Abstract
Arthrobacter sp. BCC 72131, an obligate aerobic bacterium, was used to create anaerobic conditions, and Clostridium beijerinckii TISTR 1461 was used as a butanol producer in an acetone-butanol–ethanol (ABE) fermentation. Sweet sorghum juice (SSJ) medium containing 60 g/L of total sugar supplemented with 1.27 g/L of (NH4)2SO4 was used as a butanol production (BP) medium. Arthrobacter sp. was inoculated into the BP medium in 1-L screw-capped bottles. After 2, 4, 6 and 12 h of Arthrobacter sp. cultivation at 30 °C, C. beijerinckii was transferred into the BP medium to start butanol production at 37 °C. The results showed that C. beijerinckii inoculation after 6 h of Arthrobacter sp. cultivation gave the highest butanol titer (PB) at 12.56 g/L, with a butanol yield (YB/S) and volumetric butanol productivity (QB) of 0.34 g/g and 0.23 g/L·h, respectively. These values are approximately 10–27% higher than those of the control experiment using a single culture of C. beijerinckii TISTR 1461 and oxygen-free nitrogen (OFN) gas flushing to create anaerobic conditions. Field emission scanning electron microscopic (FE-SEM) images of Clostridium cells, as well as protein and free amino nitrogen concentrations in the broth during butanol fermentation were also studied to confirm the results. The butanol fermentation was then carried out in a 5.6-L stirred-tank and a 1.2-L low-cost gas-lift bioreactor by the mixed cultures using the optimal time of Clostridium inoculation. The PB, YB/S and QB values obtained were not significantly different from those in the 1-L screw-capped bottles. Hence, Arthrobacter sp. can be used as a novel method to create anaerobic conditions instead of a traditional method employing OFN gas flushing. Using mixed cultures of Arthrobacter sp. BCC 72131 and C. beijerinckii TISTR 1461 is a practical method to produce butanol on a large-scale, both in complex and low-cost bioreactors.
1. Introduction
Butanol is widely used as a chemical starting material for making plastics, plasticizers, polymers, paints, lubricants, brake fluids and synthetic rubber [1,2]. Butanol has a high energy density of 29.2 MJ/L and can be substituted for gasoline (energy density of 32 MJ/L) with no modifications to the current internal combustion engines [3]. Presently, butanol is most often obtained from crude oil [4]. However, with the increasing fluctuations in petroleum oil availability and costs as well accumulating environmental dilemmas, butanol fermentations are alternatives of renewed interest. Additionally, it is an excellent renewable fuel that can be considered a better transportation fuel than bioethanol in future fuel systems [5], mainly due to its higher number of carbon atoms and consequently higher energy content, miscibility with gasoline and less corrosivity [6]. Additionally, the strong renewed attention given to butanol as a sustainable vehicle fuel has induced progress in improving biobutanol production by developing biotechnological production and separation processes [1].
Biobutanol can be produced by Clostridia strains via an ABE fermentation under strictly anaerobic conditions. In batch ABE fermentations, biphasic metabolism is observed. Clostridium sp. produces hydrogen, carbon dioxide, acetate and butyrate during the initial growth phase in an acidogenic phase, resulting in a decreased pH of the culture medium. Cell metabolism then shifts to solvent production to form acetone, butanol and ethanol in a solventogenic phase [7,8,9].
Oxygen-free nitrogen (OFN) gas is generally used to create anaerobic conditions for ABE fermentations at a laboratory scale [10]. It is, however, impractical in larger bioreactors because of the high operational costs and complexity of applying OFN gas. Our preliminary research found that an obligate aerobic bacterium, Arthrobacter sp., could consume oxygen in a bioreactor and create anaerobic conditions for ABE fermentation by Clostridium sp. [11]. Nevertheless, the optimum parameters for using this anaerobic bacterium to create anaerobic conditions for butanol fermentation by C. beijerinckii TISTR 1461 have not been reported.
Sweet sorghum is a non-competitive substrate that can be cultivated with lower water requirements and is tolerant to salinity and drought. It can be harvested 3–4 times per year. Sweet sorghum stalks contain up to 78% juice [12]. The juice from its stalks consists of fermentable sugars (sucrose, glucose and fructose) that can be directly fermented to produce acetone, butanol and ethanol by Clostridium spp. Daengbussadee et al. [11] reported that a simplified SSJ medium (60 g/L of sugar) supplemented with only 1.27 g/L of (NH4)2SO4 could be used as a butanol production medium yielding a product concentration (PB) of 9.88 g/L.
Laboratory-scale bioreactors, screw-capped bottles, air-locked flasks and stirred-tank bioreactors are generally used to study ABE fermentations. Mixing, mass transfer and heat transfer are completely obtained using a magnetic stirring bar in screw-capped bottles and air-locked flasks. In larger vessels, they are entirely achieved by mechanical agitation with impellers and baffles in stirred-tank bioreactors. However, the fabrication and operation of stirred tank bioreactors are costly. Such reactors have many accessories, are complex in their operation and are difficult to clean and set up. Thus, a low-cost gas-lift bioreactor used as a tower bioreactor for large-scale production was applied in the current study to evaluate butanol production.
