Yeast Hybrids in Brewing
Abstract
1. Species of Saccharomyces
2. Hybrid Nature of Yeast
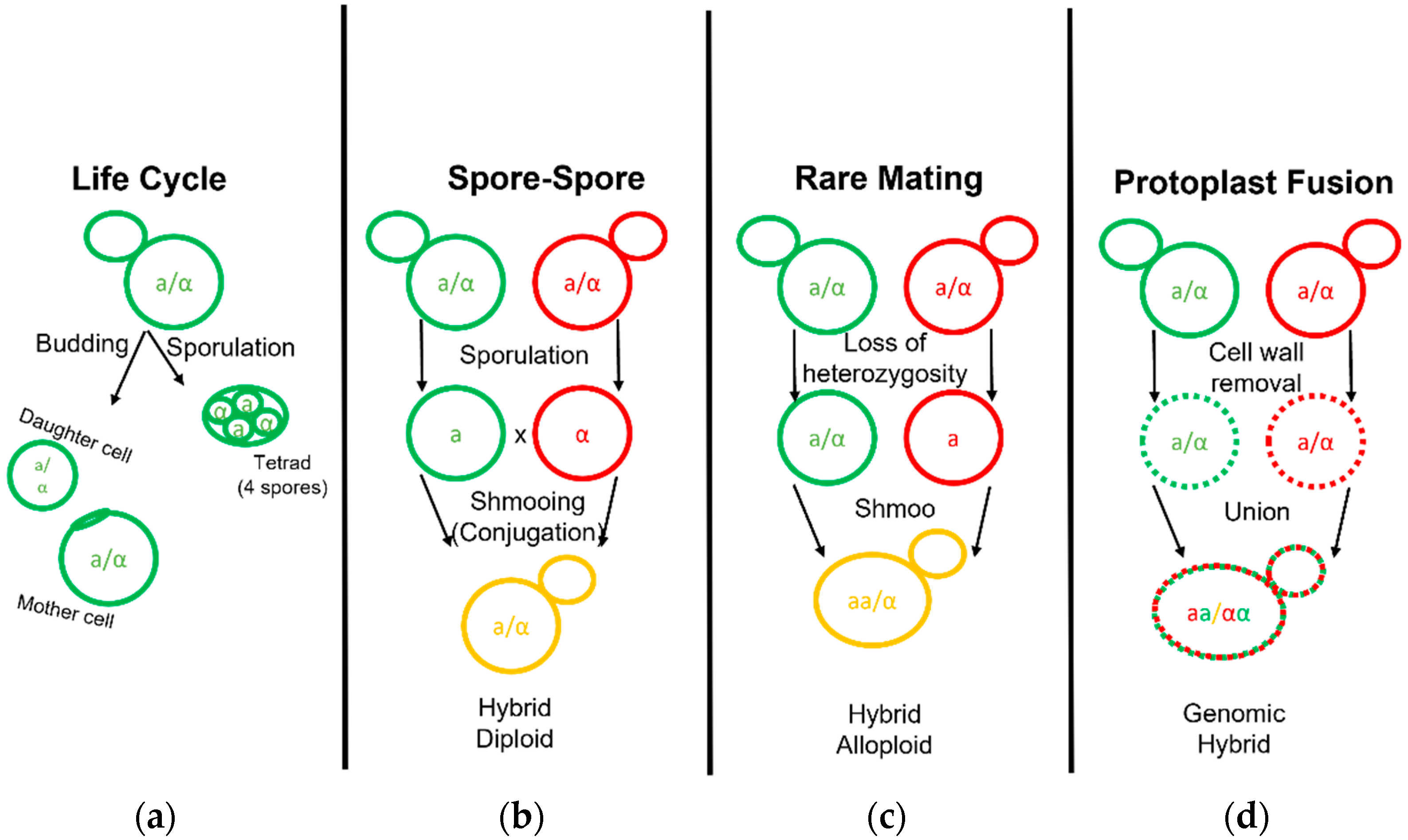
3. Novel Hybrid Development
| History | Parents | Reference |
|---|---|---|
| Isolated | S. cerevisiae × S. eubayanus | [11] |
| Isolated | S. cerevisiae × S. eubayanus × S. uvarum | [41] |
| Isolated | S. cerevisiae × S. uvarum | [68] |
| Isolated | S. cerevisiae × S. kudriavzevii | [27,28,42] |
| Isolated | S. uvarum × S. eubayanus | [27,42] |
| Developed | S. cerevisiae × S. eubayanus | [43,45,46,47,69] |
| Developed | S. cerevisiae × S. mikatae | [48] |
| Developed | S. cerevisiae × S. kudriavzevii × S. paradoxus | [49] |
| Developed | S. cerevisiae × S. kudriavzevii | [52] |
| Developed | S. cerevisiae × S. arboricola | [48,51] |
| Developed | S. cerevisiae × S. jurei | [70] |
4. Natural Considerations and State of the Field
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| Terminology | Description |
| a/α | The two mating types of S. cerevisiae that enable cellular fusion when the complimentary pheromone is detected. The mating type and sexual state is largely determined by the MAT locus on chromosome III. |
| Allele | A variant of any particular gene found at the same genomic location. The mating type of yeast is determined by which allele, MATa or MATα, is present at the MAT locus. |
| Alloploids | A hybrid organism or cell composed of two or more sets of chromosomes obtained from two separate species. |
| Auxotrophy | The loss of a functional gene needed for growth. This is particularly relevant for amino acid synthesis and metabolism during yeast genetics experiments where controlling growth is needed. |
| Ascus | The sack like structure enclosing the four spores of Saccharomyces, this characteristic is important in classification of the Ascomycota (sac fungi) yeast. |
| Brassica oleracea | Important food crop plant species. It is wild cabbage in the uncultivated form, but cultivation has yielded varieties over time to include broccoli, brussels sprouts, kale, cauliflower, cabbage, and collards. |
| Crabtree Positive | Microorganism that preferentially metabolizes sugar into ethanol in the presence of oxygen instead of cellular respiration. |
| Diauxic Shift | The shift from fermentation to utilizing ethanol for cellular respiration and cell growth. |
| Diploid | An organism with a paired set of chromosomes creating a (2n) genome, such as a human with a set of genomes from each parent. |
| Fagaceae spp. | Family of Angiosperms (flowering plants) that include the beech and oak trees. |
| Frohberg | One of two primary lineages of modern lager yeast known to brewers originally used in Germany. |
| Gene | The basic unit of heredity which is composed of a sequence of DNA. It is characterized by a start sequence, genomic segment that often is translatable into a protein of function, and a stop sequence. |
