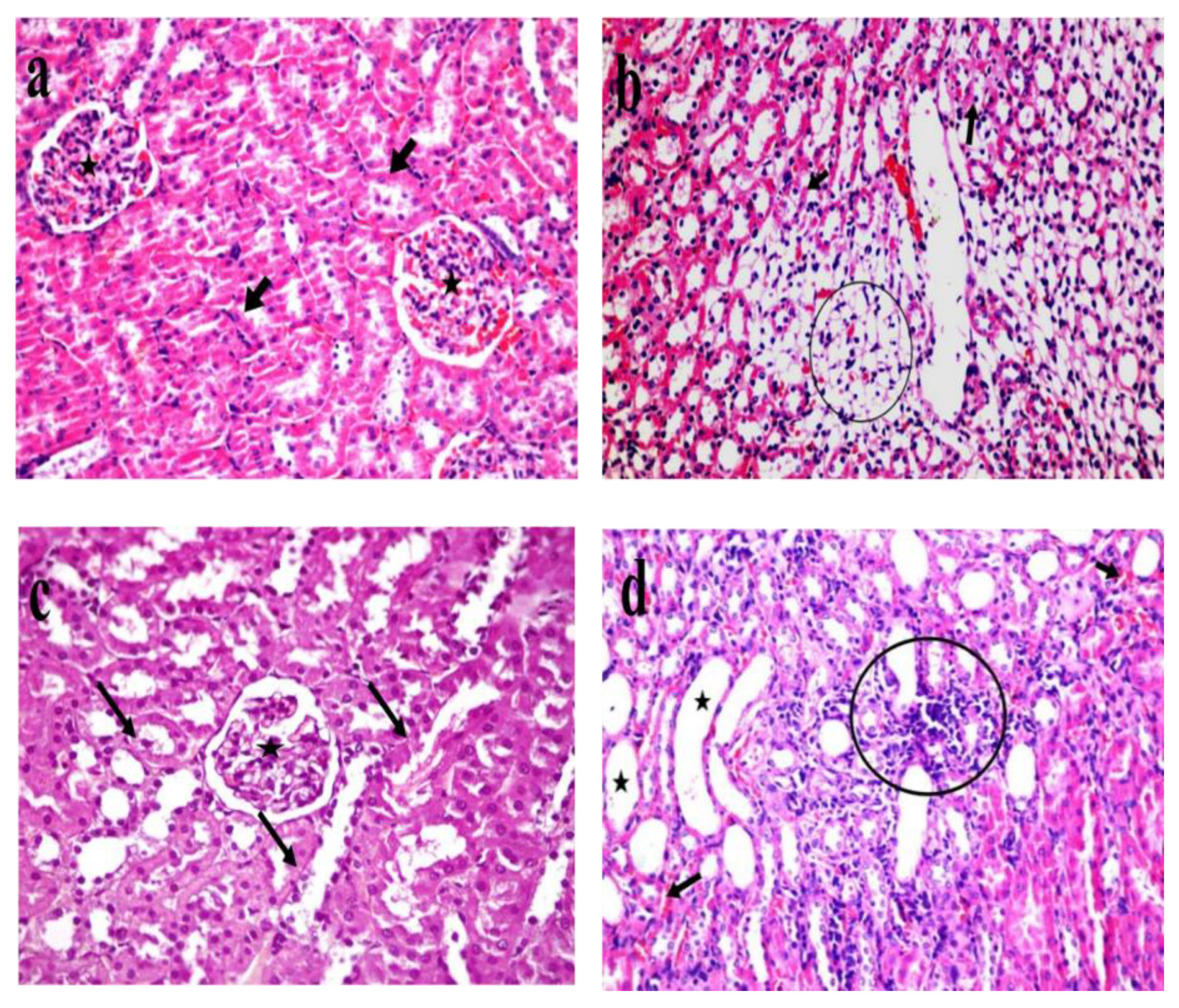
3.5. Light Microscopic Histological Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxidative Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomsa, A.M.; Alexa, A.L.; Junie, M.L.; Rachisan, A.L.; Ciumarnean, L. Oxidative stress as a potential target in acute kidney injury. PeerJ 2019, 7, e8046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Young, M.R.; Bobe, G.; Colburn, N.H.; Milner, J.A. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prev. Res. 2009, 2, 200–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ďuračková, Z. Some current insights into oxidative stress. Physiol. Res. 2010, 59, 459–469. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Free radicals: Health implications and their mitigation by herbals. J. Adv. Med. Med. Res. 2015, 7, 438–457. [Google Scholar] [CrossRef]
- Samuel, S.A.; Francis, A.O.; Anthony, O.O. Role of the kidneys in the regulation of intra-and extra-renal blood pressure. Ann. Clin. Hypertens. 2018, 2, 048–058. [Google Scholar]
- Cosme, P.; Rodríguez, A.B.; Espino, J.; Garrido, M. Plant phenolics: Bioavailability as a key determinant of their potential health-promoting applications. Antioxidants 2020, 9, 1263. [Google Scholar] [CrossRef]
- Vuolo, M.M.; Lima, V.S.; Junior, M.R.M. Phenolic compounds: Structure, classification, and antioxidant power. In Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–50. [Google Scholar]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef] [Green Version]
- Hosseini-Ara, R.; Mokhtarian, A.; Karamrezaei, A.H.; Toghraie, D. Computational analysis of high precision nano-sensors for diagnosis of viruses: Effects of partial antibody layer. Math. Comput. Simul. 2022, 192, 384–398. [Google Scholar] [CrossRef]
- Elkot, W.F.; Ateteallah, A.H.; Al-Moalem, M.H.; Shahein, M.R.; Mohamed AAlblihed, M.A.; Abdo, W.; Elmahallawy, E.K. Functional, Physicochemical, Rheological, Microbiological, and Organoleptic Properties of Synbiotic Ice cream Produced from Camel Milk Using Black Rice Powder and Lactobacillus acidophilus LA-5. Fermentation 2022, 8, 187. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Elkot, W.F.; Hijazy, H.H.A.; Kassab, R.B.; Alblihed, M.A.; Elmahallawy, E.K. The impact of date syrup on the physicochemical, microbiological, and sensory properties, and antioxidant activity of bio-fermented camel milk. Fermentation 2022, 8, 192. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; Radwan, H.A.; Elmeligy, A.A.; Hafiz, A.A.; Albrakati, A.; Elmahallawy, E.K. Production of a Yogurt Drink Enriched with Golden Berry (Physalis pubescens L.) Juice and Its Therapeutic Effect on Hepatitis in Rats. Fermentation 2022, 8, 112. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Atwaa, E.S.H.; Shahein, M.R.; El-Sattar, E.S.A.; Hijazy, H.H.A.; Albrakati, A.; Elmahallawy, E.K. Bioactivity, Physicochemical and Sensory Properties of Probiotic Yoghurt Made from Whole Milk Powder Reconstituted in Aqueous Fennel Extract. Fermentation 2022, 8, 52. [Google Scholar] [CrossRef]
