Improved Spectrophotometric Method for Determination of High-Range Volatile Fatty Acids in Mixed Acid Fermentation of Organic Residues
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Fermentation Process
2.3. Sample Preparation
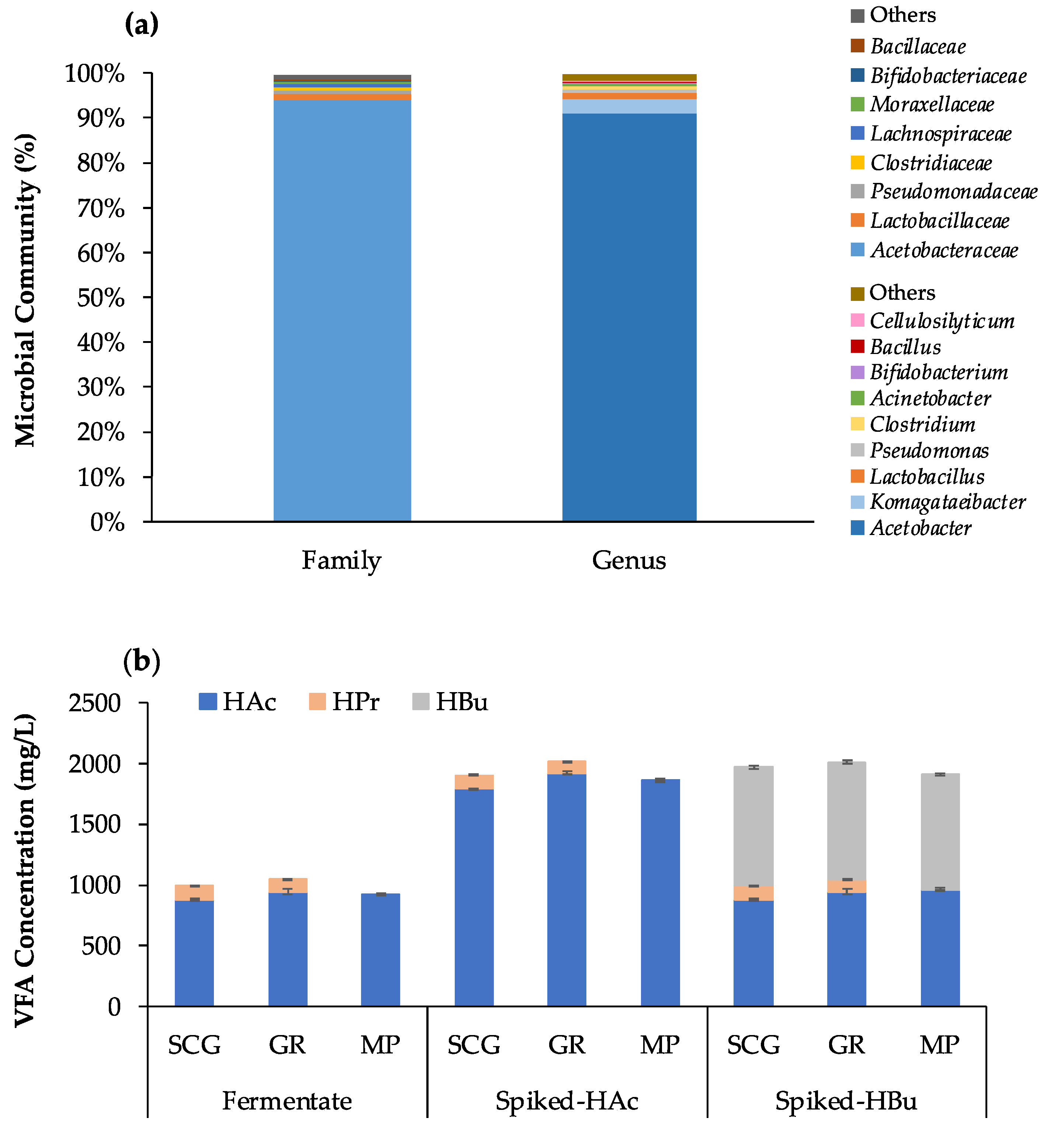
2.4. Bacteria Community Analysis by 16S rRNA Gene Sequencing
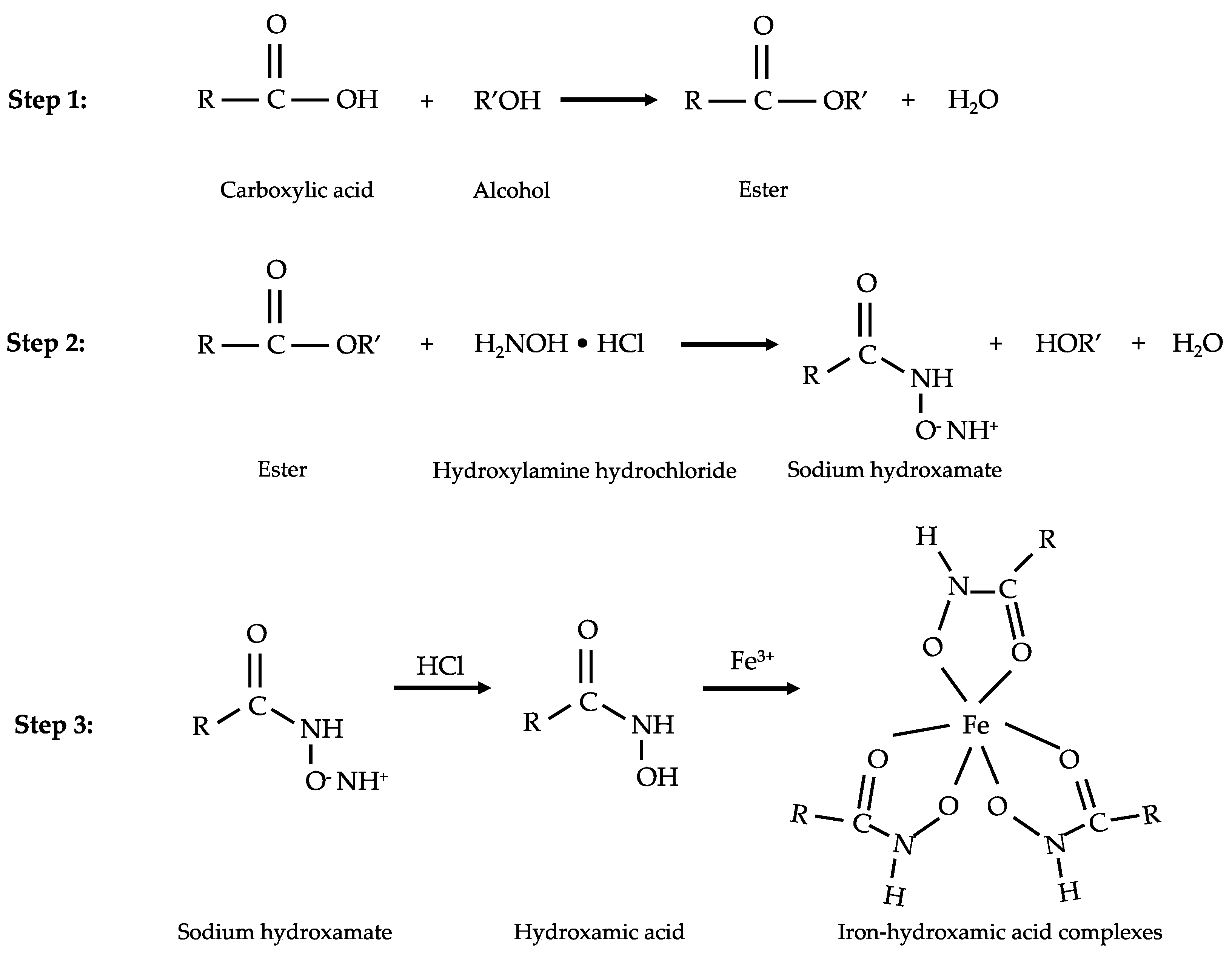
2.5. Determination of VFAs by the Modified Spectrophotometric Method
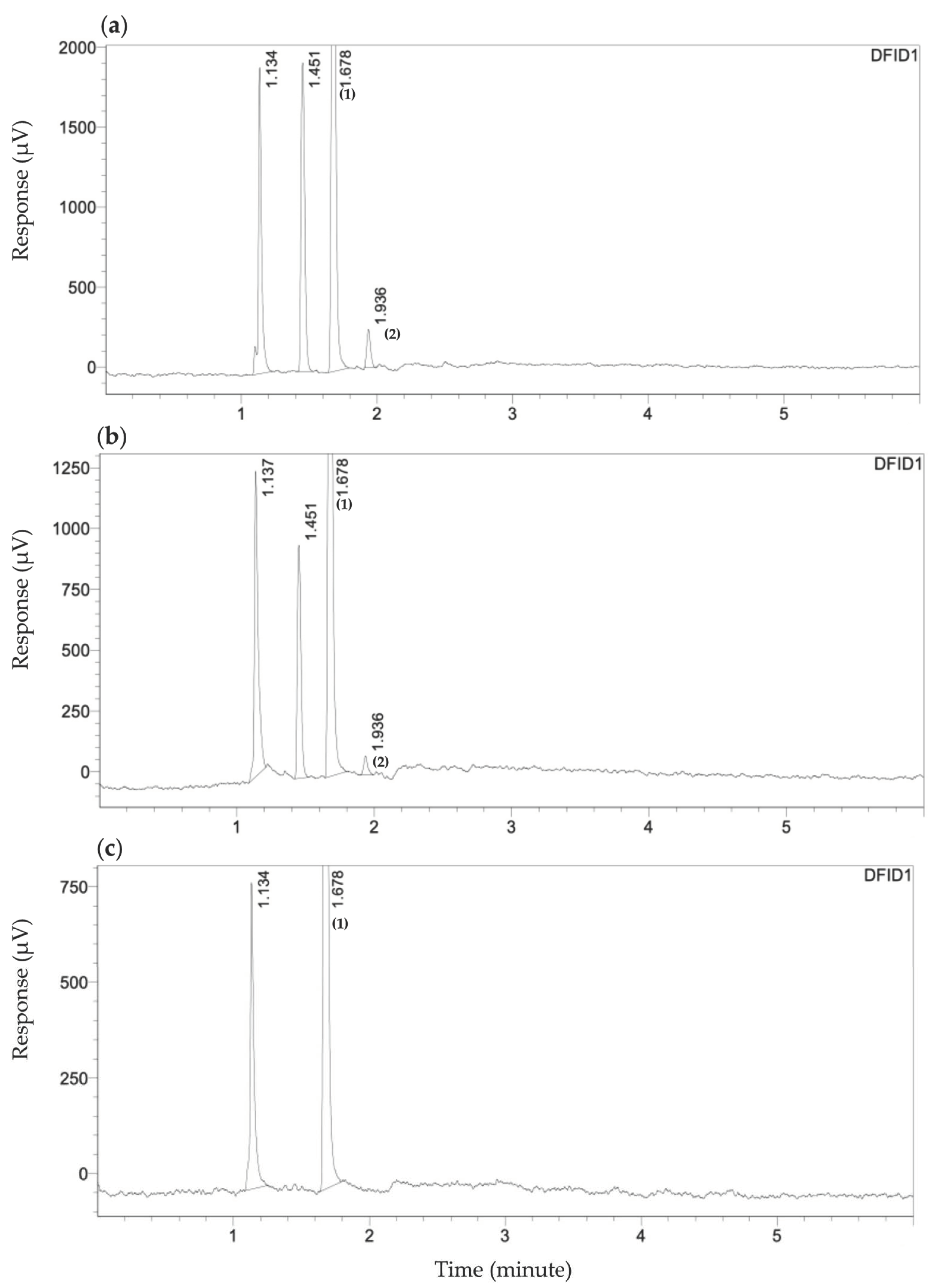
2.6. Determination of VFAs by Gas Chromatograph
2.7. Assays Validation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Figures of Merit of the Modified Spectrophotometric Methods
3.2. Determination of C2–C6 Volatile Fatty Acids
3.3. Application of the Developed Methods to Fermentation Samples
3.4. The Improvement of the Modified Spectrophotometric Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fritts, R.K.; McCully, A.L.; McKinlay, J.B. Extracellular metabolism sets the table for microbial cross-feeding. Microbiol. Mol. Biol. Rev. 2021, 85, e00135-20. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Kim, N.; Kang, J. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Munir, S.; Jamil, N. Polyhydroxyalkanoates (PHA) production in bacterial co-culture using glucose and volatile fatty acids as carbon source. J. Basic Microbiol. 2018, 58, 247–254. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, R. Production of Polyhydroxyalkanoates (PHA) by Haloferax mediterranei from Food Waste Derived Nutrients for Biodegradable Plastic Applications. J. Microbiol. Biotechnol. 2020, 31, 338–347. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Kim, N.J.; Lim, S.J.; Chang, H.N. Volatile Fatty Acid Platform: Concept and Application; Wiley: Hoboken, NJ, USA, 2018; Volume 1, pp. 173–190. [Google Scholar]
- Van Aarle, I.M.; Perimenis, A.; Lima-Ramos, J.; De Hults, E.; George, I.F.; Gerin, P.A. Mixed inoculum origin and lignocellulosic substrate type both influence the production of volatile fatty acids during acidogenic fermentation. Biochem. Eng. J. 2015, 103, 242–249. [Google Scholar] [CrossRef]