Most earlier research in mixed cultures for ABE fermentations was conducted using Clostridium spp. and facultative aerobic bacteria (Bacillus spp.) [13,14,15,16,17]. This study was performed to develop an effective technique to create anaerobic conditions by cultivating the obligate aerobic bacterium, Arthrobacter sp. BCC 72131, prior to ABE fermentation by C. beijerinckii TISTR 1461—which has never been reported. The optimum conditions of ABE fermentation for the mixed cultures were determined. Butanol production by the mixed cultures using a low-cost bioreactor (a gas-lift bioreactor) was first used to appraise its butanol production efficiency compared to using a complex bioreactor (a stirred-tank bioreactor). Additionally, the relationships between cell morphology and product formation during an ABE fermentation by mixed cultures were first reported in this paper.
2. Materials and Methods
2.1. Raw Materials
Sweet sorghum juice (SSJ) (cv. KKU 40) extracted from sorghum stalks was obtained from the Faculty of Agriculture, Khon Kaen University, Thailand. It was concentrated from a total soluble solids content of 17 to 68° Bx by heating at 80–85 °C and stored at −20 °C to protect it against bacterial growth [18]. The composition of concentrated SSJ in this study consisted of total soluble sugars (677.02 g/L), sucrose (236.63 g/L), glucose (201.61 g/L), and fructose (233.14 g/L).
2.2. Butanol Production (BP) Medium Preparation
Concentrated SSJ was diluted with distilled water to obtain a juice containing 60 g/L of total sugars. The juice was then supplemented with 1.27 g/L of (NH4)2SO4 (BDH, Poole, UK) and autoclaved before use as a butanol production (BP) medium [11].
2.3. Microorganisms and Inoculum Preparation
C. beijerinckii TISTR 1461 was purchased from the Thailand Institute of Scientific and Technology Research (TISTR), Khlong Luang, Pathumthani, Thailand. It was maintained as a spore suspension and stored at 4 °C in sterile distilled water. One milliliter of the spore suspension (~1×106 spores/mL) was activated by heat shocking and rejuvenating under anaerobic conditions in a sterile cooked meat medium (CMM) (Oxoid, Basingstoke, Hants, UK) at 37 °C for 16–19 h. The vegetative cells (5% v/v) in CMM medium were inoculated into sterile tryptone–glucose–yeast extract (TGY) medium and incubated at 37 °C for 4–6 h before use as an inoculum for the ABE fermentation [11,19,20]. TGY medium is comprised of 5 g tryptone (Oxoid, Basingstoke, Hants, UK), 1 g glucose (BDH, Leuvn, Belgium), 5 g yeast extract (Oxoid, Basingstoke, Hants, UK) and K2HPO4 (BDH, Leuvn, Belgium) in 1 L distilled water. The CMM and TGY media were autoclaved and purged with OFN gas before use.
Arthrobacter sp. BCC 72131 was purchased from the Thailand Bioresource Research Center (TBRC), Khlong Luang, Pathumthani, Thailand. Cells were grown in a sterile nutrient broth (NB) under shaking conditions at 200 rpm and 30 °C for 6–7 h. Afterwards, the culture was transferred to fresh NB under the same conditions to obtain an optical density at 600 nm of 0.5 (~0.8 g/L of cell dry weight) [11] before use as an inoculum to consume oxygen in a fermentation broth and thereby create anaerobic conditions.
2.4. Experiments
2.4.1. Butanol Production by a Single Culture of C. beijerinckii TISTR 1461
For a positive control experiment, OFN gas at a flow rate of 0.5 vvm was purged for 10 min into a sterile BP medium (Section 2.2) in 1-L screw-capped bottles with a working volume of 700 mL to create anaerobic conditions before fermentation [21]. Five percent (v/v) of the active growing cells (Section 2.3) was then transferred to the BP medium. The fermentation was operated at 37 °C with an agitation rate of 150 rpm until the end of fermentation. For a negative control experiment, the fermentation was carried out in the same manner as the positive control experiment, except there was no OFN gas flushing.
2.4.2. Butanol Production by Mixed Cultures of Arthrobacter sp. BCC 72131 and C. beijerinckii TISTR 1461
First, 5% (v/v) of Arthrobacter sp. BCC 72131 in NB (Section 2.3) was inoculated in sterile BP medium in 1-L screw-capped bottles and incubated at 30 °C with 150 rpm for 2, 4, 6 and 12 h. After each time, the active growing cells of C. beijerinckii TISTR 1461 were transferred to a BP medium to start the ABE fermentation. The fermentation was controlled at 37 °C with agitation at 150 rpm.
2.4.3. Butanol Production by Mixed Cultures in Stirred-Tank and Gas-Lift Bioreactors
A 5.6-L stirred-tank bioreactor (BioFlo® 320 Vessels, Eppendorf, Hamburg, Germany) and a 1.2-L low-cost gas-lift bioreactor, shown in Figure 1, were sterilized before adding a sterile BP medium with working volumes of 3.6 and 0.8 L, respectively. Arthrobacter sp. BCC 72131 (5% v/v) was then inoculated into the sterile BP medium in both bioreactors and incubated at 30 °C with agitation rate of 150 rpm using a six-blade dish turbine and four baffles in the stirred-tank bioreactor. Mixing in the gas-lift bioreactor was controlled with a draft tube and the circulation of gases in the head space was achieved using a peristaltic pump (Masterflex®, Radnor, PA, USA) at a flow rate of 0.25 vvm (modified from [22]). After the optimum time of Arthrobacter cultivation (obtained as described in Section 2.4.2), active C. beijerinckii TISTR 1461 cells were added into the bioreactors to start ABE fermentations. Butanol fermentations were performed at 37 °C. During fermentation, samples were taken at predetermined time intervals for analyses.
