Abstract
Eukaryotic algae represent a highly heterogeneous group in terms of organization, lifestyle, and metabolic capabilities. Unicellular green microalgae are capable of biohydrogen production through direct and indirect photolysis as well as dark fermentation. Most algae hydrogen studies focus on axenic algal cultures, although these are difficult and expensive to maintain for continuous operation. Moreover, the complex interplays and metabolic fluxes between algae and bacteria in natural ecosystems provide a number of clear biological and technological benefits to large-scale functional algae-based systems. Two green algae species from the Chlamydomonas and Chlorella genera were used to engineer stable synthetic communities by incorporating a starch-degrading bacterium from the Bacillus genus into the inter-kingdom consortium. Continuous photoheterotrophic biohydrogen production was achieved by elaborating an appropriate algal–bacterial ratio and fine-tuning the culture conditions for the synthetic consortia. Medium with starch as only carbon source served as a simple model of cheap substrate for algal hydrogen generation. The engineered pairwise algal–bacterial associations showed increased biomass and biohydrogen yield compared to the axenic control conditions. Chlorella sp. MACC-360 produced a significantly higher amount of hydrogen when both the bacterium partner and starch were added to the media compared to the axenic algae. Continuous, elevated algal hydrogen production was achieved in media supplemented with 8 g L−1 starch as sole carbon source when carefully selected initial cell number values were used for the Chlorella sp. MACC-360–B. amlyloliquefaciens co-cultures.
1. Introduction
As a renewable energy source, hydrogen (H2) is a promising alternative to fossil fuels. Hydrogen is a clean and sustainable energy carrier, and no greenhouse gases are generated at the site of use [1,2,3]. Most biologically produced hydrogen in the biosphere is evolved in microbial fermentation processes. Biological hydrogen production is an attractive renewable method for energy generation, which can benefit from both photosynthesis and fermentation processes. Certain green algae, blue green algae (cyanobacteria), fermentative and photosynthetic bacteria, as well as archaea, are capable of hydrogen photoproduction using either light energy and/or various organic substrates [4,5]. Eukaryotic green algae are capable of absorbing sunlight and directly converting solar energy into hydrogen gas using Fe-Fe hydrogenases [6]. This process takes advantage of the photosynthetic system of the algae, which links water splitting to hydrogen production [7,8,9]. Chlamydomonas reinhardtii is the most studied unicellular green alga capable of hydrogen photoproduction. C. reinhardtii can produce hydrogen through two light-dependent pathways and through dark fermentation. Direct photolysis is able to channel the protons and electrons (from the water-splitting) to their Fe-Fe hydrogenases instead of utilizing them to create photosynthates by fixing and converting carbon dioxide (CO2) into organic carbon sources. Moreover, indirect biophotolysis intends to eliminate the oxygen sensitivity of the hydrogenases by separating the hydrogen-producing reactions from the oxygen-evolving ones. Finally, algae can catabolize endogenous carbohydrates or secondary metabolites through dark fermentation under anaerobic conditions, generating organic acids, ethanol, CO2, and hydrogen gas [8,10,11]. Hydrogen production using sulfur-deprived photoheterotrophic cultures of C. reinhardtii is the most studied and widespread approach [12,13].
The vast majority of research on biohydrogen production were conducted using axenic algae cultures or pure bacterial cultures. Recently, the use of artificial microbial co-cultures and consortia, composed of pre-defined and characterized species, has attracted particular interest in the biohydrogen research and industry. Co-cultures can perform complex functions, such as simultaneous pentose or hexose consumption, which generally cannot be performed by a single species [14]. Being potentially more robust to changes in environmental conditions, co-cultures can resist periods of nutrient limitation better; what is typically combined with the exchange of metabolites between the different partners. While a number of studies describe hydrogen-producing systems relying on bacterial co-cultures, only a few studies applied inter-kingdom communities, where selected bacterial strains enhance the hydrogen production of eukaryotic algae [7,8,15,16,17,18,19].
Biohydrogen production from organic waste sources represents an emerging area of bioenergy production. Agricultural and domestic wastes can be used for biohydrogen production through the combination of dark fermentation and photolysis called photofermentation [18]. Starch is a major and common component of agricultural by-products or crop wastes (corn, rice, potato, sweet potato, etc.) [4,5,7,8,9,20]. The production of biohydrogen by eukaryotic microalgae through photofermentation is of high interest, since it generates hydrogen from the most plentiful resources, light and water. However, the adaptation of the algae to an anaerobic atmosphere is an important prerequisite. It is important to note that hydrogen production through photofermentation is considered ineffective since the simultaneously produced oxygen inhibits the algal hydrogenase enzyme. A possible solution to overcome this barrier is the application of an appropriately selected, actively respiring bacterial partner able to grow and propagate in the media designed for algae cultivation.
Carbohydrates such as glucose and starch are especially good substrates for most of the hydrogen-producing fermentative microorganisms [20]. A number of studies investigated the possibility of biohydrogen production using starch as the main substrate, and both dark and photofermentation approaches were reported using either mono- or mixed bacterial cultures [1,18,20,21,22,23]. However, in most cases the hydrogen conversion ratio was found to be insufficient [22,23]. Hydrogen was produced from the waste flows of a starch factory under different conditions in respect to reactor design, substrate composition, pH value, inoculum, temperature, and bacterial partner [24]. Biohydrogen production from organic wastes such as whey was reported where selected Rhodopseudomonas sp. (BHU strains 1–4) was used for fermentation [25]. Bacillus licheniformis was also shown to produce hydrogen when grown on wheat grain slurries [26]. Isolates of Bacillus spp. (Bacillus subtilis, Bacillus coagulans, Bacillus cereus, B. licheniformis, and Bacillus amyloliquefaciens) were shown to be the primary producers of α-amylases [27,28,29]. The α-amylase enzyme of B. amyloliquefaciens plays the main role in the hydrolysis of complex carbohydrates (including starch) [30]. B. amyloliquefaciens displays higher ability to secrete proteins and higher growth rate than other Bacillus spp. [29]. A continuous-flow anaerobic fermentation system was elaborated to produce hydrogen from starch using phosphate-buffered medium containing cassava starch (15 g/L) as the feed [31]. A hydrogen content of nearly 50% (i.e., a CO2/H2 ratio of 1.0), and a H2 yield of 0.97–1.43 mol of H2/mol of hexose, was achieved through continuous operation. The hydrogen production rate observed in this work was significantly higher than those indicated in comparable studies that used starch to produce hydrogen via dark fermentation.