- Aramrueang, N.; Zhang, R.; Liu, X. Application of biochar and alkalis for recovery of sour anaerobic digesters. J. Environ. Manag. 2022, 307, 114538. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Aramrueang, N.; Rapport, J.; Zhang, R. Effects of hydraulic retention time and organic loading rate on performance and stability of anaerobic digestion of Spirulina platensis. Biosyst. Eng. 2016, 147, 174–182. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Chen, Y. Propionic acid-rich fermentation (PARF) production from organic wastes: A review. Bioresour. Technol. 2021, 339, 125569. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Tu, W.; Wu, M.; Zhang, Z.; Wang, H. Pilot-scale fermentation of urban food waste for volatile fatty acids production: The importance of pH. Bioresour. Technol. 2021, 332, 125116. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Cetecioglu, Z. Butyric acid dominant volatile fatty acids production: Bio-augmentation of mixed culture fermentation by Clostridium butyricum. J. Environ. Chem. Eng. 2020, 8, 104496. [Google Scholar] [CrossRef]
- Pratto, B.; Chandgude, V.; de Sousa Junior, R.; Cruz, A.J.G.; Bankar, S. Biobutanol production from sugarcane straw: Defining optimal biomass loading for improved ABE fermentation. Ind. Crops Prod. 2020, 148, 112265. [Google Scholar] [CrossRef]
- Panda, S.K.; Mishra, S.S.; Kayitesi, E.; Ray, R.C. Microbial-processing of fruit and vegetable wastes for production of vital enzymes and organic acids: Biotechnology and scopes. Environ. Res. 2016, 146, 161–172. [Google Scholar] [CrossRef]
- Tripetchkul, S.; Kusuwanwichid, S.; Koonsrisuk, S.; Akeprathumchai, S. Utilization of wastewater originated from naturally fermented virgin coconut oil manufacturing process for bioextract production: Physico-chemical and microbial evolution. Bioresour. Technol. 2010, 101, 6345–6353. [Google Scholar] [CrossRef] [PubMed]
- Jayakrishnan, U.; Deka, D.; Das, G. Enhancing the volatile fatty acid production from agro-industrial waste streams through sludge pretreatment. Environ. Sci. Water Res. Technol. 2019, 5, 334–345. [Google Scholar] [CrossRef]