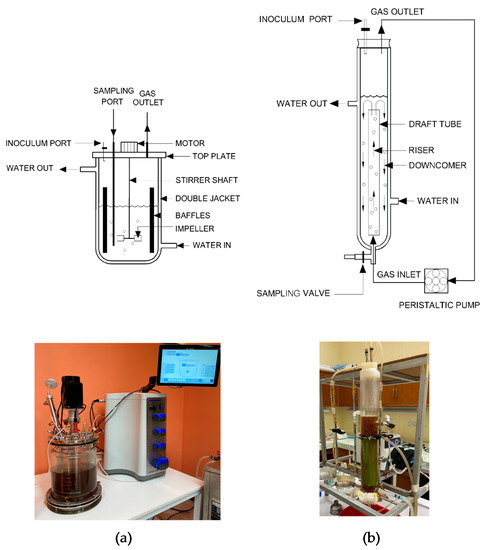
Figure 1.
Experimental setup for butanol fermentation in (a) stirred-tank bioreactor and (b) gas-lift bioreactor.
2.5. Analytical Methods
Samples were centrifuged at 12,000 rpm for 10 min to the separate cells and particles from the supernatant (Sigma 1–14, Sartorius, Osterode am Harz, Germany). The supernatant was filtered through a 0.45 µm nylon membrane (Sartorius, Goettingen, Germany) for analysis. Acetone, butanol, ethanol, acetic acid, and butyric acid in the supernatant were analyzed using a gas chromatograph (Shimadzu, GC-2014, Kyoto, Japan) employing a Porapak Q column (80/100 mesh, 3 m × 2 mm, Resteck, PA, USA), and nitrogen was used as a carrier gas [11]. pH was measured using a pH meter. The total sugar concentration was determined using a phenol–sulfuric acid method [23]. A quantitative analysis of fructose, glucose and sucrose was performed with an HPLC-RI (Waters, MA, USA) using an Inertsil® NH2 column (5 µm, 250 × 4.6 mm, GL Sciences, Tokyo, Japan). The analysis was performed at 35 °C with a flow rate of 0.8 mL/min using an isocratic system with a mixture ratio of 75 parts acetonitrile: 25 parts water (v/v) as a mobile phase [24,25]. Protein and free amino nitrogen (FAN) concentrations under optimal conditions and control experiments were also measured using the Bradford method [26] and a ninhydrin assay [27], respectively.
The morphology of C. beijerinckii TISTR 1461 during fermentation was observed using field emission scanning electron microscopic (FE-SEM) analysis. Samples were taken from the bioreactors, quickly filtered through a 0.2 µm cellulose acetate membrane (Whatman®, Maidstone, England), and washed twice with distilled water. The filtrate and bacterial cells remaining on the membrane were then dried overnight at 50 °C in a hot air oven. Then, the dried material was sputter-coated with gold. An accelerating voltage of 10 kV under a vacuum was used to yield high-resolution images. Microscopic analysis was performed with a FEI Helios Nanolab G3 CX Dual Beam FIB/SEM, Thermo Fisher Scientific, Hillsboro, OR, USA.
All experiments were performed in triplicate, and the results are expressed as mean values ± SD. The butanol yield (YB/S, g/g), ABE yield (YABE/S, g/g), volumetric butanol productivity (QB, g/L∙h) and sugar consumption (SC, %) were calculated as described in Daengbussadee et al. [11].
3. Results and Discussion
3.1. Butanol Production from SSJ Medium by Single and Mixed Cultures
Butanol fermentations by a single culture of C. beijerinckii TISTR 1461 with and without OFN gas flushing were carried out as positive and negative control treatments, respectively. The pH changes during the fermentation of both control treatments were similar (Figure 2a). However, the PB (9.88 ± 0.38 g/L), ABE concentrations (PABE, 17.61 ± 0.63 g/L) and sugar utilization (53.46 ± 0.46%) were clearly higher for the positive control with OFN gas flushing than those of the negative control with no OFN gas flushing (Figure 2b–d and Table 1). This confirms that C. beijerinckii TISTR 1461 produced higher butanol levels under strictly anaerobic conditions.
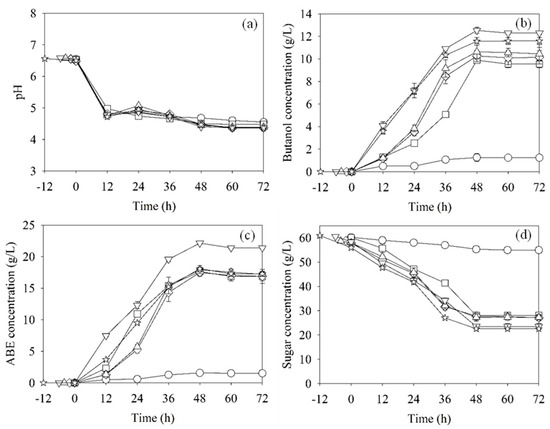
Figure 2.
Profiles of pH (a), butanol (b), ABE (c) and sugar (d) concentrations during ABE fermentation by the mixed cultures and single culture (positive control (□); negative control (○)). In the mixed cultures, C. beijerinckii TISTR 1461 was inoculated after 2 (◇), 4 (△), 6 (▽) and 12 h (☆) of Arthrobacter sp. cultivation.

Table 1.