Our goal was to achieve sustainable, continuous algal hydrogen production through photofermentation using starch as the sole carbon source. To reach this objective, it was essential to select appropriate eukaryotic green algae strains for the co-cultivation with B. amyloliquefaciens, to optimize algal–bacterial ratio and density of the starting co-cultures, as well as to fine-tune the gas-to-liquid ratio in the applied fed-batch lab-scale photobioreactors. It is important to note that, in our system, the specific algal hydrogen production was in the focus. The lack of hydrogenase enzymes in the partner bacterium was an important criterion, so that the applied B. amyloliquefaciens did not directly contribute to the photofermentative hydrogen yield.
2. Materials and Methods
2.1. Growth of Algae Strains
Chlamydomonas reinhardtii cc124 (from Chlamydomonas Resource Center) and Chlorella sp. MACC-360 (from the Mosonmagyaróvár Algal Culture Collection (MACC)) green algae were used for the experiments. Algae cultures were pre-grown on TAP (TRIS-Acetate-Phosphate) plates at 25 °C under illumination. Algae colonies were harvested from TAP plates and transferred into liquid TAP medium. Microalgae were cultured for a period of 7 days in different volumes (50, 100, 150, 200, and 250 mL) in closed 300 mL Erlenmeyer flasks at 25 °C, shaken at 180 rpm, and incubated under 50 µmol m−2 s−1 light density of 16 h light: 8 h dark photoperiod. Algal density (OD680) was measured using a HIDEX Sense microplate reader (Hidex, Turku, Finland).
2.2. Bacterial Partner
Bacillus amyloliquefaciens (DSM 1060) was selected to use in the algal–bacterial co-culture experiments. B. amyloliquefaciens was pre-grown on LB (Luria-Bertani medium) plates at 30 °C, then harvested and transferred into liquid LB medium for overnight growth. Bacterial density (OD600) was measured using a HIDEX Sense microplate reader.
2.3. Algal and Bacterial Co-Cultures
2.3.1. General Culture Analyses
Algae cultures were pre-cultured in TAP medium in 250 mL Erlenmeyer flasks at 25 °C, shaken at 180 rpm and incubated under 50 µmol m−2 s−1 light density. Algae suspensions were generated using fresh cultures by centrifugation and re-suspending the cells in fresh TAP medium. All bacterial cultures were pre-cultured in LB then re-suspended in TAP to prepare concentrated bacterial suspensions. TAP or TRIS-Phosphate media (TP was the modified TAP where acetic acid was replaced by HCl to obtain pH 7) with various starch (starch from potatoes) concentrations of 0, 4, 8, 16, 24, and 32 g L−1 (corresponding to 0, 24, 49, 98, 148, and 197 mM) were prepared.
For hydrogen measurement experiments axenic algae cultures, pure bacterial suspensions, as well as the various co-cultures, were established in 40 mL Hypo-Vial serum bottles with tightly closed butyl rubber stoppers and aluminum caps. Co-cultures were prepared as follows: 1 mL algae suspension was measured into the 40 mL bottles, in which the cell number of C. reinhardtii cc124 was 6 × 106 mL−1 culture, while the cell number of Chlorella sp. MACC-360 was 5 × 107 mL−1 culture. The final optical densities of the axenic alga suspensions were set to 0.7 (OD680). Moreover, 1 mL bacterial suspension was measured into the bottles, in which the cell number of B. amyloliquefaciens bacterial partner was either 2 × 105 or 5 × 105 mL−1 culture. The final optical densities of bacterial solutions were set to either 0.07 (OD600) or 0.175 (OD600). The starch-containing media (TAP or TP) were added to the algal–bacterial co-cultures to reach a final volume of 30 mL in all bottles. Axenic algae liquid cultures and pure bacterial suspensions contained algae or bacterial suspension, respectively, and starch-containing media, while co-cultures contained both algal and bacterial suspensions and starch-containing media. All mono- and co-cultures were incubated under 50 µmol m−2 s−1 light density at 25 °C shaken at 180 rpm. Samples for gas and liquid analysis were taken daily. Bottles were opened for 5 min under sterile conditions in every 24 h (aeration) to release the H2 partial pressure and replace the gas composition in the headspace with atmospheric air [32]. The H2 production and O2 levels in the headspace were measured before aeration of the bottles. All experiments were performed in three replicates.
2.3.2. Fed-Batch Cultures
All fed-batch algae cultures and algal–bacterial co-cultures were maintained at 25 °C shaken at 180 rpm and incubated in 50 µmol m−2 s−1 light intensity. B. amyloliquefaciens was used as partner bacterium, co-cultures were prepared as described above. Half of the mono- and co-cultures (15 mL) were taken out from the bottles in every 72 h and 15 mL fresh media was added to the axenic algae cultures, while 14 mL fresh medium and 1 mL bacterial suspension were added to the algal–bacterial co-cultures. All mono- and co-cultures were sampled and analyzed for hydrogen and oxygen production as well as for starch degradation every 24 h.
2.4. Gas Phase Analyses
The hydrogen and oxygen levels in the headspace of the Hypo-Vial bottles were routinely measured using gas chromatography. An Agilent 7890A gas chromatograph (Agilent, Santa Clara, CA, United States) equipped with a thermal conductivity detector and a HP-Molsieve column (length 30 m, diameter 0.320 mm, film 12.0 µm) was used for the hydrogen and oxygen measurements. The temperature of the injector, the TCD detector, and column were kept at 170 °C, 190 °C, and 60/55 °C, respectively. Samples of 50 µL volumes were analyzed in splitless mode. Three biological replicates were used for each measurement. Hydrogen and oxygen calibration curves were used to determine accurate gas volumes.
2.5. Starch Measurement
A spectrophotometric-based measurement was elaborated for the quantitative analysis of starch content in the culture medium. 1 mL samples were taken from the mono- and co-cultures for starch analysis. Whole culture samples were centrifuged at 13,300 rpm for 5 min, then 40 µL were taken from the supernatants, which were diluted up to 5× by adding distilled water. These final volumes of 200 µL were transferred into the wells of a 96-well microplate. 50 µL of Lugol’s solution was added to each well, the suspension was mixed, the absorbance of the solution was read at 580 nm using a HIDEX Sense microplate reader, and the starch concentration was calculated from a starch calibration curve.