- Shahein, M.R.; Atwaa, E.S.H.; El-Zahar, K.M.; Elmaadawy, A.A.; Hijazy, H.H.A.; Sitohy, M.Z.; Albrakati, A.; Elmahallawy, E.K. Remedial Action of Yoghurt Enriched with Watermelon Seed Milk on Renal Injured Hyperuricemic Rats. Fermentation 2022, 8, 41. [Google Scholar] [CrossRef]
- Swelam, S.; Zommara, M.A.; Abd El-Aziz, A.E.-A.M.; Elgammal, N.A.; Baty, R.S.; Elmahallawy, E.K. Insights into Chufa Milk Frozen Yoghurt as Cheap Functional Frozen Yoghurt with High Nutritional Value. Fermentation 2021, 7, 255. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.; Hernández-Mendoza, A.; Torres-Llanez, M.; González-Córdova, A.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef] [Green Version]
- Butnariu, M.; Grozea, I. Antioxidant (antiradical) compounds. J. Bioequiv. Availab. 2012, 4, 17–19. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C.M. The food systems in the era of the coronavirus (COVID-19) pandemic crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef]
- Al-Shawi, S.G.; Ali, H.I.; Al-Younis, Z.K. The effect of adding thyme extacts on microbiological, chemical and sensory characteristics of yogurt. J. Pure Appl. Microbiol 2020, 14, 1367–1376. [Google Scholar] [CrossRef]
- Bertolino, M.; Belviso, S.; Dal Bello, B.; Ghirardello, D.; Giordano, M.; Rolle, L.; Gerbi, V.; Zeppa, G. Influence of the addition of different hazelnut skins on the physicochemical, antioxidant, polyphenol and sensory properties of yogurt. LWT-Food Sci. Technol. 2015, 63, 1145–1154. [Google Scholar] [CrossRef] [Green Version]
- Benabderrahmane, W.; Lores, M.; Benaissa, O.; Lamas, J.P.; de Miguel, T.; Amrani, A.; Benayache, F.; Benayache, S. Polyphenolic content and bioactivities of Crataegus oxyacantha L. (Rosaceae). Nat. Prod. Res. 2021, 35, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E. Phytochemical and pharmacological activity profile of Crataegus oxyacantha L. (hawthorn)-A cardiotonic herb. Curr. Med. Chem. 2018, 25, 4854–4865. [Google Scholar] [CrossRef]
- Venskutonis, P.R. Phytochemical composition and bioactivities of hawthorn (Crataegus spp.): Review of recent research advances. J. Food Bioact. 2018, 4, 69–87. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Rodríguez, J.L.; Reyes-Estrada, C.A.; Gutiérrez-Hernández, R.; Lopez, J.A. Antioxidant, hypolipidemic and preventive effect of Hawthorn (Crataegus oxyacantha) on alcoholic liver damage in rats. J. Pharmacogn. Phytother. 2016, 8, 193–202. [Google Scholar]
- Yang, T.; Zhang, X.; Pan, S.; Zhang, X.; Dang, Z. Effect of hawthorn Jiangzhi powder on blood lipids in patients with hyperlipidemia: A pathological analysis of 484 cases. Chin. J. Integr. Tradit. West. Med. Intensive Crit. Care 2017, 24, 166–169. [Google Scholar]