Kinetic parameters of ABE fermentation by the mixed cultures of C. beijerinckii TISTR 1461 and Arthrobacter sp. BCC 72131 at different times of Arthrobacter sp. cultivation prior to C. beijerinckii inoculation and by a single culture of C. beijerinckii TISTR 1461 using OFN gas flushing.
For mixed cultures, after 2, 4, 6 and 12 h of Arthrobacter sp. cultivation, C. beijerinckii TISTR 1461 was then inoculated to start the ABE fermentation. The results showed that the ABE fermentation profiles of all conditions of the mixed cultures were similar to those of the positive control treatment using OFN gas flushing (Figure 2). The PB, sugar utilization and QB of the conditions under which C. beijerinckii TISTR 1461 was inoculated after 2 and 4 h were not significantly different compared to the positive control treatment (Table 1). These results clearly indicated that an obligate aerobe, Arthrobacter sp., could be used to create anaerobic conditions. The inoculation of C. beijerinckii TISTR 1461 after 6 h gave the highest PB (12.56 ± 0.55 g/L) and PABE (21.18 ± 0.44 g/L). Surprisingly, the PB and PABE of the mixed cultures under this condition were approximately 20–27% higher than those of the positive control treatment. The PB when C. beijerinckii was inoculated after 12 of Arthrobacter sp. cultivation was not significantly different from inoculation after 6 h (Table 1). This suggests that 6 h of Arthrobacter sp. cultivation was appropriate for developing anaerobic conditions for butanol production by C. beijerinckii TISTR 1461. The PABE value when C. beijerinckii was inoculated after 12 h of Arthrobacter sp. cultivation was lower than that after 6 h, but the sugar utilization of the former was higher than the latter. These observations might be due to the differences in the by-products produced by the pyruvate metabolism pathway that were not detected in this study such as hydrogen, isopropanol, lactic and propionic acids, carbon dioxide and lipids [28].
It was found that sugar consumption by Arthrobacter sp. before C. beijerinckii TISTR 1461 inoculation in all experiments ranged from 1.54 to 3.96 g/L. This implies that Arthrobacter sp. consumed some sugar and all the oxygen in the fermentation broth to grow or survive. During ABE fermentation, C. beijerinckii TISTR 1461 continuously consumed sugars in the fermentation broth. Under inoculating C. beijerinckii after 6 h of Arthrobacter sp. cultivation, the total remaining sugars at the end of fermentation were 24.42 g/L (~60% sugar consumption). The residual sugar fraction consisted of glucose (0.40 g/L), fructose (8.90 g/L), sucrose (9.57 g/L) and non-fermentable sugars (5.55 g/L). The fermentable sugars in the broth were not completely consumed, which might have been due to butanol toxicity (PB, 12.56 ± 0.55 g/L and PABE, 21.18 ± 0.44 g/L). This was supported by Xu et al. [29] who reported that the cell growth stopped when the butanol concentration reached ~10 g/L and the fermentation stopped at ~12 g/L of butanol, although there was still plenty of glucose present in the medium.
After 12 h of fermentation by the mixed cultures, the cell debris of Arthrobacter sp. was observed under a light microscope. Therefore, the protein and free amino nitrogen (FAN) in the fermentation broth were determined during the ABE fermentation (Figure 3). It was found that the initial values of protein and FAN in the SSJ medium using the mixed cultures and single cultures were not different. Protein and FAN increased within 24 h and decreased after that in the mixed cultures. In the fermentation by the single culture, the protein content in the broth was relatively constant and the FAN continuously decreased as the ABE fermentation progressed. In the fermentation by the mixed cultures, the increase in protein and FAN within 24 h after C. beijerinckii inoculation might have been due to the autolysis of Arthrobacter sp. This might promote the growth and butanol production of C. beijerinckii TISTR 1461 in a way that is not possible using a single culture of the Clostridium.
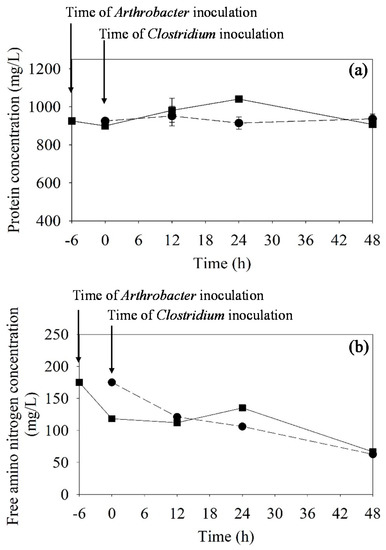
Figure 3.
Profiles of protein (a) and free amino nitrogen (FAN) (b) concentrations in the fermentation broth during ABE fermentation by mixed cultures of Arthrobacter sp. and C. beijerinckii TISTR 1461 (inoculating C. beijerinckii after 6 h of Arthrobacter cultivation, ■) and by a single culture of C. beijerinckii using OFN gas flushing (●).
3.2. Morphology of C. beijerinckii TISTR 1461 during ABE Fermentation by a Single Culture and Mixed Cultures
The morphologies of C. beijerinckii TISTR 1461 during ABE fermentation by single and mixed cultures under optimal conditions were compared. The FE-SEM micrographs of a single culture using OFN gas flushing are shown in Figure 4. In the first phase—the acidogenesis phase (0–12 h) (Figure 2a)—the cells appeared as short and highly motile rods (Figure 4a,b). During 24–36 h of ABE fermentation, the cells were enlarged, swollen and moved sluggishly (Figure 4c,d). In this phase—the solventogenesis phase—high butanol levels were produced (Figure 2b). After that, forespores were observed (Figure 4e and Figure 5a), and the maximal PB value was achieved at 48 h of fermentation (Figure 2b). The occurrence of forespores might have been due to butanol toxicity. Then, no butanol was produced until the end of fermentation. During this period, the cells had two survival mechanisms: solventogenesis and sporulation (Figure 4f) [30].