| Genomics | The study of all of an organism’s complete sequences of genetic materials composed of DNA (deoxyribonucleic acid) including structure, function, evolution, mapping, editing, and environmental interactions. |
| Haploid | An organism with one set of chromosomes composing their genome (1n). |
| Hemizygous | A condition of diploid cells where only one copy of a gene or other genetic component is present. |
| Hybrid Vigor (syn. Heterosis) | The tendency for the hybrid progeny between two species to outperform their parents in traits such as strength, size, or yield. |
| Interspecific | Arising or existing between separate species. |
| Intraspecific | Arising or existing within a species or individuals from the same species. |
| Locus | A specific fixed position on an individual chromosome where particular genetic content is present. |
| Make Accumulate Consume | S. cerevisiae yeast survival strategy that invokes the ability to make ethanol from saccharides, the ability to survive accumulation of the toxin, and the ability to consume ethanol for energy post fermentation. |
| Maltotriose | Prominent trisaccharide typical of beer wort and is enzymatically derived from starch. It is composed of three glucose molecules linked together by α-1,4 glycosidic bonds. |
| Nomenclature | The terminology and language used to categorize and communicate in various disciplines. |
| Out of Asia/Silk Road Hypothesis | The idea that Saccharomyces yeast originate from an Asian geographical region because of the abundance natural biodiversity found in that region. |
| Phenotype | A characterized trait, best exemplified by measurable qualities such as color or yield. |
| Phylogeny | The lineage and evolution of relative organisms. |
| Progeny | the offspring or descendants of an organism. |
| Quiescence | A state of inactivity, dormancy, or a period of idleness in yeast ecology. |
| Saaz | one of two primary lineages of modern lager yeast known to brewers originally used in Bohemia region of the Czech Republic where the town Žatec/Saaz is located. |
| Shmoo | The distinct physical form of two Saccharomyces yeast cells mating. This terminology originated from the morphological similarity to an Al Capp cartoon popular in America during the early 1950s. |
| Synanthropic Species | An undomesticated organism that habitually exists with human populations and benefit from non-natural environments. Their life cycles are adapted fully or in part to conditions created by human activity. |
| Tetrad | Four spores produced via meisis of Ascomycota yeast, specifically focused on yeast S. cerevisiae in this article. |
| Transgressive Phenotype | Formation of extreme phenotypes that surpass the ability of parental lineages, often found in hybrids and can be positive or negative for fitness of the individual. |