- Rasheed, H.A.; Hussien, N.R.; Al-Naimi, M.S.; Al-Kuraishy, H.M.; Al-Gareeb, A.I. Fenofibrate and Crataegus oxyacantha is an effectual combo for mixed dyslipidemia. Biomed. Biotechnol. Res. J. BBRJ 2020, 4, 259. [Google Scholar]
- Pakseresht, S.; Mazaheri Tehrani, M.; Razavi, S.M.A. Optimization of low-fat set-type yoghurt: Effect of altered whey protein to casein ratio, fat content and microbial transglutaminase on rheological and sensorial properties. J. Food Sci. Technol. 2017, 54, 2351–2360. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Chemists, A.O.O.A.; Horwitz, W. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1975; Volume 222. [Google Scholar]
- Maksimović, Z.; Malenčić, Đ.; Kovačević, N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour. Technol. 2005, 96, 873–877. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innov. Food Sci. Emerg. Technol. 2007, 8, 46–54. [Google Scholar] [CrossRef]
- Ali, H.I.; Dey, M.; Alzubaidi, A.K.; Alneamah, S.J.A.; Altemimi, A.B.; Pratap-Singh, A. Effect of rosemary (Rosmarinus officinalis L.) supplementation on probiotic yoghurt: Physicochemical properties, microbial content, and sensory attributes. Foods 2021, 10, 2393. [Google Scholar] [CrossRef] [PubMed]
- Kujawska, M.; Ignatowicz, E.; Murias, M.; Ewertowska, M.; Mikołajczyk, K.; Jodynis-Liebert, J. Protective effect of red beetroot against carbon tetrachloride-and N-nitrosodiethylamine-induced oxidative stress in rats. J. Agric. Food Chem. 2009, 57, 2570–2575. [Google Scholar] [CrossRef] [PubMed]
- Dougnon, T.V.; Bankolé, H.S.; Klotoé, J.R.; Sènou, M.; Fah, L.; Koudokpon, H.; Akpovi, C.; Dougnon, T.J.; Addo, P.; Loko, F. Treatment of hypercholesterolemia: Screening of Solanum macrocarpon Linn (Solanaceae) as a medicinal plant in Benin. Avicenna J. Phytomedicine 2014, 4, 160. [Google Scholar] [CrossRef]
- Devi, R.; Sharma, D. Hypolipidemic effect of different extracts of Clerodendron colebrookianum Walp in normal and high-fat diet fed rats. J. Ethnopharmacol. 2004, 90, 63–68. [Google Scholar] [CrossRef]
- Bachorik, P.S.; Walker, R.E.; Kwiterovich, P.O. Determination of high density lipoprotein-cholesterol in human plasma stored at −70 degrees C. J. Lipid Res. 1982, 23, 1236–1242. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Bergmeyer, H.; Harder, M. A colorimetric method of the determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Clin. Biochem. 1986, 24, 458–481. [Google Scholar]
- Koracevic, D.; Koracevic, G.; Djordjevic, V.; Andrejevic, S.; Cosic, V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001, 54, 356–361. [Google Scholar] [CrossRef] [Green Version]
- Hemieda, F.A.-K.E.-S. Influence of gender on tamoxifen-induced biochemical changes in serum of rats. Mol. Cell. Biochem. 2007, 301, 137–142. [Google Scholar] [CrossRef]