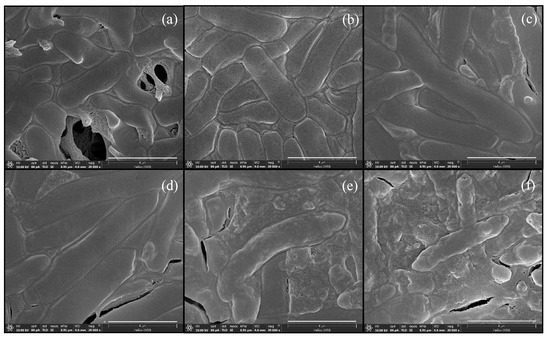
Figure 4.
FE-SEM micrograph of C. beijerinckii TISTR 1461 during ABE fermentation in SSJ medium by a single culture at 0 h (a), 12 h (b), 24 h (c), 36 h (d), 48 h (e) and 60 h (f) of fermentation. Magnification of 20,000×. Scale bar: 4 µm.
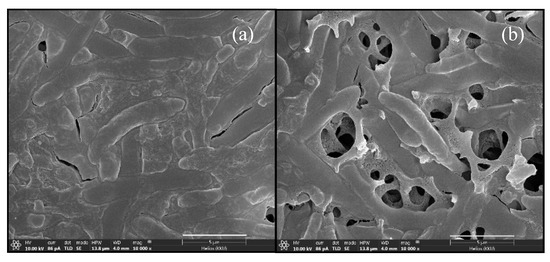
Figure 5.
FE-SEM micrograph of C. beijerinckii TISTR 1461 at 48 h of ABE fermentation in SSJ medium by a single culture (a) and mixed cultures (inoculating C. beijerinckii after 6 h of Arthrobacter cultivation) (b). Magnification of 10,000×. Scale bar: 5 µm.
In ABE fermentations by the mixed cultures, the morphology of C. beijerinckii TISTR 1461 appeared perfect with strongly motile long rods (Figure 5b), and a higher PB (12.56 ± 0.55 g/L) was obtained at 48 h after C. beijerinckii TISTR 1461 inoculation (Table 1). In contrast, the morphology of single culture C. beijerinckii TISTR 1461 at 48 h was imperfect and forespores were observed (Figure 5a). A lower PB value (9.88 ± 0.38 g/L) was obtained in a single culture (Table 1). The results of FE-SEM indicated that Arthrobacter sp. could not only be used to effectively create anaerobic conditions, but its use also promoted butanol production from the SSJ medium by C. beijerinckii TISTR 1461.
3.3. Butanol Production by the Mixed Cultures in Stirred-Tank and Gas-Lift Bioreactors
To study the capability of butanol production by the mixed culture in a low-cost bioreactor, i.e., a gas-lift bioreactor, the fermentation profiles of butanol production from the SSJ medium by mixed cultures of C. beijerinckii TISTR 1461 and Arthrobacter sp. BCC 72131 (inoculating C. beijerinckii after 6 h of Arthrobacter cultivation) in the screw-capped bottle, stirred-tank and gas-lift bioreactors were compared in Figure 6. The pHs profiles in the three bioreactors were similar (Figure 6a). The pHs decrease corresponded to the production of acids (Figure 6b,d), indicating that bacterial cells were active. Moreover, the movement of cells during this period under the microscope was swift (data not shown). In a stirred-tank bioreactor, the sugar consumption and product formation during the first period of fermentation were slightly higher than those in the screw-capped bottle and gas-lift bioreactor (Figure 6e–i). These might be due to the positive effect of the mixing. The results suggested that mixing, mass transfer and heat transfer phenomena in the stirred-tank bioreactor occurred faster than those in the screw-capped bottle and gas-lift bioreactor. However, the PB of each mixing method in screw-capped bottle (12.56 ± 0.55 g/L), stirred-tank bioreactor (12.59 ± 0.45 g/L) and gas-lift bioreactor (12.00 ± 0.48 g/L) were not significantly different at the end of fermentation (Table 2). The kinetic parameters of ABE fermentation in the various bioreactors tested were not significantly different, suggesting that the mass transfer phenomenon in the bioreactors successfully occurred via mixing. In addition, the ABE yield (YABE/S) and YB/S under all tested conditions was not significantly different, indicating that the types of bioreactor used did not affect the metabolic pathway of ABE fermentation by the mixed cultures (Table 2). With regard to the sizes of the bioreactors and the head space during ABE fermentation, the working volumes of the 1-L screw-capped bottle, 5.6-L stirred-tank bioreactor and 1.2-L gas-lift bioreactor were 0.7, 3.6 and 0.8 L, respectively. These correspond to head space volumes of 30, 36 and 33%, respectively. Similar ABE fermentation results in Table 2 imply that the different sizes of three bioreactors with a similar head space did not have any impact on ABE fermentation. Therefore, Arthrobacter sp., an obligate aerobe bacterium, is an effective microorganism for creating anaerobic conditions for butanol production by C. beijerinckii TISTR 1461 in all bioreactors, including the low-cost bioreactor, i.e., the gas-lift bioreactor.