References
- Gallone, B.; Steensels, J.; Baele, G.; Maere, S.; Verstrepen, K.J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; et al. Domestication and Divergence of Saccharomyces cerevisiae Beer Yeasts. Cell 2016, 166, 1397–1410.e16. [Google Scholar] [CrossRef] [PubMed]
- Preiss, R.; Tyrawa, C.; Krogerus, K.; Garshol, L.M.; Van Der Merwe, G. Traditional Norwegian Kveik are a genetically distinct group of domesticated Saccharomyces cerevisiae brewing yeasts. Front. Microbiol. 2018, 9, 2137. [Google Scholar] [CrossRef] [PubMed]
- Pasteur, L. Nouveaux faits concernant l’histoire de la fermentation alcoolique. Comptes Rendus Chim. 1858, 47, 1011–1013. [Google Scholar]
- Meyen, F.J.F. Jahresbericht über die Resultate der Arbeiten im Felde der physiologischen Botanik von dem Jahre 1837. In Berlin: Nicolai’sche Buchhandlung; Nicolai: Berlin, Germany, 1838; pp. 1–186. [Google Scholar]
- Martini, A.V.; Martini, A. Three newly delimited species of Saccharomyces sensu stricto. Antonie Van Leeuwenhoek 1987, 53, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.A.; Bai, F.Y. Saccharomyces arboricolus sp. nov., a yeast species from tree bark. Int. J. Syst. Evol. Microbiol. 2008, 58, 510–514. [Google Scholar] [CrossRef]
- Naumova, E.S.; Roberts, I.N.; James, S.A.; Naumov, G.I.; Louis, E.J. Three new species in the Saccharomyces sensu stricto complex: Saccharomyces cariocanus, Saccharomyces kudriavzevii and Saccharomyces mikatae. Int. J. Syst. Evol. Microbiol. 2015, 50, 1931–1942. [Google Scholar] [CrossRef]
- Naumov, G.I.; Naumova, E.S.; Hagler, A.N.; Mendonça-Hagler, L.C.; Louis, E.J. A new genetically isolated population of the Saccharomyces sensu stricto complex from Brazil. Antonie Van Leeuwenhoek 1995, 67, 351–355. [Google Scholar] [CrossRef]
- Naumov, G.I.; Lee, C.F.; Naumova, E.S. Molecular genetic diversity of the Saccharomyces yeasts in Taiwan: Saccharomyces arboricola, Saccharomyces cerevisiae and Saccharomyces kudriavzevii. Antonie Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2013, 103, 217–228. [Google Scholar] [CrossRef]
- Naumov, G.I.; Naumova, E.S.; Louis, E.J. Two New Genetically Isolated Popoulations of the Saccharomyces Sensu Stricto Complex from Japan. J. Gen. Appl. Microbiol. 1995, 41, 499–505. [Google Scholar] [CrossRef]
- Libkind, D.; Hittinger, C.T.; Valério, E.; Gonçalves, C.; Dover, J.; Johnston, M.; Gonçalves, P.; Sampaio, J.P. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. USA 2011, 108, 14539–14544. [Google Scholar] [CrossRef]
- Boynton, P.J.; Greig, D. The ecology and evolution of non-domesticated Saccharomyces species Primrose. Yeast 2014, 31, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Sipiczki, M. Interspecies hybridisation and genome chimerisation in Saccharomyces: Combining of gene pools of species and its biotechnological perspectives. Front. Microbiol. 2018, 9, 3071. [Google Scholar] [CrossRef] [PubMed]
