Abstract
This study aimed to investigate the effects of low-temperature-tolerant lactic acid bacteria on the fermentation quality and bacterial community of oat silage. Silage treatments were designed as control (with no additives), with FO3, FO5, FO8, and L214 inoculants. After 60 days of ensiling, the fermentation characteristics and bacterial community were analyzed. The results showed that the low-temperature-tolerant lactic acid bacteria were able to reduce the pH and NH3-N and increase crude protein and lactic acid contents. It is worth noting that the addition of FO3 also significantly inhibited butyric acid production. High-throughput sequencing technique showed that at the genus level, Lactiplantibacillus were the dominant bacteria in all oat silages, while at the species level, the bacterial abundance in the treated silages differed significantly from the control. The highest abundance of Lactiplantibacillus sp. was found in the control and L214 groups, while the abundance of Lactiplantibacillus curvatus was most abundant in the silage treated with low-temperature-tolerant lactic acid bacteria. The results indicated the potential effectiveness of low-temperature-tolerant lactic acid bacteria in improving fermentation quality and reducing protein losses.
1. Introduction
Forage oat is one of the oldest cereals in the history of world cultivation [1]. Due to its nutritional composition and the versatility of selected bioactive compounds [1], oat has been proven to be economically viable not only as a staple food for human consumption but also as a forage crop for animals. Nowadays, forage oat is one of the major crops in China and the sixth largest grain crop in the world [2]. It is known for its adoption to cold, infertile soil, drought, and salinity [3]. The increased demand for animal products has led to an increase in the use of feed crops, such as forage oat.
Ensiling has long been a vital method of preserving fresh oat [4]. The palatability and better nutrient preservation of processed silage crops make silage one of the most popular feedstuffs today. Well-preserved silage depends on proper fermentation during storage, which produces a low pH and enough acid to inhibit the growth of undesirable micro-organisms [5]. During ensiling, the fermentation temperature plays an important role in the production of high-quality silage, and different fermentation temperatures have a significant impact on the silage quality [6]. According to the regions where forage oats are grown in China, the average temperature is generally lower than 15 °C when forage oat is harvested from September to November. Low temperatures could inhibit microbial activity, prolong ensiling time, and reduce silage quality [6]. Until now, the effects of cold-tolerant lactic acid bacteria (LAB) strains on oat silage quality have not been well studied.
In recent years, high-throughput sequencing technologies have already been applied to quantify and analyze silage microbiota [7]. As next-generation sequencing (NGS) technology can generate much more readings than traditional stand-alone culture methods, it can facilitate the discovery of a wider diversity of the microbiota, as well as the detection of bacteria of unknown origin with high or low involvement [8]. This technique can restrict the classification of community micro-organisms to the genus level based on sequence length and the level of taxonomic resolution [9]. These contribute to finding specific strains of bacteria adapted to low-temperature silage. Unfortunately, only a few studies have been conducted on oat silage stored at low temperatures.
For this reason, this study aimed to investigate the effect of low-temperature-tolerant lactic acid bacteria on the fermentation quality and bacterial community of oat silage at 5 °C vs. 15 °C.
2. Materials and Methods
2.1. Oat Silage Preparation
Forage oat was cultivated in the experimental field located in Hebei Province and harvested at the grain-filling stage in September 2020. Silage was produced in a small-scale system in which approximately 500 g portions of silage oat material were cut into parts with a length of about 20 mm and packed into plastic bags (N-9, Asahi Kasei Co., Ltd., Tokyo, Japan). FO3, FO5, and FO8, isolated from fresh oat, were used as additives to oat silage at 1.0 × 105 colony-forming units (cfu)/g of fresh matter (FM). The above strains were cultivated in MRS broth for 24 h at 30 °C. Then, the inoculum was standardized using a spectrophotometer (600 nm) at an optical density of 1.0. Experimental treatments included: control silage without LAB, oat + FO3 silage, oat + FO5 silage, oat + FO8 silage and oat + L214 silage. Plastic bags were sealed with Sharp Vacuum Seal/Pack (SQ-202, Sharp Ltd., Tokyo, Japan), and bags of the same treatment were divided equally into two groups and stored at 5 °C and 15 °C for 60 days. FO3, FO5, and FO8 were identified as Leuconostoc mesenteroides. Strain L214 (Lactiplantibacillus plantarum) was from Gaofuji company.