2.6. Microscopy Analysis and Cell Number Determination
2.6.1. Morphological Studies
The algae cells were observed using a Zeiss Observer Z1 microscope (Carl Zeiss AG, Oberkochen, Germany). 50 algae cells per culture were measured to calculate average area (cell size) and diameter data from optical microscopy images. Confocal Laser Scanning Microscopy (CLSM, Olympus Fluoview FV-300, Olympus Optical Co., Ltd., Tokyo, Japan) was also used in this study. 50 μL cultures were taken to Eppendorf tubes and stained with Calcofluor White (CFW) and Concanavalin A (Con A), both for a final concentration of 10 µg/μL. After 30 min incubation in dark, the samples (8 μL) were spotted on microscope slides and covered with 2% (w/v) agar slice and observed with an Olympus Fluoview FV 1000 confocal laser scanning microscope with 40× magnification objective. Sequential scanning was used to avoid crosstalk of the fluorescent dyes and chlorophyll autofluorescence.
2.6.2. Determination of Algal and Bacterial Cell Numbers
The number of algal cells were counted with a Luna-FL instrument (Logos Biosystems, Villeneuve d’Ascq, France) using the “Fluorescence Cell Counting mode”. To determine the cell numbers, 100 μL culture samples were taken from the flasks and were diluted to a volume of 1 mL using distilled water. Next, 10 μL of the diluted cultures were placed in the Luna slides and cell numbers were determined with the fluorescent algae protocol. The number of living bacterial cells were counted on LB plates. The colony forming units (CFU) were counted using serial dilutions. All experiments were repeated three times.
2.7. Chlorophyll Measurements
Algae chlorophyll measurements were conducted for normalization. 1 mL culture samples were taken and centrifuged at 13,300 rpm for 3 min and re-suspended in 1 mL 96% ethanol. After 24 h incubation at 4 °C, and being centrifuged at 13,300 rpm for 3 min, the optical densities were measured at 664, 647, and 750 nm using a HIDEX Sense microplate reader. Chlorophyll content was then quantified using the equations described in Porra et al. [18].
2.8. Statistical Analysis
All the graphs, calculations, and statistical analyses were performed using GraphPad Prism software version 8.0 for Windows PC (GraphPad Software, San Diego, CA, USA). All data were submitted to one-way analysis of variance (ANOVA). All analyses were performed at 5% statistical significance level.
3. Results
3.1. Algal Hydrogen Production in Acetate-Containing Medium
Algal long-term hydrogen evolution approach was conducted in starch-containing TAP medium. Half of the culture broth (15 mL) was exchanged for fresh medium containing starch (in different concentrations) with an interval of 72 h. Only the medium was refreshed for axenic algae cultures, while bacterial partner was also re-supplied in co-cultures. The final optical densities of partner bacteria were always set to OD600: 0.07.
Hydrogen production of C. reinhardtii cc124 and Chlorella sp. MACC-360 green algae was investigated in detail. Firstly, the B. amyloliquefaciens bacterial partner was able to completely degrade the added starch within 48 h and to grow continuously in starch-containing TAP media (Figure S5). In the case of C. reinhardtii cc124, a moderate increase in cell number was detected in starch-containing TAP medium (Figure S5b). C. reinhardtii cc124 was not able to degrade starch, moreover, this alga showed a moderate starch production in TAP media in the first 48 h of growth (Figure S5a). Chlorella sp. MACC-360 co-cultures in TAP fully consumed the added starch by the end of the 3rd day, which correlated well with the daily hydrogen production pattern achieved by this alga (Figure S5a). Importantly, acetic acid was also totally consumed on the 2nd day by Chlorella sp. MACC-360, which also correlates with the daily hydrogen yields in TAP medium without starch (as previously published by our group in [11]). Thus, hydrogen production was strongly influenced by the presence of acetic acid and by the bacterial partner in TAP medium.
C. reinhardtii cc124 produced moderate amount of hydrogen regardless the presence of bacterial partner and starch (Figure 1a). The average daily algal hydrogen production under axenic conditions was 26.1 mL L−1, and very similar yields were obtained in co-cultures (27.1 mL L−1) (Figure 1a).
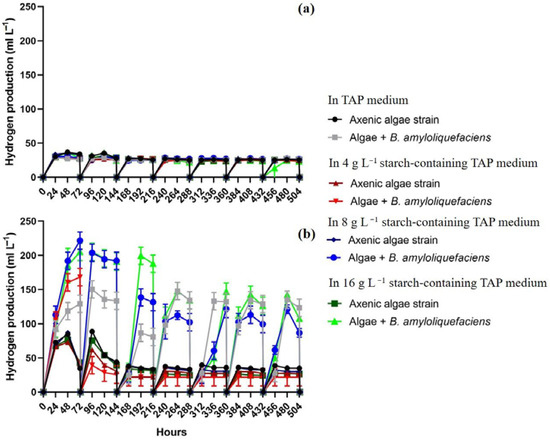
Figure 1.
Hydrogen production of C. reinhardtii cc124 (a) and of Chlorella sp. MACC-360 (b) in fed-batch experiments in TAP media (the initial bacterial OD600 of 0.07). Hydrogen was measured every 24 h in the headspace and 5 min aerated after each measurement every 72 h. Error bars are standard deviations based on three replicates.