- Naseeb, S.; James, S.A.; Alsammar, H.; Michaels, C.J.; Gini, B.; Nueno-Palop, C.; Bond, C.J.; McGhie, H.; Roberts, I.N.; Delneri, D. Saccharomyces jurei sp. Nov., isolation and genetic identification of a novel yeast species from Quercus robur. Int. J. Syst. Evol. Microbiol. 2017, 67, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Batschinskaya, A. Entwicklungsgeschichte und Kultur des neuen Hefepilzes Saccharomyces paradoxus. J. Microbiol. Epidemiol. Immunobiol. 1914, 1, 231–247. [Google Scholar]
- Beijerinck, M. Uber Regeneration der Sporenbildung bei Alkohol Hefen, wo diese Funktion im Verchwinden begriffen ist. Cent. Bakt 1898, 4, 657–731. [Google Scholar]
- Beijerinck, M. Sur la generation de la faculte de produire des spores chez des levures en voie de la perdre. Extr. Des Arch. Neelandaises Des Sci. Exactes Nat. Ser. II 1898, 2, 1–81. [Google Scholar]
- Kaneko, Y.; Banno, I. Re-examination of Saccharomyces bayanus strains by DNA-DNA hybridization and electrophoretic karyotyping. IFO Res. Commun. 1991, 15, 30–41. [Google Scholar]
- Yamada, Y.; Mikata, K.; Banno, I. Reidentification of 121 strains of the genus Saccharomyces. Bull JFCC 1993, 9, 95–119. [Google Scholar]
- Masneuf, I.; Hansen, J.; Groth, C.; Piskur, J.; Dubourdieu, D. New hybrids between Saccharomyces sensu stricto yeast species found among wine and cider production strains. Appl. Environ. Microbiol. 1998, 64, 3887–3892. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Legras, J.L.; Neuvéglise, C.; Gaillardin, C. Deciphering the hybridisation history leading to the lager lineage based on the mosaic genomes of Saccharomyces bayanus strains NBRC1948 and CBS380 T. PLoS One 2011, 6, e25821. [Google Scholar] [CrossRef]
- Querol, A.; Bond, U. The complex and dynamic genomes of industrial yeasts: MINIREVIEW. FEMS Microbiol. Lett. 2009, 293, 1–10. [Google Scholar] [CrossRef]
- Chen, Z.J. Genomic and epigenetic insights into the molecular bases of heterosis. Nat. Rev. Genet. 2013, 14, 471–482. [Google Scholar] [CrossRef]
- Fu, D.; Xiao, M.; Hayward, A.; Jiang, G.; Zhu, L.; Zhou, Q.; Li, J.; Zhang, M. What is crop heterosis: New insights into an old topic. J. Appl. Genet. 2015, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schnable, P.S.; Springer, N.M. Progress toward understanding heterosis in crop plants. Annu. Rev. Plant Biol. 2013, 64, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Crow, J.F. 90 Years Ago: The Beginning of Hybrid Maize. Genetics 1998, 148, 923–928. [Google Scholar] [CrossRef]
- Gallone, B.; Steensels, J.; Mertens, S.; Dzialo, M.C.; Gordon, J.L.; Wauters, R.; Theßeling, F.A.; Bellinazzo, F.; Saels, V.; Herrera-Malaver, B.; et al. Interspecific hybridization facilitates niche adaptation in beer yeast. Nat. Ecol. Evol. 2019, 3, 1562–1575. [Google Scholar] [CrossRef] [PubMed]