2.2. DNA Extraction and Bacterial Composition Analysis based on NGT Method
Ten grams of silage sample per bag was dissolved in 90 mL of sterile 0.85% NaCl solution and then shaken vigorously at 120 rpm at 4 °C for 2 h. The sample was filtered through two layers of sterile gauze. The filtered solution was centrifuged at 10,000 rpm at 4 °C for 10 min. The supernatant was discarded, and 1 mL of sterile 0.85% NaCl solution was added to the precipitate, which was then centrifuged at 12,000 rpm at 4 °C for 10 min, thus obtaining the cells. After centrifugation at 15,000× g for 2 min, bacterial cells were lysed with 180 μL of lysozyme solution (20 g/L lysozyme, 0.02 M Tris-HCl (pH 8.0), 0.002 M sodium EDTA (pH 8.0), 1.2 g/L Triton X-100) at 37 °C for 1 h. Subsequent bacterial DNA purification was performed using a commercial kit (DP214-02, Tiangen, Beijing, China). Extracted DNA was examined for DNA quality and concentration by 1% agarose gel electrophoresis and spectrophotometrically (with an optical density of 260/280 nm ratio) and stored at −20 °C until further analysis.
The same DNA samples were amplified using primers 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGT WTCTAAT-3′) targeting the V3–V4 region. The PCR procedure consisted of an initial denaturation at 98 °C for 2 min, followed by completion of 30 cycles of denaturation at 98 °C for 30 s, annealing at 50 °C for 30 s, extension at 72 °C for 60 s, and a final extension at 72 °C for 5 min. Each treatment was conducted in triplicate, and the mixture was purified.
The paired-end sequencing (2 × 300) of the purified amplicons was performed on an Illumina MiSeq platform of Hokkaido System Science Co., Ltd. (Hokkaido, Japan). Raw sequences were processed using the QIIME (version 1.80) microbial ecology pipeline. The 300 bp reads were truncated at any site receiving an average quality score <20 over a 20 bp sliding window, and truncated reads that were shorter than 50 bp were discarded. Similarly, two nucleotide miss-matches in primer matches were removed, sequences that overlapped by more than 50 bp were assembled, and chimeric sequences were identified and removed. Only sequences with at least 200 bp after quality filtering were grouped into operational taxonomic units (OTUs) using a 97% similarity threshold. Sequences were categorized from phylum to genus level by using the default settings of the Ribosome Database Project classifier.
In order to study the effect of silage additives and silage temperature as variables on microbial communities, we clustered the sequences after high-throughput sequencing and classified them into different OTUs (Operational Taxonomic Units). OTU is an artificially assigned uniform marker for a taxonomic unit (strain, genus, species, group, etc.) in phylogenetic or population genetics studies to facilitate analysis. In this experiment, OTUs at 97% similarity level were analyzed bioinformatically using the platform Uparse (version 7.0.1090, available online: http://drive5.com/uparse/ (accessed on 12 December 2021).
Species diversity of microbial communities in silage material was measured by the Shannon and Simpson indices. Both the Simpson Index and the Shannon Index are used to estimate the microbial diversity of sample; the Simpson Index was introduced by Edward Hugh Simpson (1949) and is commonly used in ecology to quantify the biodiversity of an area. The Shannon Index is similar to the Simpson Index and is often used to reflect the alpha diversity of a community, with higher Shannon values indicating higher community diversity.
2.3. Fermentation Quality and Chemical Composition Analysis
A sample of silage (20 g) per bag was mixed with 180 mL of sterilized water and stored at 4 °C for 24 h [7]. The supernatant was filtered through 0.22 µm filter paper and used to determine the fermentation quality of the oat silage. The pH was determined using a glass electrode pH meter (PHS-3C, INESA Scientific Instruments, Shanghai, China). The concentrations of lactic acid, acetic acid, propionic acid, and butyric acid were determined by high-performance liquid chromatography (HPLC) (column: Shodex RS Pak KC-811; Showa Denko K.K., Kawasaki, Japan; detector: DAD, 210 nm, SPD-20A; Shimadzu Co., Ltd., Kyoto, Japan; eluent: 3 mmol L−1 HClO4, 10 mL min−1; temperature: 50 °C). Approximately 200 g of each pre-ensiled material and silage sample was dried at 65 °C for 48 h to determine DM (dry matter) content, then the material was ground and passed through a 1.0 mm sieve to determine the chemical composition. The content of OM (organic matter) and EE (ether extract) was analyzed according to the AOAC method [10]. The Kjeldahl method (FOSS KjeltecTM 2300) was used to determine the total nitrogen (TN) content. CP (crude protein) was calculated by multiplying TN by 6.25 [10]. NDF (neutral detergent fiber) and ADF (acid detergent fiber) were both determined using the method of VanSoest et al. [11]. The contents of DM, ADF, NDF, and WSC in fresh oat were 18.66%, 328.88 g kg DM−1, 567.56 g kg DM−1, and 150.56 g kg DM−1, respectively.