- Wijngaard, H.H.; Rößle, C.; Brunton, N. A survey of Irish fruit and vegetable waste and by-products as a source of polyphenolic antioxidants. Food Chem. 2009, 116, 202–207. [Google Scholar] [CrossRef]
- Aramrueang, N.; Asavasanti, S.; Khanunthong, A. Chapter 1—Leafy Vegetables. Academic Press: Cambridge, MA, USA, 2019; pp. 245–272. [Google Scholar]
- Aramrueang, N.; Zicari, S.M.; Zhang, R. Characterization and compositional analysis of agricultural crops and residues for ethanol production in California. Biomass Bioenergy 2017, 105, 288–297. [Google Scholar] [CrossRef]
- Ye, H.; Wang, Q.; Zhu, F.; Feng, G.; Yan, C.; Zhang, J. Antifungal Activity of Alpha-Mangostin against Colletotrichum gloeosporioides In Vitro and In Vivo. Molecules 2020, 25, 5335. [Google Scholar] [CrossRef]
- Silva, C.S.; Gabriel, C.; Cerqueira, F.; Manso, M.C.; Vinha, A. Coffee Industrial Waste as a Natural Source of Bioactive Compounds with Antibacterial and Antifungal Activities; Formatex Research Center: Badajoz, Spain, 2015; pp. 131–136. [Google Scholar]
- Zhou, Y.-Q.; Liu, H.; He, M.-X.; Wang, R.; Zeng, Q.-Q.; Wang, Y.; Ye, W.-C.; Zhang, Q.-W. A Review of the Botany, Phytochemical, and Pharmacological Properties of Galangal, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 7, pp. 351–396. [Google Scholar]
- Chouni, A.; Paul, S. A review on phytochemical and pharmacological potential of Alpinia galanga. Pharmacogn. J. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Li, Q.; Xu, S.; Zhao, X.; Xue, P.; Feng, X. Antifungal activity of zedoary turmeric oil against Phytophthora capsici through damaging cell membrane. Pestic. Biochem. Physiol. 2019, 159, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; Dinsdale, R.M.; Guwy, A.J.; Premier, G.C. Critical analysis of methods for the measurement of volatile fatty acids. Crit. Rev. Environ. Sci. Technol. 2016, 46, 209–234. [Google Scholar] [CrossRef]
- Mu, Z.-X.; He, C.-S.; Jiang, J.-K.; Zhang, J.; Yang, H.-Y.; Mu, Y. A modified two-point titration method for the determination of volatile fatty acids in anaerobic systems. Chemosphere 2018, 204, 251–256. [Google Scholar] [CrossRef]