- González, S.S.; Barrio, E.; Querol, A. Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in brewing. Appl. Environ. Microbiol. 2008, 74, 2314–2320. [Google Scholar] [CrossRef]
- Borneman, A.R.; Desany, B.A.; Riches, D.; Affourtit, J.P.; Forgan, A.H.; Pretorius, I.S.; Egholm, M.; Chambers, P.J. The genome sequence of the wine yeast VIN7 reveals an allotriploid hybrid genome with Saccharomyces cerevisiae and Saccharomyces kudriavzevii origins. FEMS Yeast Res. 2012, 12, 88–96. [Google Scholar] [CrossRef]
- Le Jeune, C.; Lollier, M.; Demuyter, C.; Erny, C.; Legras, J.-L.; Aigle, M.; Masneuf Pomarède, I. Characterization of natural hybrids of Saccharomyces cerevisiae and Saccharomyces bayanus var. uvarum. FEMS Yeast Res. 2007, 7, 540–549. [Google Scholar] [CrossRef][Green Version]
- Heil, C.S.; Burton, J.N.; Liachko, I.; Friedrich, A.; Hanson, N.A.; Morrise, C.L.; Schacherer, J.; Shendurer, J.; Thomas, J.H.; Maitreya, J. Dunham Identification of a novel interspecific hybrid yeast from a metagenomic spontaneously inoculated beer sample using Hi-C. Yeast 2018, 35, 71–84. [Google Scholar] [CrossRef]
- Steensels, J.; Gallone, B.; Verstrepen, K.J. Interspecific hybridization as a driver of fungal evolution and adaptation. Nat. Rev. Microbiol. 2021, 19, 485–500. [Google Scholar] [CrossRef] [PubMed]
- Liti, G.; Peruffo, A.; James, S.A.; Roberts, I.N.; Louis, E.J. Inferences of evolutionary relationships from a population survey of LTR-retrotransposons and telomeric-associated sequences in the Saccharomyces sensu stricto complex. Yeast 2005, 22, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Sherlock, G. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 2008, 18, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Glendinning, T. Some Practical Aspects of the Fermentable Matter. J. Fed. Inst. Brew. 1898, 5, 20–34. [Google Scholar] [CrossRef]
- Walther, A.; Hesselbart, A.; Wendland, J. Genome Sequence of Saccharomyces carlsbergensis, the World’s First Pure Culture Lager Yeast. G3: Genes|Genomes|Genet. 2014, 4, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Barnett, J.A. A history of research on yeasts 10: Foundations of yeast genetics. Yeast 2007, 24, 799–845. [Google Scholar] [CrossRef]
- Hammond, B.J.R.M.; Eckersley, K.W. Fermentation Properties of Brewing Yeast with Killer Character. J. Insta. Brew. 1984, 90, 167–177. [Google Scholar] [CrossRef]
- Johnston, J.R. Breeding yeasts for brewing. J. Inst. Brew. 1965, 71, 135–137. [Google Scholar] [CrossRef]
- Spencer, J.F.T.; Spencer, D.M. Hybridization of non-sporulating and weakly sporulating strains of brewer’s and distiller’s yeasts. J. Inst. Brew. 1977, 83, 287–289. [Google Scholar] [CrossRef]
- Peŕez-Través, L.; Lopes, C.A.; Querol, A.; Barrio, E. On the complexity of the Saccharomyces bayanus taxon: Hybridization and potential hybrid speciation. PLoS ONE 2014, 9, e93729. [Google Scholar] [CrossRef]