2.4. Statistical Analysis
Chemical composition, fermentation quality, and microbial counts data were analyzed using a two-way analysis of variance, with silage additives and ensiling temperature as the main variables. The level of statistical significance was set at p < 0.05 using the SAS program version 9.1 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Chemical Composition of Oat Silage
The fermentation quality of oat silage stored at 60 d is shown in Table 1. In the fermentation environment at 5 °C, the final pH of the control group (CK) was 6.33, while the pH values of silages treated with FO3, FO5, and FO8 were 4.43, 4.44, and 4.66, respectively, all of which were significantly lower than that in the control group (p < 0.05). Silage samples supplemented with FO3, F05, and F08 had a higher lactic acid content than the CK group.

Table 1.
Chemical composition of oat silage after 60 days of ensiling.
The effect of each additive on the quality of oat silage at 15 °C was similar to that at 5 °C. The pH in the treatment groups with the addition of FO3, FO5, and FO8 was lower than that in the CK and L214 groups (p < 0.05); the lactic acid content in the oat silage treated with FO3 and FO4 was significantly higher than that in the group with FO8 and CK groups (p < 0.05). More importantly, it is noteworthy that the addition of FO3 and FO5 produced the lowest pH and highest lactic acid content in the silages at both 5 °C and 15 °C.
NH3-N content in the FO3, FO5, and FO8 treatments was lower than that in CK and L214 silages (p < 0.05). In addition, FO3 treatment had the lowest NH3-N content among all silages at 5 °C. The contents of DM, NDF, and ADF ranged between 17 and 19%, 537 and 570 g/kg DM, and 305 and 334 g/kg DM, respectively. According to the NH3-N development, higher CP contents were observed in FO3, FO5, and FO8 treatments at 5 °C (p < 0.05), but that did not occur at 15 °C.
The WSC content of FO3, FO5, and FO8 was lower than that of the CK group. There was no significant difference in DM, NDF, and EE between treatments.
3.2. Bacterial Community during the Ensiling Process
As shown in Table 2, the coverage values of all samples were around 0.99, indicating that the analysis of the microbial composition was credible. The number of OTUs per sample ranged from 42 to 78. The Shannon Index varied between 0.95 and 2.72, reflecting the diversity index of the microbial community. The factors of temperature (T) and additives (A) had a significant effect on Shannon diversity. The trend in species diversity reflected by the Simpson Index coincided with the Shannon Index.

Table 2.
Alpha diversity of oat silages at different temperatures and additives during the ensiling process.
3.3. Analysis of the Microbial Community of Oat Silage
The bacterial community of oat silage at the genus level is shown in Figure 1. In the fermentation environment at 5 °C, the dominance of Lactiplantibacillus in all additive groups was significantly higher than that in the CK group. The Lactiplantibacillus abundance in FO3 and FO5 silages accounted for more than 95% of the total bacteria. Enterobacter and Leuconostoc were also the dominant bacteria in the FO8 group. Lactiplantibacillus still represented the largest proportion in FO3 and FO5. It is noteworthy that as the temperature increased, the proportion of Lactococcus increased, and Enterbobacter decreased in all groups.
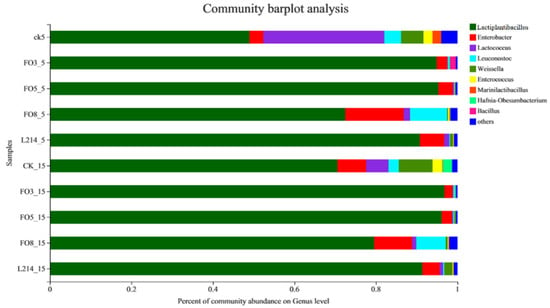
Figure 1.