Chlorella sp. MACC-360 produced higher amounts of hydrogen compared to that of C. reinhardtii cc124, especially when co-cultivated with B. amyloliquefaciens (Figure 1b). Hydrogen production of Chlorella sp. MACC-360 was strongly enhanced by the presence of B. amyloliquefaciens both in TAP and in TAP containing starch (Figure 1b). The average daily algal hydrogen yield of Chlorella sp. MACC-360 in co-cultures was approximately three times higher than in axenic algae cultures in TAP, containing at least 8 g L−1 starch (Figure 1b). The maximum daily hydrogen production was observed on the second and third days of each cycle in the Chlorella sp. MACC-360-B. amyloliquefaciens co-culture cultivated in TAP medium supplemented with starch (although minor differences were observed among the samples containing different amounts of starch). In the first three cycles (the first 216 h), the hydrogen production of Chlorella sp. MACC-360 co-cultures in TAP containing at least 8 g L−1 starch was higher compared to co-cultures in starch-free TAP medium. In the following cycles (after 216 h), the hydrogen production remained at a similar level in all co-cultured samples, including co-cultures in starch-free TAP. Thus, algal–bacterial co-cultures showed continuous, stable hydrogen production where the hydrogen production was not influenced by starch addition in TAP medium. Again, hydrogen production was influenced by the presence of the bacterial partner in TAP medium. The addition of 4 g L−1 starch to the TAP increased algal hydrogen production only in the first 3 days, and in later stages of the fed-batch experiments no enhancement could be observed. However, stable and continuously high algal hydrogen production was detected in almost all co-cultures throughout the 21-day long fed-batch experiments, except when 4 g L−1 starch was added and re-supplied in the co-culture TAP medium (Figure 1b). The B. amyloliquefaciens partner was able to completely metabolize starch within 48 h in all co-cultures (Figure 2). In the case of axenic Chlorella sp. MACC-360, slow gradual starch accumulation was detected during the first three days. In the first two days Chlorella sp. MACC-360 totally consumed acetic acid, then Chlorella sp. MACC-360 was able to utilize a significant amount of starch even in axenic cultures, however, this was still insufficient to increase hydrogen production [11]. Thus, the applied bacterial partner is supposed to have an important role in utilizing excessive starch and the generated excess reducing power might have been utilized in algal (Chlorella) hydrogen production.
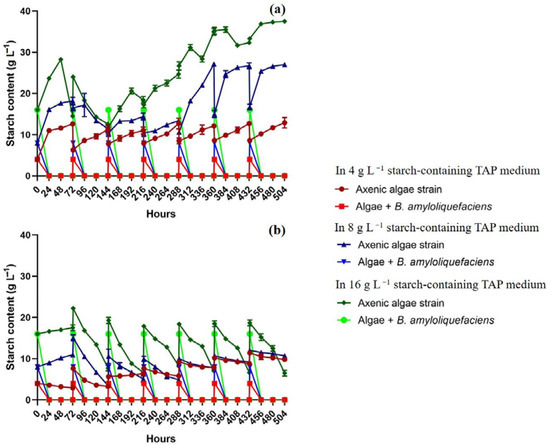
Figure 2.
Starch content in the axenic and co-cultures of C. reinhardtii cc124 (a) and Chlorella sp. MACC-360 (b) in fed-batch experiments (the initial bacterial OD600 of 0.07). Starch content was measured every 24 h. Error bars are standard deviations based on three replicates.
3.2. Algal Hydrogen Production in Acetate-Free Medium
Algae monocultures and algal–bacterial co-cultures were tested for continuous biohydrogen production in acetate-free TP medium using starch as the sole organic carbon source. The fresh TP media, starch, and the bacterium B. amyloliquefaciens were added at 72-h intervals similarly to the fed-batch experiments performed in TAP, and the final optical density of the partner bacteria was set to OD600: 0.07 in the first experiments with acetate-free medium.
Hydrogen production of C. reinhardtii cc124 and Chlorella sp. MACC-360 green algae was investigated in acetate free medium (TP, which is a modified TAP without acetate). Again, the B. amyloliquefaciens bacterial partner was able to completely degrade the added starch within 48 h and to grow continuously in starch-containing TP media (Figure S6). Interestingly, none of the axenic algae cultures were able to degrade starch in TP medium and the cell number of both algae species decreased continuously in starch-containing TP medium (Figure S6a,b). Neither C. reinhardtii cc124 nor Chlorella sp. MACC-360 were capable of producing hydrogen under axenic conditions in TP (Figure 3). In the case of C. reinhardtii cc124 the algae were not able to produce hydrogen even when co-cultured with B. amyloliquefaciens and supplemented with starch in TP (Figure 3a), while hydrogen production by Chlorella sp. MACC-360 was observed at starch concentrations of 4, 8, and 16 g L−1 when co-cultured with B. amyloliquefaciens (Figure 3b). Thus, C. reinhardtii cc124 was not able to produce hydrogen using hydrolized starch under the applied conditions, while Chlorella sp. MACC-360 was suitable to establish a continuous starch-to-biohydrogen process (Figure 3a,b). Glucose is generated during starch degradation by B. amyloliquefaciens, which can be utilized by Chlorella sp. MACC-360 for both growth and hydrogen production, while C. reinhardtii cc124 is known to be unable to use glucose as carbon source [16,32,33]. In the fed-batch system the Chlorella sp. MACC-360 co-culture showed the most stable hydrogen production in TP medium containing 8 g L−1 starch. Unfortunately, the amount of generated hydrogen was rather low and after 12 days the algae cells stopped producing hydrogen. The algae cells were actively propagating, the algae cell numbers as well as the bacterial CFU (colony forming unit) were stable and growing (Figure S7a,b). CLSM analysis also revealed that the algae cells were alive and intact (Figure S8e–h). The size (diameter) of the Chlorella sp. MACC-360 algae cells were similar to what was observed in TAP medium (either axenic or in co-culture) (Figure S8a–d). The combined addition of starch and B. amyloliquefaciens to the TP medium clearly enhanced algae growth and resulted in elevated algae cell number (Figures S7 and S8h). The images of the culture bottles also confirmed the viability and healthy status of the co-cultured Chlorella sp. MACC-360 algae (Figure 4).
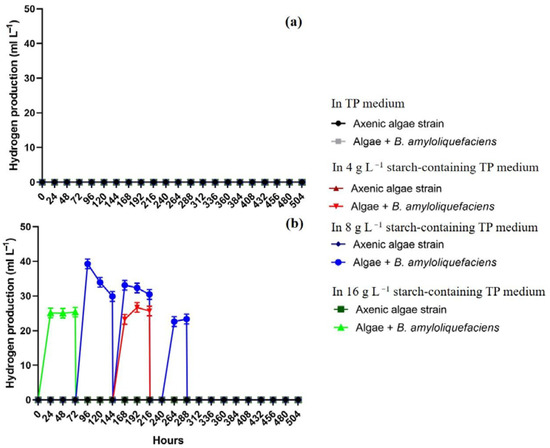
Figure 3.