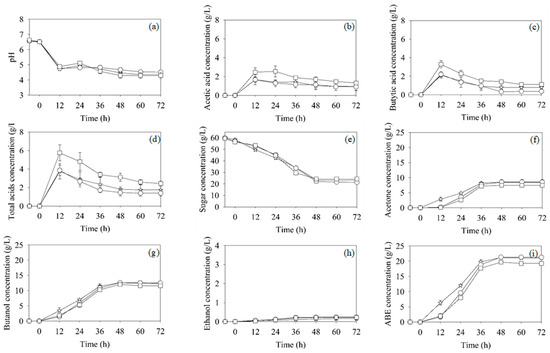
Figure 6.
Profiles of pH (a), acetic acid (b), butyric acid (c), total acids (d), total sugar (e), acetone (f), butanol (g), ethanol (h) and ABE concentrations (i) during batch ABE fermentation by the mixed cultures in screw-capped bottles (○), stirred-tank bioreactor (☆) and gas-lift bioreactor (□).

Table 2.
Kinetic parameters of ABE fermentation by the mixed cultures of C. beijerinckii TISTR 1461 and Arthrobacter sp. BCC 72131 (inoculating C. beijerinckii after 6 h of Arthrobacter cultivation) in various bioreactors after 54 h of fermentation.
3.4. Comparison of Butanol Production by Mixed Cultures with Other Studies
In this study, Arthrobacter sp. BCC 72131 was used to create anaerobic conditions for butanol production by C. beijerinckii TISTR 1461. Earlier research was performed using other microorganisms (e.g., Bacillus subtilis, B. cereus, B. cellolyticus and Saccharomyces cerevisiae) to create anaerobic conditions for butanol production by Clostridium. Butanol production levels by mixed cultures are summarized in Table 3. It was found that the PB values in all previous research ranged from 3.77 to 14.90 g/L, while QB and YB/S ranged from 0.02 to 0.23 g/L·h and from 0.21 to 0.34 g/g, respectively. The PB values in the current study were relatively high compared to earlier works. In our study, the PB was slightly lower than that reported by Abd-Alla and Elsadek El-Enany [15], which might have been due to the differences in initial substate concentration, the bacterial cells used and environmental conditions for ABE fermentation. However, QB and YB/S in our study were higher than those in other studies by approximately 1.1–11.5-fold. This implies that Arthrobacter sp. has high potential for use to create anaerobic conditions for butanol production. Butanol production by the mixed cultures of Arthrobacter sp. and C. beijerinckii TISTR 1461 has high potential for use in large-scale production.
An alternative method to create anaerobic conditions for ABE fermentations is using chemicals that strongly react with oxygen such as sodium dithionite (STDN). It was reported that 0.25 mM SDTN could be used to create anaerobic conditions for C. beijerinckii to produce butanol (8.51 g/L) but very low butanol productivity (0.1 g/L·h) was obtained [11]. According to our results, Arthrobacter sp. is suitable to be used to create anaerobic conditions for butanol production. During anaerobic condition creation, sugar consumption by Arthrobacter sp. in various bioreactors was only 2.20–2.76 g/L, corresponding to 3.67–4.60% of the total sugar. However, the economics of using the mixed cultures (Arthrobacter sp. and C. beijerinckii TISTR 1461) and a single culture of C. beijerinckii TISTR 1461 with OFN gas flushing needs to be further evaluated [31].


Table 3.
Batch butanol production by mixed cultures in earlier research.
Table 3.
Batch butanol production by mixed cultures in earlier research.
| Substrate | Mixed Cultures | Bioreactor | Butanol Production | References | ||
|---|---|---|---|---|---|---|
| PB (g/L) | QB (g/L·h) | YB/S (g/g) | ||||
| SSJ (60 g/L of sugar) | C. beijerinckii TISTR 1461 + Arthrobacter sp. | 1-L screw-capped bottle | 12.56 | 0.23 | 0.34 | The current study |
| SSJ (60 g/L of sugar) | C. beijerinckii TISTR 1461 + Arthrobacter sp. | 5.6-L stirred-tank bioreactor | 12.59 | 0.23 | 0.34 | The current study |
| SSJ (60 g/L of sugar) | C. beijerinckii TISTR 1461 + Arthrobacter sp. | 1.2-L gas-lift bioreactor | 12.00 | 0.22 | 0.33 | The current study |
| Cassava starch (40 g/L of starch) | C. butylicum TISTR 1032 + B. subtilis WS 161 | 120-mL serum bottle | 6.70 | 0.10 | 0.21 | [13] |
| Pretreated palm pressed fiber (5 g/L) | C. acetobutylicum DSM 1731 + B. cellolyticus JCM 9156 | 60-mL serum bottle | 3.77 | 0.02 | - | [14] |
| Spoilage date fruit (75 g/L) | C. acetobutylicum ATCC 824 + B. subtilis DSM 4451 | 2-L fermenter | 14.90 | 0.21 | 0.29 | [15] |
| Corn mash (6.5% by wt. of corn flour) | C. beijerinckii NCIMB 8052 + B. cereus CGMCC 1.895 | 5-L fermenter | 6.78 | 0.07 | - | [16] |
| Agave hydrolysate (52 g/L) | C. acetobutylicum ATCC 824 + B. subtilis CDBB 555 | 120-mL glass bottle | 8.28 | 0.10 | 0.29 | [17] |
| Mixed-sugars (25 g/L of xylose and 25 g/L of glucose) | C. acetobutylicum CH02 + S. cerevisiae | 250-mL flask | 8.33 | 0.09 | 0.18 | [32] |
PB, butanol concentration; QB, volumetric butanol productivity; and YB/S, butanol yield.