Microbial communities at the genus level of oat silage at day 60 revealed by high-throughput sequencing.
The relative abundance of silage bacteria at the species level is shown in Figure 2. After 60 days of ensiling, Lactiplantibacillus curvatus was the predominant strain in the FO3, FO5, and FO8 groups at both 15 °C and 5 °C. Lactiplantibacillus sp. was extremely abundant in the L214 and control groups. In addition, the abundance of Enterobacter cloacae only reached more than 10% in the FO8 group. There was a noteworthy increase in the abundance of Lactiplantibacillus brevis with an increasing silage temperature, evident in the control and all lactic acid bacteria groups.
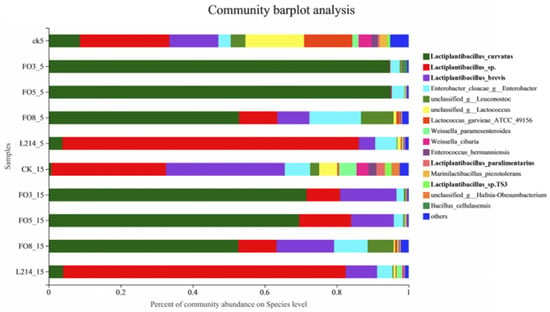
Figure 2.
The microbial community of oat silages at the species level revealed by high-throughput sequencing.
3.4. Cluster Analysis of the Bacterial Community and its Correlation with Fermentation Products
Cluster analysis was performed in order to acquire an overview of the bacterial community at different treatments (Figure 3). Basically, the silage samples treated with the low-temperature-tolerant LAB can be separated well from the L214 and CK groups, indicating that the low-temperature-tolerant LAB had a significant impact on the microbial community. For FO3 and FO5 silage, the samples stored at 5 °C were clearly distinguished from 15 °C.
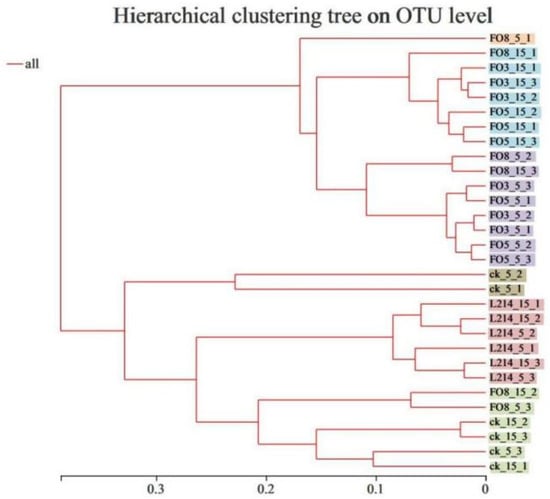
Figure 3.
The cluster analysis of microbial community of oat silages based on OTUs.
Based on the Spearman Correlation Heatmap (Figure 4), a significant positive correlation between temperature and Lactiplantibacillus brevis and Weissella cibaria was observed, with Lactiplantibacillus brevis being the most sensitive to temperature (p < 0.05). Elevated pH had a significantly positive relationship with the proliferation of Enterococcus hermanniensis, Weissella cibaria, and Lactococcus garvieae. ATCC.49156 and Lactiplantibacillus sp. (p < 0.05) and a negative relationship with Lactiplantibacillus curvatus (p < 0.05). More importantly, Lactiplantibacillus curvatus was positively correlated with LA and negatively with pH and NH3-N.
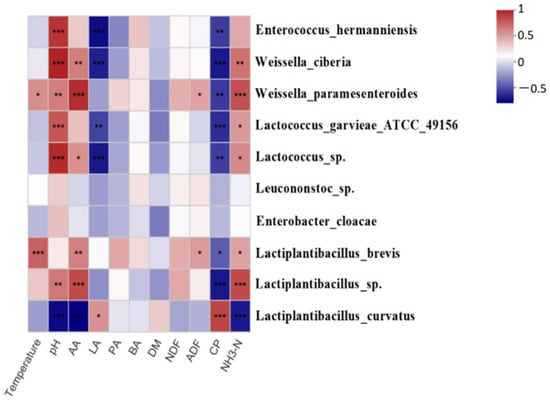
Figure 4.
