Effects of Inoculation with Lactic Acid Bacteria on the Preservation of Nannochloropsis gaditana Biomass in Wet Anaerobic Storage and Its Impact on Biomass Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wet Anaerobic Storage
2.3. Compositional Analysis
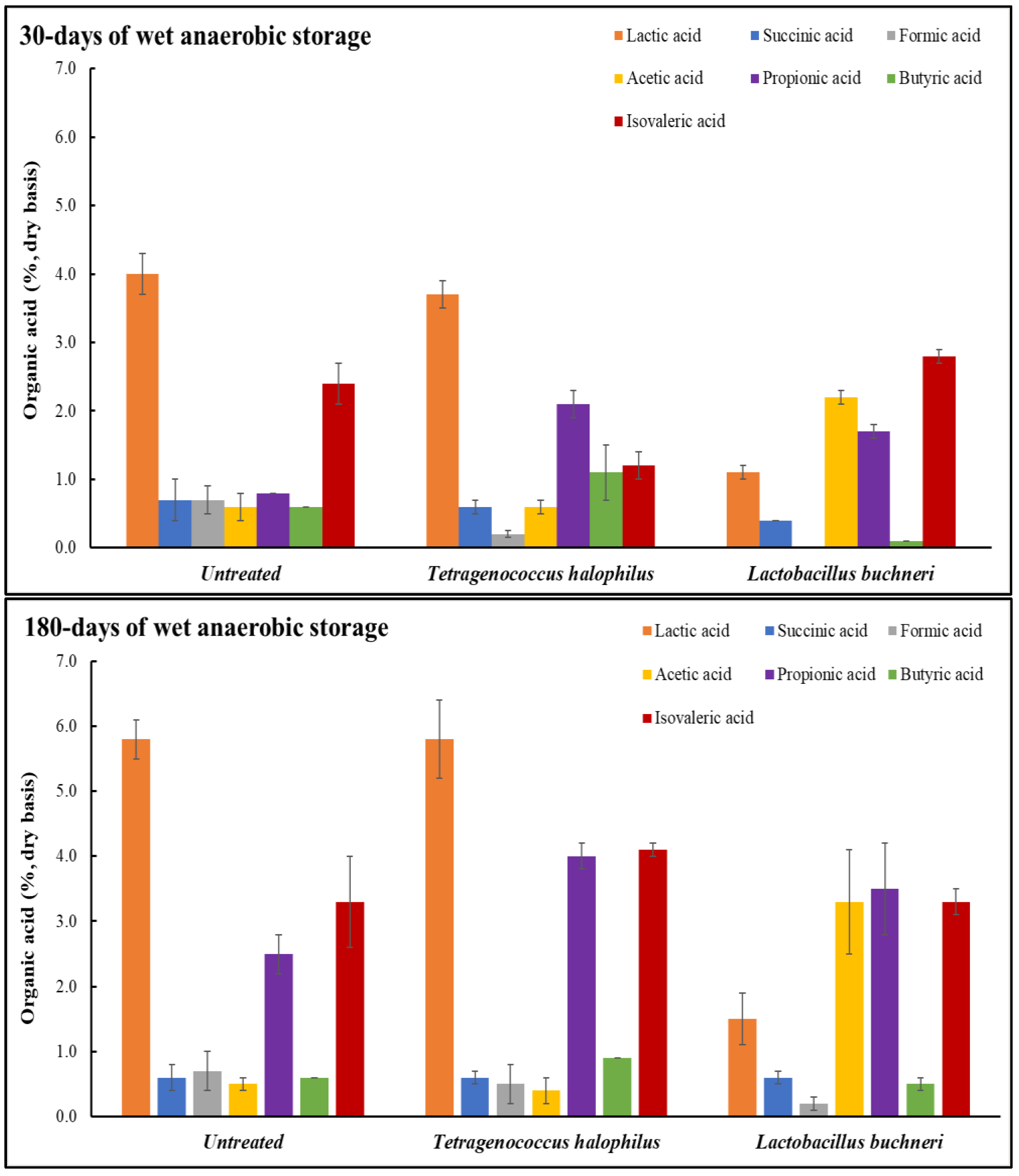
2.4. Organic Acid Determination
2.5. Energy, Proximate, and Ultimate Analysis
2.6. Statistical Analysis
3. Results
3.1. Storage Performance
3.2. Elemental and Proximate Compositions
3.3. Biochemical Composition
3.4. Carbohydrate Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wahlen, B.D.; Wendt, L.M.; Murphy, J.A.; Seibel, F. Mitigation of variable seasonal productivity in algae biomass through blending and ensiling: An assessment of compositional changes in storage. Algal Res. 2019, 42, 101584. [Google Scholar] [CrossRef]
- Wendt, L.M.; Kinchin, C.; Wahlen, B.D.; Davis, R.; Dempster, T.A.; Gerken, H. Assessing the stability and techno-economic implications for wet storage of harvested microalgae to manage seasonal variability. Biotechnol. Biofuels 2019, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Langholtz, M.H.; Stokes, B.J.; Eaton, L.M. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, Volume 1: Economic Availability of Feedstocks; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2016; 448p.