- Marsh, W.H.; Fingerhut, B.; Miller, H. Automated and manual direct methods for the determination of blood urea. Clin. Chem. 1965, 11, 624–627. [Google Scholar] [CrossRef]
- Han, K.-H.; Sekikawa, M.; Shimada, K.-i.; Hashimoto, M.; Hashimoto, N.; Noda, T.; Tanaka, H.; Fukushima, M. Anthocyanin-rich purple potato flake extract has antioxidant capacity and improves antioxidant potential in rats. Br. J. Nutr. 2007, 96, 1125–1134. [Google Scholar] [CrossRef] [Green Version]
- Hosni, M.; El Maghrbi, A.; Ganghish, K. Occurrence of Trichinella spp. in wild animals in northwestern Libya. Open Vet. J. 2013, 3, 85–88. [Google Scholar] [PubMed]
- Öztürk, N.; Tunçel, M. Assessment of phenolic acid content and in vitro antiradical characteristics of hawthorn. J. Med. Food 2011, 14, 664–669. [Google Scholar] [CrossRef]
- Šavikin, K.P.; Krstić-Milošević, D.B.; Menković, N.R.; Beara, I.N.; Mrkonjić, Z.O.; Pljevljakušić, D.S. Crataegus orientalis leaves and berries: Phenolic profiles, antioxidant and anti-inflammatory activity. Nat. Prod. Commun. 2017, 12, 1934578X1701200204. [Google Scholar] [CrossRef] [Green Version]
- Jafari, S.M.; Vakili, S.; Dehnad, D. Production of a Functional Yogurt Powder Fortified with Nanoliposomal Vitamin D Through Spray Drying. Food Bioprocess Technol. 2019, 12, 1220–1231. [Google Scholar] [CrossRef]
- Azizkhani, M.; Tooryan, F. Antimicrobial activities of probiotic yogurts flavored with peppermint, basil, and zataria against Escherichia coli and Listeria monocytogenes. J. Food Qual. Hazards Control 2016, 3, 79–86. [Google Scholar]
- Suliman, A.; Ahmed, K.; Mohamed, B.; Babiker, E. Potential of cinnamon (Cinnamomum cassia) as an anti-oxidative and anti-microbial agent in sudanese yoghurt (Zabadi). J. Dairy Vet. Sci. 2019, 12, 555833. [Google Scholar]
- Dabija, A.; Codină, G.G.; Ropciuc, S.; Gâtlan, A.-M.; Rusu, L. Assessment of the antioxidant activity and quality attributes of yogurt enhanced with wild herbs extracts. J. Food Qual. 2018, 2018, 5329386. [Google Scholar] [CrossRef]
- Olmedo, R.H.; Nepote, V.; Grosso, N.R. Preservation of sensory and chemical properties in flavoured cheese prepared with cream cheese base using oregano and rosemary essential oils. LWT-Food Sci. Technol. 2013, 53, 409–417. [Google Scholar] [CrossRef]
- Yadav, K.; Shukla, S. Microbiological, physicochemical analysis and sensory evaluation of herbal yogurt. Pharma Innov. 2014, 3, 1–4. [Google Scholar]
- Shelbaya, L.A.; Ghoneim, G.A. Biological Evaluation of Cupcake Treated with Hawthorn Leaves on Diabetic Rats. J. Food Dairy Sci. 2017, 8, 25–30. [Google Scholar] [CrossRef]