The Spearman Correlation Heatmap, which shows correlations between different species and environment in oat silage. LA, lactic acid; AA, acetic acid; PA, propionic acid; BA, butyric acid; DM, dry matter; NDF, neutral detergent fiber; ADF, acid detergent fiber; CP, crude protein; *, p < 0.05; **, p < 0.01; ***, p < 0.001.
4. Discussion
Overall, butyric acid content was higher in this research compared with other acids, especially in the control silages. Even though we cannot explain the exact reason now, the relatively low DM content and low ensiling temperature might be possible reasons. As we know, Clostridium mainly produces butyric acid, which favors environments with high moisture content. The low temperature might inhibit LAB growth quickly at the beginning, allowing undesirable micro-organism multiplication at the early ensiling stage. Regarding that, a trial for examining the dynamic change of fermentation products and microbiota during the whole ensiling process of oat silage will be performed, and with the results, we will try to classify the reason for high butyric acid.
Oat silage treated with FO3, FO5, and FO8 showed relatively higher lactic acid content at both 5 °C and 15 °C. Of these treatments, FO3 showed higher fermentation quality at both 5 °C and 15 °C, indicated by the higher lactic acid and lower NH3-N content. The rapid accumulation of lactic acid and the decrease in the pH value are essential for obtaining high-quality silage [7]. Thus, FO3 might be an interesting strain for the development of microbial inoculants for cold climates. In addition, FO3-treated silage samples also had a higher WSC content, which might imply the potentially effective utilization of WSC in oat silage.
The ratio of lactic acid to acetic acid is often used as a qualitative indicator of fermentation patterns, reflecting the relationship between homogeneous and heterogeneous fermentation by LAB [12]. The present study showed that the ratios of lactic acid to acetic acid of FO3, FO5, and FO8 treatments under 5 °C fermentation and FO3 and FO5 treatments under 15 °C fermentation were beyond 3.0, indicating that the fermentation process tended to be homogeneous. Interestingly, the FO3 and FO5 treatments induced a higher ratio of lactic acid to acetic acid compared with other treatments. Therefore, FO3 and FO5 have a potential role in promoting the growth and metabolism of homofermentative LAB. Butyric acid, usually produced by Clostridium fermentation, is known to be one indicator of nutrition loss [13,14,15]. In our experiments, FO3-treated silage had a lower butyric acid content at both 5 °C, and 15 °C. We speculate that the addition of FO3 caused a rapid increase in lactic acid content and a decrease in pH during silage, which inhibited the multiplication of Clostridium and thus reduced the occurrence of butyric acid in the silage samples. Even though the LAB additions could reduce the butyric acid content at different levels, the butyric acid content was relatively high. Compared with previous reports in oat silages, the relatively high DM could partially explain the high occurrence of butyric acid in our study.
The main objective of ensiling is to maximize the retention of nutrients, especially crude protein. Analysis showed that treatments had a significant effect on both crude protein and NH3-N (p < 0.05). The crude protein values were higher in all the low-temperature-tolerant LAB groups compared to the control group after 60 days. Umana et al. (1991) concluded that well-preserved silage should contain NH3-N <100 g/kg [16], and in our study, NH3-N levels in all silages were below the recommended levels.
LAB, as the dominant bacteria in the silage process, contains a large number of diverse species and characteristics. As shown in Table 1, the addition of low-temperature tolerant LAB had different effects on silage quality. Therefore, an in-depth understanding of the microbial community and their relative abundance in silage can help to improve silage preservation and recycling. Cao et al. (2011) investigated the effect of low temperatures on the fermentation potential of TMR silage. They observed that the LAB count and corresponding activities in low-temperature silage were lower, and TMR silage could not produce sufficient lactic acid at low temperatures, resulting in poorer-quality silage. Therefore, there is a need to develop techniques for the preparation of silage to promote lactic acid fermentation during the cold season [17].
Coverage values for all samples were greater than 0.99, indicating that the sequencing depth was sufficient to understand the microbial composition. The number of OTUs and Shannon Index were significantly lower in LAB-treated silages, indicating that low-temperature-tolerant LAB could reduce the microbial diversity in oat silage. In addition, the lower bacterial diversity also suggests that bacterial selection occurred after the addition of low-temperature-tolerant LAB.