- Shakya, R.; Adhikari, S.; Mahadevan, R.; Shanmugam, S.R.; Nam, H.; Hassan, E.B.; Dempster, T.A. Influence of biochemical composition during hydrothermal liquefaction of algae on product yields and fuel properties. Bioresour. Technol. 2017, 243, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Knoshaug, E.P.; Davis, R.; Laurens, L.M.L.; Van Wychen, S.; Pienkos, P.T.; Nagle, N. Combined algal processing: A novel integrated biorefinery process to produce algal biofuels and bioproducts. Algal Res. 2016, 19, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Leow, S.; Dong, T.; Nagle, N.J.; Knoshaug, E.P.; Laurens, L.M.L.; Pienkos, P.T.; Guest, J.S.; Strathmann, T.J. Demonstration and Evaluation of Hybrid Microalgae Aqueous Conversion Systems for Biofuel Production. ACS Sustain. Chem. Eng. 2019, 7, 5835–5844. [Google Scholar] [CrossRef]
- Knoshaug, E.P.; Mohagheghi, A.; Nagle, N.J.; Stickel, J.J.; Dong, T.; Karp, E.M.; Kruger, J.S.; Brandner, D.G.; Manker, L.P.; Rorrer, N.A.; et al. Demonstration of parallel algal processing: Production of renewable diesel blendstock and a high-value chemical intermediate. Green Chem. 2018, 20, 457–468. [Google Scholar] [CrossRef] [Green Version]
- Wahlen, B.D.; Roni, M.S.; Cafferty, K.G.; Wendt, L.M.; Westover, T.L.; Stevens, D.M.; Newby, D.T. Managing variability in algal biomass production through drying and stabilization of feedstock blends. Algal Res. 2017, 24, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Wendt, L.M.; Wahlen, B.D.; Li, C.; Kachurin, G.; Ogden, K.L.; Murphy, J.A. Evaluation of a high-moisture stabilization strategy for harvested microalgae blended with herbaceous biomass: Part I—Storage performance. Algal Res. 2017, 25, 567–575. [Google Scholar] [CrossRef]
- Davis, R.; Kinchin, C.; Markham, J.; Tan, E.; Laurens, L.; Sexton, D.; Knorr, D.; Schoen, P.; Lukas, J. Process Design and Economics for the Conversion of Algal Biomass to Biofuels: Algal Biomass Fractionation to Lipid- and Carbohydrate-Derived Fuel Products; NREL/TP-5100-62368 United States 10.2172/1159351 NREL English; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2014; 110p.