4. Conclusions
Mixed cultures of Arthrobacter sp. BCC 72131 and C. beijerinckii TISTR 1461 were successfully used to produce butanol from a simple sweet sorghum juice medium. Arthrobacter sp. is a highly effective bacterium for creating anaerobic conditions that can be used in place of the traditional method employing OFN gas flushing. Inoculating C. beijerinckii 6 h after Arthrobacter sp. was the most appropriate method for butanol production from sweet sorghum juice. The mixed cultures yielded higher butanol production than a single culture of C. beijerinckii TISTR 1461. Butanol production by mixed cultures of Arthrobacter sp. BCC 72131 and C. beijerinckii TISTR 1461 can be applied in large-scale stirred-tank and low-cost gas-lift bioreactors.
Author Contributions
Conceptualization, P.L.; methodology and formal analysis, C.D.; investigation and writing—original draft preparation, C.D.; writing—review and editing, L.L. and P.L.; supervision, P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fermentation Research Center for Value Added Agricultural Products (FerVAAP), Khon Kaen University, Thailand, funding number 01/2564.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Sweet sorghum juice was provided by the Division of Agronomy, Faculty of Agriculture, Khon Kaen University, Thailand.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dzida, M. Thermophysical properties of 1-butanol at high pressures. Energies 2020, 13, 5046. [Google Scholar] [CrossRef]
- Dürre, P. From Pandora’s box to cornucopia: Clostridia a historical perspective. In Clostridia: Biotechnology and Medical Applications; Bahl, H., Dürre, P., Eds.; Wiley-VCH: Weinheim, Germany, 2001; pp. 1–17. [Google Scholar]
- Xue, C.; Wu, Y.; Gu, Y.; Jiang, W.; Dong, H.; Zhang, Y.; Zhao, C.; Li, Y. Biofuels and bioenergy: Acetone and butanol. Compr. Biotechnol. 2019, 3, 79–100. [Google Scholar] [CrossRef]
- Xue, C.; Liu, F.; Xu, M.; Zhao, J.; Chen, L.; Ren, J.; Bai, F.; Yang, S.T. A novel in situ gas stripping-pervaporation process integrated with acetone-butanol-ethanol fermentation for hyper n-butanol production. Biotechnol. Bioeng. 2016, 113, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhou, Y.; Luo, Z.; Cui, Y.; Xu, Y.; Lin, L.; Zhao, M.; Guo, Y.; Pang, Z. Dual function of ammonium acetate in acetone-butanol-ethanol fermentation by Clostridium acetobutylicum. Bioresour. Technol. 2018, 267, 319–325. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, J.H.; Jang, S.H.; Nielsen, L.K.; Kim, J.; Jung, K.S. Fermentative butanol production by Clostridia. Biotechnol. Bioeng. 2008, 101, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Narueworanon, P.; Laopaiboon, L.; Phukoetphim, N.; Laopaiboon, P. Impacts of initial sugar, nitrogen and calcium carbonate on butanol fermentation from sugarcane molasses by Clostridium beijerinckii. Energies 2020, 13, 694. [Google Scholar] [CrossRef] [Green Version]
- Sirisantimethakom, L.; Thanapornsin, T.; Laopaiboon, L.; Laopaiboon, P. Enhancement of butanol production efficiency from sweet sorghum stem juice by Clostridium beijerinckii using statistical experimental design. Chiang Mai J. Sci. 2018, 45, 1235–1246. [Google Scholar]
- Pinto, T.; Flores-Alsina, X.; Gernaey, K.V.; Junicke, H. Alone or together? A review on pure and mixed microbial cultures for butanol production. Renew. Sustain. Energy Rev. 2021, 147, 111244. [Google Scholar] [CrossRef]
- Qureshi, N.; Blaschek, H.P. Production of acetone butanol ethanol (ABE) by a hyper-producing mutant strain of Clostridium beijerinckii BA101 and recovery by pervaporation. Biotechnol. Prog. 1999, 15, 594–602. [Google Scholar] [CrossRef]
- Daengbussadee, C.; Laopaiboon, L.; Kaewmaneewat, A.; Sirisantimethakom, L.; Laopaiboon, P. Novel methods using an Arthrobacter sp. to create anaerobic conditions for biobutanol production from sweet sorghum juice by Clostridium beijerinckii. Processes 2021, 9, 178. [Google Scholar] [CrossRef]
- Mathur, S.; Umakanth, A.V.; Tonapi, V.A.; Sharma, R.; Sharma, M.K. Sweet sorghum as biofuel feedstock: Recent advances and available resources. Biotechnol. Biofuels 2017, 10, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, H.T.M.; Cheirsilp, B.; Hodgson, B.; Umsakul, K. Potential use of Bacillus subtilis in a co-culture with Clostridium butylicum for acetone-butanol-ethanol production from cassava starch. Biochem. Eng. J. 2010, 48, 260–267. [Google Scholar] [CrossRef]