Stratified cluster analysis of samples clearly reflected the degree of variation in the distribution of the bacterial community. After 60 days of ensiling, the bacterial community in the control and L214 treatment groups were clearly separated from the FO3 and FO5 groups, indicating that the addition of FO3 and FO5 resulted in significant changes in the bacterial community. However, it is noteworthy that as the ensiling temperature increased, the bacterial community was significantly separated at different temperatures. The results indicated that silage temperature and low-temperature-tolerant LAB both influenced the fermentation quality.
To investigate the effect of the addition of LAB on the microbial community of oat silage, we used high-throughput sequencing techniques to reveal changes in the bacterial community. As we know, Lactiplantibacillus plays a key role in lowering the silage pH [18]. After 60 days of ensiling, the abundance of Lactiplantibacillus was highest when FO3 and FO5 were added. Enterococcus is a coccus-type LAB that is usually present during silage fermentation. Several studies have shown that Enterococcus plays an important role in accelerating silage fermentation, but it can only survive in the early stages of fermentation due to its intolerance to acid [7,19]. Therefore, its high abundance implies a low lactic acid content in the silage, which is similar to the results we found in the CK group.
To better understand the bacterial structure of the oat silage samples, we carried out species identification of the microbial species. Lactiplantibacillus curvatus was dominant in silage in FO3, FO5, and FO8 silages. Similar results were also found in vacuum-packed and processed meat products stored at low temperatures [20]. In fact, Lactiplantibacillus curvatus acted as the dominant bacteria for homolactic fermentation, and it also has been suggested as a promising inoculant for sorghum silage stored at 4 °C [21,22]. Leuconostoc mesenteroides belongs to cocci-shaped bacteria. As we know, the cocci-shaped LAB plays a more important role in the primary stage of ensiling [19]. In the later fermentation process, as the pH is reduced, the cocci-shaped LAB is gradually replaced by Lactiplantibacillus. In this regard, Leuconostoc mesenteroides was probably present at the beginning of the ensiling process. Unfortunately, we did not examine the bacterial community at the beginning.
Enterobacter could ferment lactic acid into products such as acetic acid, resulting in nutritional losses. In our experiments, the abundance of Enterobacter cloacae in the control group was 7.0% at 15 °C, while the abundance of Enterobacter cloacae in the FO3 and FO5 groups was 2.0% and 2.6%, respectively. That difference may be due to the different concentrations of lactic acid. Li et al. (2021) reported that sufficient lactic acid concentrations were effective in inhibiting the growth of Enterobacter in silage [23].
5. Conclusions
The results of the study showed that the additives had a significant effect on the bacterial diversity and fermentation parameters of oat silage. The selected lactic acid bacteria significantly improved the fermentation quality by reducing the pH, NH3-N content, and loss of crude protein. In addition, they significantly modulated the bacterial community during ensiling. With the addition of low-temperature-tolerant inoculants, the proportion of Lactococcus increased, and Enterbobacter decreased. In comparison with other additive groups, the fermentation parameters of oat silage treated with the FO3 additive were most suitable for ensiling at low temperatures. This study provides new insights into the sustainable improvement of low-temperature oat silage fermentation.