- Nelson, J.A. Postharvest Degradation of Microalgae: Effect of Temperature and Water Activity; Utah State University: Logan, UT, USA, 2015. [Google Scholar] [CrossRef]
- Aliyu, A.; Lee, J.G.M.; Harvey, A.P. Microalgae for biofuels via thermochemical conversion processes: A review of cultivation, harvesting and drying processes, and the associated opportunities for integrated production. Bioresour. Technol. Rep. 2021, 14, 100676. [Google Scholar] [CrossRef]
- Pereira, A.P.; Dong, T.; Knoshaug, E.P.; Nagle, N.; Spiller, R.; Panczak, B.; Chuck, C.J.; Pienkos, P.T. An alternative biorefinery approach to address microalgal seasonality: Blending with spent coffee grounds. Sustain. Energy Fuels 2020, 4, 3400–3408. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage review: Factors affecting dry matter and quality losses in silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendt, L.M.; Wahlen, B.D.; Knoshaug, E.P.; Nagle, N.J.; Dong, T.; Spiller, R.; Panczak, B.; Van Wychen, S.; Dempster, T.A.; Gerken, H.; et al. Anaerobic Storage and Conversion of Microalgal Biomass to Manage Seasonal Variation in Cultivation. ACS Sustain. Chem. Eng. 2020, 8, 13310–13317. [Google Scholar] [CrossRef]
- Wahlen, B.D.; Wendt, L.M.; Murphy, A.; Thompson, V.S.; Hartley, D.S.; Dempster, T.; Gerken, H. Preservation of Microalgae, Lignocellulosic Biomass Blends by Ensiling to Enable Consistent Year-Round Feedstock Supply for Thermochemical Conversion to Biofuels. Front. Bioeng. Biotech. 2020, 8, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wychen, S.V.; Laurens, L. Determination of Total Carbohydrates in Algal Biomass: Laboratory Analytical Procedure (LAP); No. NREL/TP-5100-60957; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2016.
- Van Wychen, S.; Ramirez, K.; Laurens, L.M.L. Determination of Total Lipids as Fatty Acid Methyl Esters (FAME) by In Situ Transesterification: Laboratory Analytical Procedure (LAP); NREL/TP-5100-60958 United States 10.2172/1118085 NREL English; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2016.
- Lourenço, S.O.; Barbarino, E.; Lavín, P.L.; Lanfer Marquez, U.M.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- ASTM D5865/D5865M-19; Standard Test Method for Gross Calorific Value of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D7582-15; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D5373-10; Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM D4239-10; Standard Test Method for Sulfur in the Analysis Sample of Coal and Coke Using High-Temperature Tube Furnace Combustion. ASTM International: West Conshohocken, PA, USA, 2010.
- Kumazawa, T.; Nishimura, A.; Asai, N.; Adachi, T. Isolation of immune-regulatory Tetragenococcus halophilus from miso. PLoS ONE 2018, 13, e0208821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, P.; Henderson, N.; Heron, S. The Biochemistry of Silage, 2nd ed.; Cambrian Printers, Ltd.: Aberystwyth, UK, 1981. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Muck, R.E. New trends and opportunities in the development and use of inoculants for silage. FEMS Microbiol. Rev. 1996, 19, 53–68. [Google Scholar] [CrossRef]