- Vikas, O.; Mridul, U. Bioconversion of papaya peel waste into vinegar using Acetobacter aceti. Int. J. Sci. Res. 2014, 3, 409–411. [Google Scholar]
- Lahav, O.; Morgan, B. Titration methodologies for monitoring of anaerobic digestion in developing countries—A review. J. Chem. Technol. Biotechnol. 2004, 79, 1331–1341. [Google Scholar] [CrossRef]
- Sun, H.; Guo, J.; Wu, S.; Liu, F.; Dong, R. Development and validation of a simplified titration method for monitoring volatile fatty acids in anaerobic digestion. Waste Manag. 2017, 67, 43–50. [Google Scholar] [CrossRef]
- Chatterjee, B.; Radhakrishnan, L.; Mazumder, D. New approach for determination of volatile fatty acid in anaerobic digester sample. Environ. Eng. Sci. 2018, 35, 333–351. [Google Scholar] [CrossRef]
- Lützhøft, H.-C.H.; Boe, K.; Fang, C.; Angelidaki, I. Comparison of VFA titration procedures used for monitoring the biogas process. Water Res. 2014, 54, 262–272. [Google Scholar] [CrossRef]
- Li, X.-Y.; Feng, Y.; Duan, J.-L.; Feng, L.-J.; Wang, Q.; Ma, J.-Y.; Liu, W.-Z.; Yuan, X.-Z. Model-based mid-infrared spectroscopy for on-line monitoring of volatile fatty acids in the anaerobic digester. Environ. Res. 2022, 206, 112607. [Google Scholar] [CrossRef]
- Stockl, A.; Lichti, F. Near-infrared spectroscopy (NIRS) for a real time monitoring of the biogas process. Bioresour. Technol. 2018, 247, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, H.; Zheng, H.; Uhrin, D.; Dewhurst, R.J.; Roehe, R. Comparison of HPLC and NMR for quantification of the main volatile fatty acids in rumen digesta. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Zhu, J. Optimizing secondary electrospray ionization high-resolution mass spectrometry (SESI-HRMS) for the analysis of volatile fatty acids from gut microbiome. Metabolites 2020, 10, 351. [Google Scholar] [CrossRef]
- Ghidotti, M.; Fabbri, D.; Torri, C.; Piccinini, S. Determination of volatile fatty acids in digestate by solvent extraction with dimethyl carbonate and gas chromatography-mass spectrometry. Anal. Chim. Acta 2018, 1034, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, H.; Dymock, J.F.; Thom, N. The rapid colorimetric determination of organic acids and their salts in sewage-sludge liquor. Analyst 1962, 87, 949–955. [Google Scholar] [CrossRef]