Continuous hydrogen production of C. reinhardtii cc124 (a) and Chlorella sp. MACC-360 (b) in acetate-free TP medium (the initial bacterial OD600 of 0.07). Hydrogen was measured in the headspace at every 24 h. Bottles were aerated for 5 min after hydrogen measurement at every 72 h. Error bars are standard deviations based on three replicates.
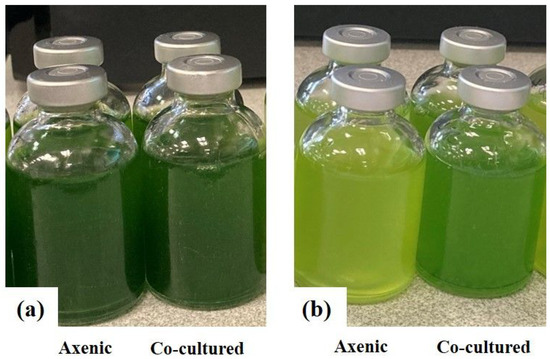
Figure 4.
Chlorella sp. MACC-360 cultivation in TAP (a) and TP (b) media supplemented with 8 g L−1 starch (the initial bacterial OD600 of 0.07). Axenic and co-cultured algae are shown. Images were taken on the 7th day of growth.
Interestingly, when the amount of B. amlyloliquefaciens bacterial partner was increased in the co-cultures (to a starting bacterial OD600: 0.175 instead of the earlier applied OD600: 0.07), the hydrogen production of Chlorella sp. MACC-360 became stable and continuous in the acetate-free TP medium containing 8 g L−1 starch (Figure 5).
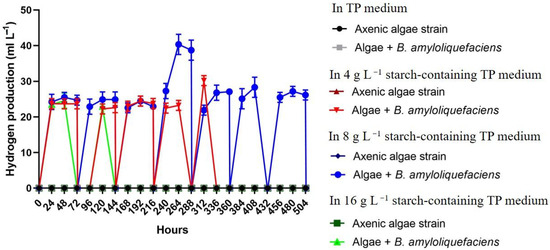
Figure 5.
Continuous hydrogen production of Chlorella sp. MACC-360 in acetate-free TP medium supplemented with elevated amount of bacterial partner (the initial bacterial OD600 of 0.175). Hydrogen was measured in the headspace at every 24 h. Bottles were aerated for 5 min after hydrogen measurement at every 72 h. Error bars are standard deviations based on three replicates.
The average yield of daily hydrogen production was 25.5 mL L−1, which was fully stable throughout the 21-day long experiment. Again, the co-cultures were thoroughly investigated. Interestingly, a more stable algae/bacteria rate was found. In the previous long-term experiments, starting with a bacterial OD600 of 0.07, the algal cell number grew continuously, shifting the algae/bacteria ratio from 250 (5 × 107/2 × 105 mL−1) to 1.9 × 10−3 (8.85 × 109/4.5 × 1012 mL−1), while in the new experimental setup with an increased initial bacterial OD600 of 0.175, the algae/bacteria ratio changed from 45.45 (5 × 107/1.1 × 106 mL−1) to 4.3 × 10−6 (5.86 × 106/1.35 × 1012 mL−1) by the 11th day (Figure 6).
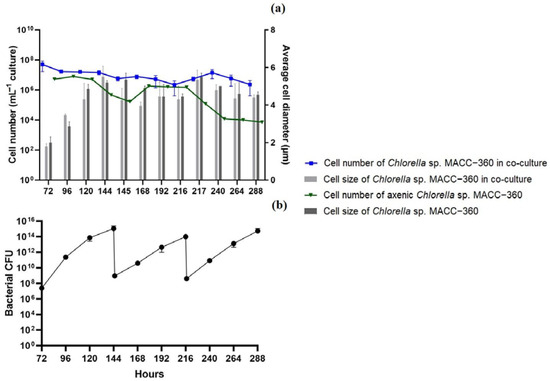
Figure 6.
Algal cell number and average cell size (diameter) of Chlorella sp. MACC-360 in acetate-free TP medium containing 8 g L−1 starch in experiments where the initial B. amyloliquefaciens OD600 value was 0.175 (a). Colony forming units (CFU) of the partner bacterium throughout the experiment (b). Error bars are standard deviations based on three replicates.
The size (diameter) of the Chlorella sp. MACC-360 algae cells was similar to what was observed either in axenic or in co-culture in TP media (Figure 6). CLSM analysis confirmed the higher algal cell number in the presence of B. amyloliquefaciens bacterial partner in starch-containing acetate-free TP medium compared to that observed under the axenic conditions (Figure S8g,h).
4. Discussion
The complex interactions between eukaryotic green algae and bacteria are ubiquitous in natural ecosystems, and these mutually beneficial interactions might be utilized for long-term, stable biohydrogen production [7,8,15]. Thus, the application of algal–bacterial consortia for biohydrogen generation has a number of advantages over using axenic algae cultures or pure bacterial isolates for hydrogen evolution [8]. The well-chosen heterotrophic bacterial partners share nutrients, vitamins with the algae, and increase their photosynthetic efficiency by directly providing CO2 to the green algae and boosting algal hydrogen production by efficiently respiring dissolved oxygen [34].
Starch is readily available in a number of agricultural and industrial wastewater flows, so that Tris-Phosphate (TP) supplemented with starch served as a simple model of cheap substrate for algal propagation and algae-based hydrogen generation. The main goal of this study was the establishment of a stable, continuous algal–bacterial co-culture based photofermentative hydrogen production approach by utilizing starch as the sole organic carbon source.