- Krishnan, A.; Likhogrud, M.; Cano, M.; Edmundson, S.; Melanson, J.B.; Huesemann, M.; McGowen, J.; Weissman, J.C.; Posewitz, M.C. Picochlorum celeri as a model system for robust outdoor algal growth in seawater. Sci. Rep. 2021, 11, 11649. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K.; Zondlo, J.W. Pyrolysis of dedicated bioenergy crops grown on reclaimed mine land in West Virginia. J. Anal. Appl. Pyrolysis 2017, 123, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Oginni, O.; Singh, K. Pyrolysis characteristics of Arundo donax harvested from a reclaimed mine land. Ind. Crops Prod. 2019, 133, 44–53. [Google Scholar] [CrossRef]
- Ren, X.; Meng, J.; Chang, J.; Kelley, S.S.; Jameel, H.; Park, S. Effect of blending ratio of loblolly pine wood and bark on the properties of pyrolysis bio-oils. Fuel Process. Technol. 2017, 167, 43–49. [Google Scholar] [CrossRef]
- Oginni, O.; Singh, K. Effect of carbonization temperature on fuel and caffeine adsorption characteristics of white pine and Norway spruce needle derived biochars. Ind. Crops Prod. 2021, 162, 113261. [Google Scholar] [CrossRef]
- Oasmaa, A.; Solantausta, Y.; Arpiainen, V.; Kuoppala, E.; Sipilä, K. Fast Pyrolysis Bio-Oils from Wood and Agricultural Residues. Energy Fuels 2010, 24, 1380–1388. [Google Scholar] [CrossRef]
- Laurens, L.M.L. Summative Mass Analysis of Algal Biomass—Integration of Analytical Procedures. NREL 2013, 303, 275–3000. [Google Scholar]
- Templeton, D.W.; Laurens, L.M.L. Nitrogen-to-protein conversion factors revisited for applications of microalgal biomass conversion to food, feed, and fuel. Algal Res. 2015, 11, 359–367. [Google Scholar] [CrossRef] [Green Version]
| Treatment | Storage Duration (Days) | Dry Matter Loss (% Dry Basis) | pH | Organic Acid (% Dry Basis) |
|---|---|---|---|---|
| Untreated | 30 | 6.2 a ± 1.1 | 4.34 c ± 0.10 | 9.8 ab ± 0.7 |
| Tetragenococcus halophilus | 6.5 a ± 0.3 | 4.60 b ± 0.03 | 9.8 ab ± 0.4 | |
| Lactobacillus buchneri | 4.5 b ± 0.2 | 4.76 a ± 0.01 | 8.3 b ± 0.3 | |
| Untreated | 180 | 9.3 a ± 0.8 | 4.29 b ± 0.16 | 14.0 b ± 0.4 |
| Tetragenococcus halophilus | 8.8 a ± 0.5 | 4.29 b ± 0.10 | 16.7 a ± 1.3 | |
| Lactobacillus buchneri | 7.1 a ± 1.6 | 4.77 a ± 0.12 | 13.2 b ± 1.0 |
| Treatment | Storage Duration | Ash (% d.b.) | C (% d.b.) | H (% d.b.) | N (% d.b.) | O (% d.b.) | S (% d.b.) | HHV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|
| Unstored | 0 | 7.0 | 57.6 c ± 0.6 | 9.1 b ± 0.1 | 2.5 b ± 0.0 | 23.5 a ± 0.7 | 0.31 a ± 0.02 | 28.0 ab ± 0.2 |
| Untreated | 30 | 7.0 | 58.3 c ± 0.1 | 9.2 b ± 0.0 | 2.7 b ± 0.0 | 22.5 b ± 0.1 | 0.35 a ± 0.01 | 27.8 bc ± 0.1 |
| L. buchneri | 7.0 | 58.3 c ± 0.1 | 9.0 c ± 0.1 | 2.9 ab ± 0.0 | 22.5 b ± 0.1 | 0.32 a ± 0.03 | 28.2 a ± 0.2 | |
| T. halophilus | 8.1 | 58.4 ab ± 0.1 | 9.1 b ± 0.1 | 3.0 a ± 0.0 | 21.1 c ± 0.2 | 0.29 a ± 0.02 | 27.6 c ± 0.2 | |
| Untreated | 180 | 7.3 | 59.1 a ± 0.1 | 9.3 b ± 0.0 | 2.8 b ± 0.0 | 21.2 c ± 0.1 | 0.32 a ± 0.04 | 28.2 a ± 0.2 |