- Siedlecka, E.M.; Kumirska, J.; Ossowski, T.; Glamowski, P.; Gołębiowski, M.; Gajdus, J.; Kaczyński, Z.; Stepnowski, P. Determination of volatile fatty acids in environmental aqueous samples. Pol. J. Environ. Stud. 2008, 17, 351–356. [Google Scholar]
- Silva, M.A.; Correa, R.A.; Maria, G.d.O.; Antoniosi Filho, N.R. A new spectrophotometric method for determination of biodiesel content in biodiesel/diesel blends. Fuel 2015, 143, 16–20. [Google Scholar] [CrossRef][Green Version]
- Thompson, A.R. A colorimetric method for the determination of esters. Aust. J. Chem. 1950, 3, 128–135. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef]
- Tolbin, A.Y.; Pushkarev, V.E.; Tomilova, L.G.; Zefirov, N.S. Threshold concentration in the nonlinear absorbance law. Phys. Chem. Chem. Phys. 2017, 19, 12953–12958. [Google Scholar] [CrossRef]
- DiLallo, R.; Albertson, O.E. Volatile acids by direct titration. J. Water Pollut. Control Fed. 1961, 33, 356–365. [Google Scholar]
- Vannecke, T.; Lampens, D.; Ekama, G.; Volcke, E. Evaluation of the 5 and 8 pH point titration methods for monitoring anaerobic digesters treating solid waste. Environ. Technol. 2015, 36, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Patel, M.; Mishra, B.K.; Das, S. Short-chain fatty acid and vitamin production potentials of Lactobacillus isolated from fermented foods of Khasi Tribes, Meghalaya, India. Ann. Microbiol. 2019, 69, 1191–1199. [Google Scholar] [CrossRef]
- Innocente, N.; Moret, S.; Corradini, C.; Conte, L. A rapid method for the quantitative determination of short-chain free volatile fatty acids from cheese. J. Agric. Food Chem. 2000, 48, 3321–3323. [Google Scholar] [CrossRef]
- Nakae, T.; Elliott, J. Production of volatile fatty acids by some lactic acid bacteria. II. Selective formation of volatile fattyacids by degradation of amino acids. J. Dairy Sci. 1965, 48, 293–299. [Google Scholar] [CrossRef]