The hydrogen evolution of Chlorella sp. MACC-360 was compared to the widely used green algae C. reinhardtii cc124. Comparisons were made using axenic algae and mixed algal–bacterial co-cultures using either acetate-containing or acetate-free medium. Clear differences were observed between the hydrogen production patterns of the two algae strains in the different growth media. In TAP, both algae preferred the use of acetate over the macromolecule starch and its derivatives. Only Chlorella sp. MACC-360 was able to metabolize directly the added starch even without the B. amyloliquefaciens partner when acetate was present in the medium. It can be hypothesized from the experiments that axenic Chlorella sp. MACC-360 was able to use the electrons derived from starch degradation in the presence of acetate [33]. Nevertheless, the presence of the bacterial partner was imperative to increase the hydrogen production of Chlorella sp. MACC-360 (Figure 1b). In TAP medium, the highest increase in the hydrogen production of Chlorella sp. MACC-360 was achieved when starch was added at a concentration of 8 g L−1. The addition of the bacterial partner did not significantly increase the hydrogen production of Chlamydomonas—either TAP was supplemented with starch or not. In TP medium, none of the axenic algae strains degraded starch. However, Chlorella sp. MACC-360 was able to consume starch in TP when the bacterial partner was added. These results clearly showed that acetate is a key component for algal hydrogen evolution, and that an appropriately selected bacterial partner can further improve algal hydrogen production for certain algae species [11]. Thus, the combined application of starch and the starch-degrading heterotrophic bacterium B. amyloliquefaciens resulted in increased algal hydrogen production compared to that achieved by axenic algae cultures. However, the improvement in algal hydrogen yield was clearly dependent on the applied green algae strains. Addition of the bacterial partner to C. reinhardtii cc124 did not result in a significant increase in algal hydrogen production either in TAP or TP medium. However, Chlorella sp. MACC-360 showed a significantly increased hydrogen production when the bacterial partner was added to the algae in both media. Although the increase in absolute hydrogen production was remarkable in TAP, the relative increase in hydrogen yield was more pronounced in TP.
Interaction between algae and bacteria is very important to improve algal hydrogen yields. The algal photosynthetic products serve as substrate for the bacteria, and active bacterial respiration results in low dissolved oxygen conditions, which makes the expression and activity of algal hydrogenase enzymes possible.
The results of the TP (acetate-free) experiments indicated the importance of well-balanced algal–bacterial ratio. In the first set of the TP experiments, the B. amyloliquefaciens partner was added with an OD600 of 0.07 in the co-cultures. Under such conditions, the relatively highest hydrogen production was achieved by Chlorella sp. MACC-360 algae (having a fixed co-culture starting OD680 of 0.7) when the medium was supplemented with 8 g L−1 starch. However, the produced amount of hydrogen in TP was very low and the algal–bacterial consortium was unstable. The algae started decaying after 10 days and consequently stopped hydrogen production. As a next step, in order to reach our major goal to establish a stable, efficient continuous hydrogen producing system, important adjustments in the applied growth conditions were made. The appropriate ratio of the algae and bacterial partners proved to be an essential factor in this experiment. Continuous, higher algal hydrogen production was achieved in TP supplemented with 8 g L−1 starch when the Chlorella sp. MACC-360–B. amlyloliquefaciens co-cultures had a starting algae OD680 of 0.7 and an initial bacterial OD600 of 0.175. Thus, the 2.5× increase of the initial bacterial concentration in the co-culture was very important to reach a functional balance between the algae and bacteria.
It is to be concluded that the algal–bacterial system is stable and can be functionally maintained for long-term, however, its hydrogen production efficiency is far from being optimal. The partner bacterium B. amlyloliquefaciens highly efficiently consumed the total amount of starch even when added at a concentration of 16 g L−1, nevertheless, only a small fraction of the electrons generated from starch were converted to hydrogen by the algal hydrogenase enzymes. Most of the starch utilized by the bacterium were used for generating algal and bacterial biomass, as it was evident from the balanced algal and rapidly increasing number of bacterial cells. Thus, the results showed that the interkingdom co-culture based algal hydrogen production was strongly dependent on a number of growth parameters, as well as on the selected algal strains and partner bacterium. The applied starch concentration, the number of algal and bacterial cells, and the initial ratio of the selected algal and bacterial partners are major factors determining algal hydrogen productivity in TP medium. Nevertheless, the presence of acetate is the most important factor to determine algal hydrogen productivity in TAP medium. The stable, continuous hydrogen production rate achieved by the Chlorella sp. MACC-360–B. amyloliquefaciens co-cultures might be further improved.
One possible technological way to increase the efficiency is the immobilization of the co-cultured cells. Such biofilm reactors could significantly reduce start-up time, increase organic loading rates, and give robustness against product (hydrogen) inhibition [35,36]. A further major advantage of the immobilized cell technology might be the decreased cell washout from the bioreactors [37]. It is notable that a possible disadvantage of the biofilm reactors is that closer attention has to be paid to the mixing of the reactor in order to avoid the heterogeneous distribution of microbial activity and therefore the inconsistency of hydrogen yield [38,39]. Another interesting and novel possibility for immobilization is the incorporation of a special, self-aggregating bacterium into the co-culture in order to form natural inter-kingdom cell aggregates in stirred liquid medium (unpublished in-house data). This way, one could create naturally encapsulated algal–bacterial co-cultures, where the close physical vicinity of the interacting partners might ensure efficient oxygen scavenging [40,41], and electron channeling to the algal hydrogenase enzymes.