| L. buchneri | 8.4 | 58.9 a ± 0.2 | 9.3 b ± 0.0 | 3.0 a ± 0.0 | 20.2 d ± 0.2 | 0.29 a ± 0.03 | 28.5 a ± 0.2 | |
| T. halophilus | 7.7 | 58.9 a ± 0.3 | 9.4 a ± 0.0 | 3.0 a ± 0.0 | 20.6 cd ± 0.3 | 0.36 a ± 0.05 | 28.5 a ± 0.3 |
| Treatment | Storage Duration | Carbohydrates (%, d.b.) | Protein (%, d.b.) | FAME (%, d.b.) |
|---|---|---|---|---|
| Unstored | 0 | 21.4 a ± 0.8 | 12.1 c ± 0.2 | 41.1 c ± 0.3 |
| Untreated | 30 | 16.6 b ± 0.1 | 12.7 c ± 0.1 | 45.1 a ± 0.3 |
| Lactobacillus buchneri | 16.7 b ± 0.2 | 13.9 b ± 0.1 | 40.6 c ± 0.6 | |
| Tetragenococcus halophilus | 13.9 c ± 0.2 | 14.2 a ± 0.1 | 46.1 a ± 2.1 | |
| Untreated | 180 | 9.7 e ± 0.4 | 13.3 b ± 0.1 | 46.9 a ± 0.8 |
| Lactobacillus buchneri | 11.7 d ± 0.1 | 14.3 a ± 0.1 | 44.3 b ± 0.8 | |
| Tetragenococcus halophilus | 9.1 e ± 0.1 | 14.5 a ± 0.1 | 44.5 a ± 2.1 |
| Treatment | Storage Days | Glucose (%, d.b.) | Xylose (%, d.b.) | Galactose (%, d.b.) | Arabinose (%, d.b.) | Mannose (%, d.b.) |
|---|---|---|---|---|---|---|
| Unstored | 0 | 15.2 a ± 0.6 | 0.63 c ± 0.04 | 4.68 a ± 0.25 | 0.39 ab ± 0.02 | 0.69 a ± 0.06 |
| Untreated | 30 | 10.4 c ± 0.03 | 0.70 b ± 0.01 | 4.46 ab ± 0.09 | 0.40 ab ± 0.02 | 0.77 a ± 0.04 |
| L. buchneri | 10.8 b ± 0.05 | 0.66 bc ± 0.01 | 4.17 c ± 0.05 | 0.40 ab ± 0.04 | 0.64 a ± 0.04 | |
| T. halophilus | 8.5 e ± 0.02 | 0.61 c ± 0.02 | 3.84 de ± 0.05 | 0.32 b ± 0.10 | 0.64 a ± 0.1 | |
| Untreated | 180 | 4.2 g ± 0.1 | 0.63 bc ± 0.03 | 3.80 e ± 0.05 | 0.40 ab ± 0.04 | 0.74 a ± 0.1 |
| L. buchneri | 5.5 f ± 0.03 | 0.83 a ± 0.02 | 4.04 cd ± 0.09 | 0.52 a ± 0.06 | 0.76 a ± 0.01 | |
| T. halophilus | 3.6 h ± 0.04 | 0.65 bc ± 0.02 | 3.92 de ± 0.01 | 0.31 b ± 0.03 | 0.64 a ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oginni, O.; Wahlen, B.; Wendt, L.; Walton, M.; Dempster, T.; Gerken, H. Effects of Inoculation with Lactic Acid Bacteria on the Preservation of Nannochloropsis gaditana Biomass in Wet Anaerobic Storage and Its Impact on Biomass Quality. Fermentation 2022, 8, 159. https://doi.org/10.3390/fermentation8040159
Oginni O, Wahlen B, Wendt L, Walton M, Dempster T, Gerken H. Effects of Inoculation with Lactic Acid Bacteria on the Preservation of Nannochloropsis gaditana Biomass in Wet Anaerobic Storage and Its Impact on Biomass Quality. Fermentation. 2022; 8(4):159. https://doi.org/10.3390/fermentation8040159
Chicago/Turabian StyleOginni, Oluwatosin, Bradley Wahlen, Lynn Wendt, Michelle Walton, Thomas Dempster, and Henri Gerken. 2022. "Effects of Inoculation with Lactic Acid Bacteria on the Preservation of Nannochloropsis gaditana Biomass in Wet Anaerobic Storage and Its Impact on Biomass Quality" Fermentation 8, no. 4: 159. https://doi.org/10.3390/fermentation8040159
APA StyleOginni, O., Wahlen, B., Wendt, L., Walton, M., Dempster, T., & Gerken, H. (2022). Effects of Inoculation with Lactic Acid Bacteria on the Preservation of Nannochloropsis gaditana Biomass in Wet Anaerobic Storage and Its Impact on Biomass Quality. Fermentation, 8(4), 159. https://doi.org/10.3390/fermentation8040159