- Cho, W.-Y.; Kim, D.-H.; Lee, H.-J.; Yeon, S.-J.; Lee, C.-H. Quality characteristic and antioxidant activity of yogurt containing olive leaf hot water extract. CyTA-J. Food 2020, 18, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Shiravani, M.; Ansari, S. Yogurt fortification with walnut leaf extract and investigation of its physicochemical and sensory properties. J. Innov. Food Sci. Technol. 2020, 12, 1–17. [Google Scholar]
- Al Shammari, A.M.N. Biological Effects of Hawthorn Leaves Powder and its Extract on Biological and Biochemical Change of Induced Obese Rats. Life Sci. J. 2020, 17, 62–66. [Google Scholar]
- Qin, C.; Xia, T.; Li, G.; Zou, Y.; Cheng, Z.; Wang, Q. Hawthorne leaf flavonoids prevent oxidative stress injury of renal tissues in rats with diabetic kidney disease by regulating the p38 MAPK signaling pathway. Int. J. Clin. Exp. Pathol. 2019, 12, 3440–3446. [Google Scholar] [PubMed]
- Yilmaz, S.; Kaya, E.; Kisacam, M.A. The effect on oxidative stress of aflatoxin and protective effect of lycopene on aflatoxin damage. Aflatoxin-Control Anal. Detect. Health Risks 2017, 30, 67–90. [Google Scholar]
- Zeni, A.L.B.; Moreira, T.D.; Dalmagro, A.P.; Camargo, A.; Bini, L.A.; Simionatto, E.L.; Scharf, D.R. Evaluation of phenolic compounds and lipid-lowering effect of Morus nigra leaves extract. An. Da Acad. Bras. De Ciências 2017, 89, 2805–2815. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Fong, W.P.; Cheng, C.H. The dual actions of morin (3,5,7,2′,4′-pentahydroxyflavone) as a hypouricemic agent: Uricosuric effect and xanthine oxidase inhibitory activity. J. Pharmacol. Exp. Ther. 2006, 316, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Zhang, G.; Liao, Y.; Pan, J.; Gong, D. Dietary flavonoids as xanthine oxidase inhibitors: Structure–affinity and structure–activity relationships. J. Agric. Food Chem. 2015, 63, 7784–7794. [Google Scholar] [CrossRef]
- Nair, G.G.; Nair, C.K.K. Radioprotective effects of gallic acid in mice. BioMed Res. Int. 2013, 2013, 953079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koc, M.; Taysi, S.; Buyukokuroglu, M.E.; Bakan, N. Melatonin protects rat liver against irradiation-induced oxidative injury. J. Radiat. Res. 2003, 44, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Xu, J.; Zheng, P.; Xing, L.; Shen, H.; Yang, L.; Zhang, L.; Ji, G. Hawthorn leaf flavonoids alleviate nonalcoholic fatty liver disease by enhancing the adiponectin/AMPK pathway. Int. J. Clin. Exp. Med. 2015, 8, 17295–17307. [Google Scholar] [PubMed]
- Tatsimo, S.J.N.; Tamokou, J.d.D.; Havyarimana, L.; Csupor, D.; Forgo, P.; Hohmann, J.; Kuiate, J.-R.; Tane, P. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum. BMC Res. Notes 2012, 5, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadek, M.A.; Mousa, S.; El-Masry, S.; Demain, A.M. Influence of Hawthorn (Crataegus oxyacantha) Leaves Extract Administration on Myocardial Infarction Induced by Isoproterenol in Rats. Cardiol. Angiol. Int. J. 2018, 1–12. [Google Scholar] [CrossRef]