Additionally, a deeper molecular-level understanding of algal–bacterial interactions through metatranscriptomic analysis of the pairwise algal–bacterial combinations can provide essential information to further enhance algal biohydrogen production and to facilitate the industrial application of engineered microbial communities in wastewater treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation8070294/s1, Text S1: Optimization of algae growth conditions, Figure S1: Algae cell numbers of C. reinhardtii cc124 (a) and of Chlorella sp. MACC-360 (b) and average cell diameter of C. reinhardtii cc124 (c) and of Chlorella sp. MACC-360 (d). Erlenmeyer flasks with a total volume of 300 mL were used for cultivation. The one-way analysis of variance (ANOVA) showed significant difference between the cell size values of 2:1 and 5:1 liquid-to gas ratio cultures of C. reinhardtii cc124 (p < 0.05). Error bars are standard deviations based on three replicates, Figure S2: Optical microscopy images of C. reinhardtii cc124. Morphology of algae cells grown in 50 mL (a), in 100 mL (b), in 150 mL (c), in 200 mL (d) and in 250 mL TAP medium (e) in Erlenmeyer flasks of 300 mL total volume, Figure S3: Optical microscopy images of Chlorella sp. MACC-360. Morphology of algae cells grown in 50 mL (a), in 100 mL (b), in 150 mL (c), in 200 mL (d) and in 250 mL TAP medium (e) in Erlenmeyer flasks of 300 mL total volume, Figure S4: Chlorophyll concentrations in green algae grown under various liquid-to-gas volume ratio conditions, Figure S5: Starch degrading capacity (a) and cell numbers (b) of green algae and the partner bacterium in TAP containing 10 g L−1 starch (the initial bacterial OD600 of 0.07). Figure S6: Starch degrading capacity (a) and cell numbers (b) of green algae and the partner bacterium in TP containing 10 g L−1 starch (the initial bacterial OD600 of 0.07), Figure S7: Algal cell number and average cell size (diameter) of Chlorella sp. MACC-360 in acetate-free TP medium containing 8 g L−1 starch (a), in which algae cell number is OD680 of 0.7 and the bacterial cell number is OD600 of 0.07. Colony forming units (CFU) of the partner bacterium B. amyloliquefaciens (b), Figure S8: CLSM analysis of Chlorella sp. MACC-360 cultures in both medium and supplemented with 8 g L−1 starch. Images were taken on the 7th day of cultivation. Panel a,b show algae cultures in TAP medium. Axenic algae cells (a) and algae co-cultured with B. amyloliquefaciens (b). Panel c,d show algae cultures in TAP medium supplemented with 8 g L−1 starch. Axenic algae cells (c) and algae co-cultured with B. amyloliquefaciens (d) Panel e,f show algae cultures in TP medium. Axenic algae cells (e) and algae co-cultured with B. amyloliquefaciens (f) Panel g,h show algae cultures in TP medium supplemented with 8 g L−1 starch. Axenic algae cells (g) and algae co-cultured with B. amyloliquefaciens (h).
Author Contributions
B.H. wrote the manuscript and performed the experiments; B.P. provided useful practical hints and participated in the critical discussions; A.F. performed the confocal microscopy analyses and G.M. designed the study, wrote the manuscript and discussed the relevant literature. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following international and domestic funds: NKFI-FK-123899 (GM), 2020-1.1.2-PIACI-KFI-2020-00020, the Lendület-Programme (GM) of the Hungarian Academy of Sciences (LP2020-5/2020) and the National Laboratory Programme (RRF-2.3.1-21-2022-00008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the main text and in the figures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, K.; Hsu, Y.; Lo, Y.; Lin, P.; Lin, C.; Chang, J. Exploring optimal environmental factors for fermentative hydrogen production from starch using mixed anaerobic microflora. Int. J. Hydrogen Energy 2008, 33, 1565–1572. [Google Scholar] [CrossRef]
- Han, S.; Shin, H. Biohydrogen production by anaerobic fermentation of food waste. Int. J. Hydrogen Energy 2004, 29, 569–577. [Google Scholar] [CrossRef]
- Lin, C.; Chang, C.; Hung, C. Fermentative hydrogen production from starch using natural mixed cultures. Int. J. Hydrogen Energy 2008, 33, 2445–2453. [Google Scholar] [CrossRef]
- Kumar, A.; Jain, S.R.; Sharma, C.B.; Joshi, A.P.; Kalia, V.C. Increased H2 production by immobilized microorganisms. World J. Microbiol. Biotechnol. 1995, 11, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.S.; Tsygankov, A.A.; Rao, K.K.; Hall, D.O. Hydrogen photoproduction by Rhodobacter sphaeroides immobilised on polyurethane foam. Biotechnol. Lett. 1998, 20, 1007–1009. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Thavasi, V.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Klimov, V.V.; Ramakrishna, S.; Los, D.A.; Mimuro, M.; Nishihara, H.; Carpentier, R. Photosynthetic hydrogen production. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 101–113. [Google Scholar] [CrossRef]
- Akano, T.; Miura, Y.; Fukatsu, K.; Mlyasaka, H.; Ikuta, Y.; Matsumoto, H.; Hamasaki, A.; Shioji, N.; Mlzoguchi, T.; Yagi, K.; et al. Hydrogen production by photosynthetic microorganisms. Appl. Biochem. Biotechnol.—Part A Enzym. Eng. Biotechnol. 1996, 57–58, 677–688. [Google Scholar] [CrossRef]
- Lakatos, G.; Deák, Z.; Vass, I.; Rétfalvi, T.; Rozgonyi, S.; Rákhely, G.; Ördög, V.; Kondorosi, É.; Maróti, G. Bacterial symbionts enhance photo-fermentative hydrogen evolution of Chlamydomonas algae. Green Chem. 2014, 16, 4716–4727. [Google Scholar] [CrossRef]
- Kargi, F.; Pamukoglu, M.Y. Dark fermentation of ground wheat starch for bio-hydrogen production by fed-batch operation. Int. J. Hydrogen Energy 2009, 34, 2940–2946. [Google Scholar] [CrossRef]
- Gorman, D.S.; Levine, R.P. Cytochrome f and plastocyanin: Their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc. Natl. Acad. Sci. USA 1965, 54, 1665–1669. [Google Scholar] [CrossRef] [Green Version]
- Lakatos, G.; Balogh, D.; Farkas, A.; Ördög, V.; Tamás, P.; Bíró, T.; Maróti, G. Factors influencing algal photobiohydrogen production in algal-bacterial co-cultures. Algal Res. 2017, 28, 161–171. [Google Scholar] [CrossRef]