- Ponthein, W.; Cheirsilp, B. Development of acetone butanol ethanol (ABE) production from palm pressed fiber by mixed culture of Clostridium sp. and Bacillus sp. Energy Procedia 2011, 9, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Abd-Alla, M.H.; Elsadek El-Enany, A.-W. Production of acetone-butanol-ethanol from spoilage date palm (Phoenix dactylifera L.) fruits by mixed culture of Clostridium acetobutylicum and Bacillus subtilis. Biomass Bioenergy 2012, 42, 172–178. [Google Scholar] [CrossRef]
- Mai, S.; Wang, G.; Wu, P.; Gu, C.; Liu, H.; Zhang, J.; Wang, G. Interactions between Bacillus cereus CGMCC 1.895 and Clostridium beijerinckii NCIMB 8052 in coculture for butanol production under nonanaerobic conditions. Biotechnol. Appl. Biochem. 2017, 64, 719–726. [Google Scholar] [CrossRef]
- Oliva-Rodríguez, A.G.; Quintero, J.; Medina-Morales, M.A.; Morales-Martínez, T.K.; Rodríguez-De la Garza, J.A.; Moreno-Dávila, M.; Aroca, G.; Rios González, L.J. Clostridium strain selection for co-culture with Bacillus subtilis for butanol production from agave hydrolysates. Bioresour. Technol. 2019, 275, 410–415. [Google Scholar] [CrossRef]
- Laopaiboon, L.; Nuanpeng, S.; Srinophakun, P.; Klanrit, P.; Laopaiboon, P. Ethanol production from sweet sorghum juice using very high gravity technology: Effects of carbon and nitrogen supplementations. Bioresour. Technol. 2009, 100, 4176–4182. [Google Scholar] [CrossRef]
- Wechgama, K.; Laopaiboon, L.; Laopaiboon, P. Biobutanol production from agricultural raw materials by Clostridium spp. Chiang Mai J. Sci. 2017, 44, 394–405. [Google Scholar]
- Ezeji, T.C.; Qureshi, N.; Blaschek, H.P. Production of acetone, butanol and ethanol by Clostridium beijerinckii BA101 and in situ recovery by gas stripping. World J. Microbiol. Biotechnol. 2003, 19, 595–603. [Google Scholar] [CrossRef]
- Sirisantimethakom, L.; Laopaiboon, L.; Sanchanda, P.; Chatleudmongkol, J.; Laopaiboon, P. Improvement of butanol production from sweet sorghum juice by Clostridium beijerinckii using an orthogonal array design. Ind. Crop. Prod. 2016, 79, 287–294. [Google Scholar] [CrossRef]
- Thanapornsin, T.; Sanchanda, P.; Laopaiboon, L.; Laopaiboon, P. Batch butanol fermentation from sugarcane molasses integrated with a gas stripping system: Effects of sparger types and gas flow rates. Asia-Pac. J. Sci. Technol. 2018, 23, APST-23-04-08. [Google Scholar] [CrossRef]
- Mecozzi, M. Estimation of total carbohydrate amount in environmental samples by the phenol-sulphuric acid method assisted by multivariate calibration. Chemom. Intell. Lab. Syst. 2005, 79, 84–90. [Google Scholar] [CrossRef]
- Sirisantimethakom, L.; Laopaiboon, L.; Danvirutai, P.; Laopaiboon, P. Volatile compounds of a traditional Thai rice wine. Biotechnology 2008, 7, 505–513. [Google Scholar] [CrossRef]
- Sriputorn, B.; Laopaiboon, P.; Phukoetphim, N.; Polsokchuak, N.; Butkun, K.; Laopaiboon, L. Enhancement of ethanol production efficiency in repeated-batch fermentation from sweet sorghum stem juice: Effect of initial sugar, nitrogen and aeration. Electron. J. Biotechnol. 2020, 46, 55–64. [Google Scholar] [CrossRef]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Abernathy, D.G.; Spedding, G.; Starcher, B. Analysis of protein and total usable nitrogen in beer and wine using a microwell ninhydrin assay. J. Inst. Brew. 2009, 115, 122–127. [Google Scholar] [CrossRef]
- Dahman, Y.; Dignan, C.; Fiayaz, A.; Chaudhry, A. An Introduction to Biofuels, Foods, Livestock, and the Environment; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; ISBN 9780081024263. [Google Scholar]
- Xu, M.; Zhao, J.; Yu, L.; Tang, I.C.; Xue, C.; Yang, S.T. Engineering Clostridium acetobutylicum with a histidine kinase knockout for enhanced n-butanol tolerance and production. Appl. Microbiol. Biotechnol. 2015, 99, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Al-Hinai, M.A.; Jones, S.W.; Papoutsakis, E.T. The Clostridium sporulation programs: Diversity and preservation of endospore differentiation. Microbiol. Mol. Biol. Rev. 2015, 79, 19–37. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, A.; Valdez-Vazquez, I.; Soto, A.; Sánchez, S.; Tavarez, D. Lignocellulosic n-butanol co-production in an advanced biorefinery using mixed cultures. Biomass Bioenergy 2017, 102, 1–12. [Google Scholar] [CrossRef]
- Qi, G.X.; Xiong, L.; Huang, C.; Chen, X.F.; Lin, X.Q.; Chen, X. De solvents production from a mixture of glucose and xylose by mixed fermentation of Clostridium acetobutylicum and Saccharomyces cerevisiae. Appl. Biochem. Biotechnol. 2015, 177, 996–1002. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).