- Rojas, V.; Gil, J.V.; Piñaga, F.; Manzanares, P. Studies on acetate ester production by non-Saccharomyces wine yeasts. Int. J. Food Microbiol. 2001, 70, 283–289. [Google Scholar] [CrossRef]
- Mkhize, N.T.; Msagati, T.A.; Mamba, B.B.; Momba, M. Determination of volatile fatty acids in wastewater by solvent extraction and gas chromatography. Phys. Chem. Earth 2014, 67, 86–92. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Zaalberg, B.; Kersten, S.R.; Schuur, B. Extraction of volatile fatty acids from fermented wastewater. Sep. Purif. Technol. 2016, 161, 61–68. [Google Scholar] [CrossRef]




| Acetic Acid | Propionic Acid | Butyric Acid | Isobutyric Acid | Valeric Acid | Isovaleric Acid | Caproic Acid | |
|---|---|---|---|---|---|---|---|
| Calibration curves of the developed method | |||||||
| Linear range (mg/L) | 250–5000 | 250–5000 | 250–5000 | 250–5000 | 250–5000 | 250–5000 | 250–5000 |
| Slope ± SD | 0.1854 ± 0.0047 | 0.1227 ± 0.0017 | 0.0924 ± 0.0024 | 0.0526 ± 0.0025 | 0.0712 ± 0.0037 | 0.0294 ± 0.0011 | 0.0654 ± 0.0034 |
| Intercept ± SD | 0.0114 ± 0.0171 | 0.0124 ± 0.0089 | 0.0042 ± 0.0073 | 0.0066 ± 0.0051 | 0.0089 ± 0.0074 | 0.0032 ± 0.0022 | 0.0174 ± 0.0032 |
| Determination coefficient (R2) | 0.9984 | 0.9985 | 0.9948 | 0.9989 | 0.9993 | 0.9992 | 0.9872 |
| Precision (%) (n = 3) | 10.99 | 8.10 | 6.94 | 12.14 | 9.34 | 11.45 | 9.70 |
| Calibration curves of GC method | |||||||
| Retention time (min) | 1.677 | 1.923 | 2.286 | 2.015 | 2.947 | 2.496 | 3.927 |
| Linear range (mg/L) | 250–5000 | 250–5000 | 250–5000 | 250–5000 | 250–5000 | 250–5000 | 250–5000 |
| Slope ± SD | 10,793 ± 792 | 17,119 ± 1511 | 20,801 ± 1543 | 21,426 ± 1377 | 21,821 ± 1186 | 22,669 ± 2520 | 18,922 ± 1390 |
| Intercept ± SD | −937 ± 614 | −1035 ± 1049 | −957 ± 1705 | −1567 ± 930 | −1430 ± 1012 | −1158 ± 937 | −245 ± 1480 |
| Determination coefficient (R2) | 0.9993 | 0.9992 | 0.9995 | 0.9994 | 0.9997 | 0.9998 | 0.9997 |
| Precision (%) (n = 5) | 11.22 | 10.89 | 9.63 | 8.00 | 9.17 | 15.00 | 14.70 |
| Acid | Added 1 (mg/L) | Spectrophotometric | GC | ||||
|---|---|---|---|---|---|---|---|