- Wirth, R.; Lakatos, G.; Maróti, G.; Bagi, Z.; Minárovics, J.; Nagy, K.; Kondorosi, É.; Rákhely, G.; Kovács, K.L. Exploitation of algal-bacterial associations in a two-stage biohydrogen and biogas generation process. Biotechnol. Biofuels. 2015, 8, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, R.; Lakatos, G.; Böjti, T.; Maróti, G.; Bagi, Z. Anaerobic gaseous biofuel production using microalgal biomass—A review. Anaerobe 2018, 52, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eiteman, M.A.; Lee, S.A.; Altman, E. A co-fermentation strategy to consume sugar mixtures effectively. J. Biol. Eng. 2008, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghirardi, M.L.; Dubini, A.; Yu, J.; Maness, P.C. Photobiological hydrogen-producing systems. Chem. Soc. Rev. 2009, 38, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Fakhimi, N.; Dubini, A.; Tavakoli, O.; González-ballester, D. Acetic acid is key for synergetic hydrogen production in Chlamydomonas- bacteria co-cultures. Bioresour. Technol. 2019, 289, 121648. [Google Scholar] [CrossRef]
- Dubini, A.; Ghirardi, M.L. Engineering photosynthetic organisms for the production of biohydrogen. Photosynth. Res. 2015, 123, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.J. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Boboescu, I.Z.; Gherman, V.D.; Lakatos, G.; Pap, B.; Bíró, T.; Maróti, G. Surpassing the current limitations of biohydrogen production systems: The case for a novel hybrid approach. Bioresour. Technol. 2016, 204, 192–201. [Google Scholar] [CrossRef]
- Liu, G.; Shen, J. Effects of Culture and Medium Conditions on Hydrogen Production from Starch Using Anaerobic Bacteria. J. Biosci. Bioeng. 2004, 98, 251–256. [Google Scholar] [CrossRef]
- Yang, H.; Shen, J. Effect of ferrous iron concentration on anaerobic bio-hydrogen production from soluble starch. Int. J. Hydrogen Energy 2006, 31, 2137–2146. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, T.; Su, H. Enhanced hydrogen production from corn starch wastewater as nitrogen source by mixed cultures. Renew. Energy 2016, 96, 1135–1141. [Google Scholar] [CrossRef]
- Chen, S.; Lee, K.; Lo, Y.; Chen, W.; Wu, J.; Lin, C.; Chang, J. Batch and continuous biohydrogen production from starch hydrolysate by Clostridium species. Int. J. Hydrogen Energy 2008, 33, 1803–1812. [Google Scholar] [CrossRef]
- Júnior, A.D.N.F.; Etchebehere, C.; Zaiat, M. Mesophilic hydrogen production in acidogenic packed-bed reactors (APBR) using raw sugarcane vinasse as substrate: Influence of support materials. Anaerobe 2015, 34, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Otsuka, S.; Morimoto, M. Hydrogen production from industrial wastewater by anaerobic microflora in chemostat culture. J. Ferment. Bioeng. 1996, 82, 194–197. [Google Scholar] [CrossRef]
- Singh, S.P.; Srivastava, S.C.; Pandey, K.D. Hydrogen production by Rhodopseudomonas at the expense of vegetable starch, sugarcane juice and whey. Int. J. Hydrogen Energy 1994, 19, 437–440. [Google Scholar] [CrossRef]
- Kalia, V.C.; Jain, S.R.; Kumar, A.; Joshi, A.P. Frementation of biowaste to H2 by Bacillus licheniformis. World J. Microbiol. Biotechnol. 1994, 10, 224–227. [Google Scholar] [CrossRef]
- Tang, S.; Xu, T.; Peng, J.; Zhou, K.; Zhu, Y. Overexpression of an endogenous raw starch digesting mesophilic α-amylase gene in Bacillus amyloliquefaciens Z3 by in vitro methylation protocol. J. Sci. Food Agric. 2020, 100, 3013–3023. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Ma, L.; Blom, J.; Wu, H.; Gu, Q.; Borriss, R. The “pseudo-pathogenic” effect of plant growth-promoting Bacilli on starchy plant storage organs is due to their α-amylase activity which is stimulating endogenous opportunistic pathogens. Appl. Microbiol. Biotechnol. 2020, 104, 2701–2714. [Google Scholar] [CrossRef]
- Ahmad, A.; Mishra, R. Different unfolding pathways of homologous alpha amylases from Bacillus licheniformis (BLA) and Bacillus amyloliquefaciens (BAA) in GdmCl and urea. Int. J. Biol. Macromol. 2020, 159, 667–674. [Google Scholar] [CrossRef]
- Wang, C.H.; Chang, J.S. Continuous biohydrogen production from starch with granulated mixed bacterial microflora. Energy Fuels 2008, 22, 93–97. [Google Scholar] [CrossRef]
- Jurado-Oller, J.L.; Dubini, A.; Galván, A.; Fernández, E.; González-Ballester, D. Low oxygen levels contribute to improve photohydrogen production in mixotrophic non-stressed Chlamydomonas cultures. Biotechnol. Biofuels 2015, 8, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Miyachi, S. Effect of temperature on starch degradation in chlorella vulgaris 11h cells. Plant Cell Physiol. 1982, 23, 333–341. [Google Scholar] [CrossRef]
- Yao, S.; Lyu, S.; An, Y.; Lu, J.; Gjermansen, C.; Schramm, A. Microalgae–bacteria symbiosis in microalgal growth and biofuel production: A review. J. Appl. Microbiol. 2019, 126, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Keskin, T.; Aksöyek, E.; Azbar, N. Comparative analysis of thermophilic immobilized biohydrogen production using packed materials of ceramic ring and pumice stone. Int. J. Hydrogen Energy 2011, 36, 15160–15167. [Google Scholar] [CrossRef]
- Karadag, D.; Köroğlu, O.E.; Ozkaya, B.; Cakmakci, M. A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochem. 2015, 50, 262–271. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Factors influencing fermentative hydrogen production: A review. Int. J. Hydrogen Energy 2009, 34, 799–811. [Google Scholar] [CrossRef]
- Kumar, G.; Mudhoo, A.; Sivagurunathan, P.; Nagarajan, D.; Ghimire, A.; Lay, C.-H.; Lin, C.-Y.; Lee, D.-J.; Chang, J.-S. Recent insights into the cell immobilization technology applied for dark fermentative hydrogen production. Bioresour. Technol. 2016, 219, 725–737. [Google Scholar] [CrossRef]
- Rachman, M.A.; Furutani, Y.; Nakashimada, Y.; Kakizono, T.; Nishio, N. Enhanced hydrogen production in altered mixed acid fermentation of glucose by Enterobacter aerogenes. J. Ferment. Bioeng. 1997, 83, 358–363. [Google Scholar] [CrossRef]
- Arooj, M.F.; Han, S.-K.; Kim, S.-H.; Kim, D.-H.; Shin, H.-S. Continuous biohydrogen production in a CSTR using starch as a substrate. Int. J. Hydrogen Energy 2008, 33, 3289–3294. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Li, M.; Shi, J.; Wang, L.; Luo, S.; Liu, H. Chemical Flocculation-Based Green Algae Materials for Photobiological Hydrogen Production. ACS Appl. Bio Mater. 2022, 5, 897–903. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).