| Treatments | Chemical Compositions | Physicochemical Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Solids | Fat | Protein | Ash | Acidity % | pH | TPC | AO Activity | PV | AV | |
| PY | 10.24 ± 0.16 A | 3.12 ± 0.08 A | 3.94 ± 0.08 A | 0.72 ± 0.02 B | 0.82 ± 0.02 A | 4.68 ± 0. 02 C | 18.00 ± 2.45 C | 7.70 ± 0.57 C | 1.20 ± 0.05 A | 0.50 ± 0.02 A |
| HLEY 0.2 | 10.42 ± 0.20 A | 3.14 ± 0.08 A | 3.98 ± 0.13 A | 0.76 ± 0.03 AB | 0.76 ± 0.02 B | 4.75 ± 0.02 B | 38.00 ± 2.45 B | 12.40 ± 0.49 B | 0.90 ± 0.03 B | 0.42 ± 0.02 B |
| HLEY 0.4 | 10.66 ± 0.24 A | 3.18 ± 0.10 A | 4.02 ± 0.16 A | 0.80 ± 0.03 A | 0.70 ± 0.02 C | 4.82 ± 0.03 A | 62.00 ± 3.27 A | 20.60 ± 0.82 A | 0.60 ± 0.02 C | 0.34 ± 0.02 C |
| Groups | Initial Weight (g) | Final Weight (g) | Body Weight Gain (BWG (%)) |
|---|---|---|---|
| Group (1) | 227.40 ± 2.49 A | 287.07 ± 1.72 A | 26.25 ± 1.18 A |
| Group (2) | 229.80 ± 1.54 A | 265.40 ± 1.66 D | 15.49 ± 0.30 D |
| Group (3) | 228.60 ± 1.41 A | 271.20 ± 2.62 C | 18.63 ± 0.67 C |
| Group (4) | 227.50 ± 1.78 A | 275.48 ± 2.14 B | 21.09 ± 0.56 B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahein, M.R.; Atwaa, E.-S.H.; Babalghith, A.O.; Alrashdi, B.M.; Radwan, H.A.; Umair, M.; Abdalmegeed, D.; Mahfouz, H.; Dahran, N.; Cacciotti, I.; et al. Impact of Incorporating the Aqueous Extract of Hawthorn (C. oxyanatha) Leaves on Yogurt Properties and Its Therapeutic Effects against Oxidative Stress Induced by Carbon Tetrachloride in Rats. Fermentation 2022, 8, 200. https://doi.org/10.3390/fermentation8050200
Shahein MR, Atwaa E-SH, Babalghith AO, Alrashdi BM, Radwan HA, Umair M, Abdalmegeed D, Mahfouz H, Dahran N, Cacciotti I, et al. Impact of Incorporating the Aqueous Extract of Hawthorn (C. oxyanatha) Leaves on Yogurt Properties and Its Therapeutic Effects against Oxidative Stress Induced by Carbon Tetrachloride in Rats. Fermentation. 2022; 8(5):200. https://doi.org/10.3390/fermentation8050200
Chicago/Turabian StyleShahein, Magdy Ramadan, El-Sayed H. Atwaa, Ahmad O. Babalghith, Barakat M. Alrashdi, Hanan A. Radwan, Muhammad Umair, Dyaaaldin Abdalmegeed, Hala Mahfouz, Naief Dahran, Ilaria Cacciotti, and et al. 2022. "Impact of Incorporating the Aqueous Extract of Hawthorn (C. oxyanatha) Leaves on Yogurt Properties and Its Therapeutic Effects against Oxidative Stress Induced by Carbon Tetrachloride in Rats" Fermentation 8, no. 5: 200. https://doi.org/10.3390/fermentation8050200
APA StyleShahein, M. R., Atwaa, E.-S. H., Babalghith, A. O., Alrashdi, B. M., Radwan, H. A., Umair, M., Abdalmegeed, D., Mahfouz, H., Dahran, N., Cacciotti, I., & Elmahallawy, E. K. (2022). Impact of Incorporating the Aqueous Extract of Hawthorn (C. oxyanatha) Leaves on Yogurt Properties and Its Therapeutic Effects against Oxidative Stress Induced by Carbon Tetrachloride in Rats. Fermentation, 8(5), 200. https://doi.org/10.3390/fermentation8050200