| Measured 2 (mg/L) | Accuracy (%) | Precision (% RSD) | Measured 2 (mg/L) | Accuracy (%) | Precision (% RSD) | ||
| Acetic | 2000 | 2019 ± 91 | 100.93 | 4.49 | 1989 ± 34 | 99.46 | 1.72 |
| Propionic | 2000 | 2102 ± 110 | 105.11 | 5.24 | 2013 ± 30 | 100.65 | 1.49 |
| Butyric | 2000 | 2032 ± 39 | 101.62 | 1.92 | 1977 ± 47 | 98.85 | 2.36 |
| Isobutyric | 2000 | 2023 ± 38 | 101.14 | 1.88 | 1941 ± 18 | 97.03 | 0.92 |
| Valeric | 2000 | 2033 ± 114 | 101.66 | 5.58 | 1991 ± 11 | 99.55 | 0.55 |
| Isovaleric | 2000 | 2034 ± 34 | 101.70 | 1.67 | 1986 ± 45 | 99.29 | 2.25 |
| Caproic | 2000 | 1910 ± 77 | 95.51 | 4.03 | 1967 ± 63 | 98.35 | 3.23 |
| Sample | Initial VFAs (mg/L) | Added Acetic Acid (mg/L) | Added Butyric Acid (mg/L) | Estimated VFAs (mg/L) | Spectrophotometric | GC | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Found (mg/L) | Accuracy (%) | Precision (% RSD) | Total Found (mg/L) | Accuracy (%) | Precision (% RSD) | |||||
| SCG 1 | 992 ± 16 | - | - | 992 | 1056 ± 85 | 106.37 | 8.06 | 992 ± 16 | 100.00 | 1.58 |
| SCG 2 | 992 ± 16 | 500 | - | 1492 | 1563 ± 99 | 104.71 | 6.32 | 1436 ± 12 | 96.23 | 0.80 |
| SCG 3 | 992 ± 16 | 750 | - | 1742 | 1844 ± 104 | 105.85 | 5.64 | 1659 ± 21 | 95.23 | 1.24 |
| SCG 4 | 992 ± 16 | 1000 | - | 1992 | 2026 ± 93 | 101.71 | 4.57 | 1903 ± 3 | 95.50 | 0.17 |
| SCG 5 | 992 ± 16 | 1500 | - | 2492 | 2558 ± 163 | 102.64 | 6.35 | 2353 ± 45 | 94.42 | 1.93 |
| SCG 6 | 992 ± 16 | - | 1000 | 1992 | 2120 ± 150 | 106.43 | 7.08 | 1967 ± 24 | 98.75 | 0.59 |
| GR 1 | 1047 ± 21 | - | - | 1047 | 1077 ± 47 | 102.86 | 4.37 | 1047 ± 21 | 100.00 | 2.03 |
| GR 2 | 1047 ± 21 | 500 | - | 1547 | 1506 ± 112 | 97.39 | 7.45 | 1488 ± 5 | 96.23 | 0.32 |
| GR 3 | 1047 ± 21 | 750 | - | 1797 | 1747 ± 162 | 97.25 | 9.26 | 1736 ± 5 | 96.61 | 0.26 |
| GR 4 | 1047 ± 21 | 1000 | - | 2047 | 2013 ± 63 | 98.37 | 3.13 | 2018 ± 13 | 98.60 | 0.66 |
| GR 5 | 1047 ± 21 | 1500 | - | 2547 | 2528 ± 115 | 99.25 | 4.56 | 2419 ± 16 | 94.98 | 0.66 |
| GR 6 | 1047 ± 21 | - | 1000 | 2047 | 1972 ± 58 | 96.34 | 2.95 | 2010 ± 13 | 98.22 | 0.57 |
| MP 1 | 923 ± 8 | - | - | 923 | 942 ± 27 | 102.08 | 2.84 | 923 ± 8 | 100.00 | 0.90 |