Author Contributions
Conceptualization, K.-K.N.; methodology, X.-M.Z.; software, X.-M.Z.; validation, B.-J.Y., K.-K.N., and D.-D.J.; formal analysis, X.-M.Z.; investigation, X.-M.Z.; resources, B.-J.Y.; data curation, B.-J.Y.; writing—original draft preparation, X.-M.Z.; writing—review and editing, K.-K.N.; visualization, K.-K.N.; supervision, K.-K.N.; project administration, K.-K.N.; funding acquisition, K.-K.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Technology Project of Inner Mongolia Autonomous Region (2021GG0066), National Key Research and Development Program of China (Grant No. 2021YFD1300300) and the National Modern Agricultural Industry Technology System of the People’s Republic of China (No. CARS-07-E-3).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the first author. The data are not publicly available due to restrictions by the research group.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lásztity, R. Oat grain-a wonderful reservoir of natural nutrients and biologically active substances. Food Rev. Int. 1998, 14, 99–119. [Google Scholar] [CrossRef]
- National Research Council. Lost Crops of Africa: Volume I: Grains; National Academies Press: Washington, WA, USA, 1996. [Google Scholar]
- Wang, S. Fodder Oats in China. In Fodder Oats: A World Overview; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; pp. 123–143. [Google Scholar]
- Jia, T.; Wang, B.; Yu, Z.; Wu, Z. The effects of stage of maturity and lactic acid bacteria inoculants on the ensiling characteristics, aerobic stability and in vitro digestibility of whole-crop oat silages. Grassl. Sci. 2021, 67, 55–62. [Google Scholar] [CrossRef]
- Cao, Y.; Cai, Y.; Takahashi, T.; Yoshida, N.; Tohno, M.; Uegaki, R.; Nonaka, K.; Terada, F. Effect of lactic acid bacteria inoculant and beet pulp addition on fermentation characteristics and in vitro ruminal digestion of vegetable residue silage. J. Dairy Sci. 2011, 94, 3902–3912. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Bai, C.; Liu, L.; Cao, B. Effects of lactic acid bacteria inoculant on the fermentation quality of reed grass (Phragmites australis Cav. Trin. ex Sterd.) at low temperature. Acta Agrestia Sin. 2011, 19, 127–131. [Google Scholar]
- Ni, K.K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.; Mills, D. Next-generation approaches to the microbial ecology of food fermentations. BMB Rep. 2012, 45, 377–389. [Google Scholar] [CrossRef] [Green Version]
- Amnon, A.; Amit, Z.; Or, Z.; Michael, E.; Shay, S.; Ohad, S. High-resolution microbial community reconstruction by integrating short reads from multiple 16S rRNA regions. Nucleic Acids Res. 2013, 41, e205. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.; Robertson, J.; Lewis, B. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Jones, B.; Satter, L.; Muck, R. Influence of bacterial inoculant and substrate addition to lucerne ensiled at different dry matter contents. Grass Forage Sci. 1992, 47, 19–27. [Google Scholar] [CrossRef]
- Muck, R. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Kung, L.; Shaver, R.; Grant, R.; Schmidt, R. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef] [PubMed]
- Muck, R.; Nadeau, E.; McAllister, T.; Contreras-Govea, F.; Santos, M.; Kung, L. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef] [PubMed]
- Umana, R.; Staples, C.; Bates, D.; Wilcox, C.; Van Mahana, W. Effects of a microbial inoculants and (or) sugarcane molasses on the fermentation, aerobic stability and digestibility of Bermuda grass ensiled at two moister contents. J. Anim. Sci. 1991, 69, 4588–4601. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Cai, Y.; Hirakubo, T.; Fukui, H.; Matsuyama, H. Fermentation characteristics and microorganism composition of total mixed ration silage with local food by-products in different seasons. Anim. Sci. J. 2011, 82, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Eikmeyer, F.; Köfinger, P.; Poschenel, A.; Jünemann, S.; Zakrzewski, M.; Heinl, S.; Mayrhuber, E.; Grabherr, R.; Pühler, A.; Schwab, H.; et al. Metagenome analyses reveal the influence of the inoculant Lactiplantibacillus buchneri CD034 on the microbial community involved in grass silaging. J. Biotechnol. 2013, 167, 334–343. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Ohmomo, S.; Kumai, S.; Nakase, T. Influence of Lactiplantibacillus spp. from an inoculant and of Weissella and Leuconostoc spp. from forage crops on silage fermentation. Appl. Environ. Microb. 1998, 64, 2982–2987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dykes, G.; Cloete, T.; Von Holy, A. Taxonomy of lactic acid bacteria associated with vacuum-packaged processed meat spoilage by multivariate analysis of cellular fatty acids. Int. J. Food Microbiol. 1995, 28, 89–100. [Google Scholar] [CrossRef]
- Tanaka, O.; Mori, K.; Omomo, S. Effect of inoculation with Lactiplantibacillus curvatus on ensiling. Grassl. Sci. 2000, 41, 55–59. [Google Scholar]
- Langston, C.; Bouma, C. Types and sequence change of bacteria in orchardgrass and alfalfa silage. J. Dairy Sci. 1960, 43, 1575–1584. [Google Scholar] [CrossRef]
- Li, X.; Chen, F.; Wang, X.; Sun, L.; Guo, L.; Xiong, Y.; Wang, Y.; Zhou, H.; Jia, S.; Yang, F. Impacts of low temperature and ensiling period on the bacterial bommunity of oat silage by SMRT. Microorganisms 2021, 9, 274. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).