| MP 2 | 923 ± 8 | 500 | - | 1423 | 1408 ± 38 | 98.94 | 2.72 | 1410 ± 12 | 99.13 | 0.82 |
| MP 3 | 923 ± 8 | 750 | - | 1673 | 1618 ± 92 | 96.72 | 5.68 | 1624 ± 18 | 97.07 | 1.14 |
| MP 4 | 923 ± 8 | 1000 | - | 1923 | 2048 ± 48 | 106.50 | 2.35 | 1861 ± 14 | 96.78 | 0.75 |
| MP 5 | 923 ± 8 | 1500 | - | 2423 | 2294 ± 65 | 94.68 | 2.83 | 2314 ± 21 | 95.50 | 0.91 |
| MP 6 | 923 ± 8 | - | 1000 | 1923 | 1965 ± 47 | 102.17 | 2.38 | 1906 ± 19 | 99.13 | 1.01 |
| Acid | Concentration Range of Standard Curve (mg/L) | Total Volume of Reaction (mL) | Accuracy (%) | Precision (% RSD) | References |
|---|---|---|---|---|---|
| Acetic-Caproic | 250–5000 | 4.3 | 95.51–105.11 | 1.67–5.58 | This study |
| Acetic | 250–5000 | 4.3 | 100.93 | 4.49 | This study |
| Acetic | 10–1200 | 14.7 | 82.10–104.2 | 1.3–14.0 | [40] |
| Acetic | 100–1400 | 49.7 | 95.76–115.35 | 1.38–27.63 | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aramrueang, N.; Lomwongsopon, P.; Boonsong, S.; Kingklao, P. Improved Spectrophotometric Method for Determination of High-Range Volatile Fatty Acids in Mixed Acid Fermentation of Organic Residues. Fermentation 2022, 8, 202. https://doi.org/10.3390/fermentation8050202
Aramrueang N, Lomwongsopon P, Boonsong S, Kingklao P. Improved Spectrophotometric Method for Determination of High-Range Volatile Fatty Acids in Mixed Acid Fermentation of Organic Residues. Fermentation. 2022; 8(5):202. https://doi.org/10.3390/fermentation8050202
Chicago/Turabian StyleAramrueang, Natthiporn, Passanun Lomwongsopon, Sasiprapa Boonsong, and Papassorn Kingklao. 2022. "Improved Spectrophotometric Method for Determination of High-Range Volatile Fatty Acids in Mixed Acid Fermentation of Organic Residues" Fermentation 8, no. 5: 202. https://doi.org/10.3390/fermentation8050202
APA StyleAramrueang, N., Lomwongsopon, P., Boonsong, S., & Kingklao, P. (2022). Improved Spectrophotometric Method for Determination of High-Range Volatile Fatty Acids in Mixed Acid Fermentation of Organic Residues. Fermentation, 8(5), 202. https://doi.org/10.3390/fermentation8050202