- Langdon, Q.K.; Peris, D.; Baker, E.C.P.; Opulente, D.A.; Nguyen, H.V.; Bond, U.; Gonçalves, P.; Sampaio, J.P.; Libkind, D.; Hittinger, C.T. Fermentation innovation through complex hybridization of wild and domesticated yeasts. Nat. Ecol. Evol. 2019, 3, 1576–1586. [Google Scholar] [CrossRef] [PubMed]
- Mertens, S.; Steensels, J.; Saels, V.; De Rouck, G.; Aerts, G.; Verstrepen, K.J. A large set of newly created interspecific Saccharomyces hybrids increases aromatic diversity in lager beers. Appl. Environ. Microbiol. 2015, 81, 8202–8214. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, F.; Krogerus, K.; Vidgren, V.; Sandell, M.; Gibson, B. Improved cider fermentation performance and quality with newly generated Saccharomyces cerevisiae × Saccharomyces eubayanus hybrids. J. Ind. Microbiol. Biotechnol. 2017, 44, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, K.; Magalhães, F.; Vidgren, V.; Gibson, B. New lager yeast strains generated by interspecific hybridization. J. Ind. Microbiol. Biotechnol. 2015, 42, 769–778. [Google Scholar] [CrossRef]
- Krogerus, K.; Arvas, M.; De Chiara, M.; Magalhães, F.; Mattinen, L.; Oja, M.; Vidgren, V.; Yue, J.X.; Liti, G.; Gibson, B. Ploidy influences the functional attributes of de novo lager yeast hybrids. Appl. Microbiol. Biotechnol. 2016, 100, 7203–7222. [Google Scholar] [CrossRef]
- Hebly, M.; Brickwedde, A.; Bolat, I.; Driessen, M.R.M.; de Hulster, E.A.F.; van den Broek, M.; Pronk, J.T.; Geertman, J.M.; Daran, J.M.; Daran-Lapujade, P. S. cerevisiae × S. eubayanus interspecific hybrid, the best of both worlds and beyond. FEMS Yeast Res. 2015, 15, 1–14. [Google Scholar] [CrossRef]
- Nikulin, J.; Krogerus, K.; Gibson, B. Alternative Saccharomyces interspecies hybrid combinations and their potential for low-temperature wort fermentation. Yeast 2018, 35, 113–127. [Google Scholar] [CrossRef]
- Bellon, J.R.; Eglinton, J.M.; Siebert, T.E.; Pollnitz, A.P.; Rose, L.; De Barros Lopes, M.; Chambers, P.J. Newly generated interspecific wine yeast hybrids introduce flavour and aroma diversity to wines. Appl. Microbiol. Biotechnol. 2011, 91, 603–612. [Google Scholar] [CrossRef]
- Bellon, J.R.; Schmid, F.; Capone, D.L.; Dunn, B.L.; Chambers, P.J. Introducing a New Breed of Wine Yeast: Interspecific Hybridisation between a Commercial Saccharomyces cerevisiae Wine Yeast and Saccharomyces mikatae. PLoS ONE 2013, 8, e62053. [Google Scholar] [CrossRef]
- Winans, M.J.; Yamamoto, Y.; Fujimaru, Y.; Kusaba, Y.; Gallagher, J.E.G.; Kitagaki, H. Saccharomyces arboricola and Its Hybrids’ Propensity for Sake Production: Interspecific hybrids reveal increased fermentation abilities and a mosaic metabolic profile. Fermentation 2020, 6, 14. [Google Scholar] [CrossRef]
- Bizaj, E.; Cordente, A.G.; Bellon, J.R.; Raspor, P.; Curtin, C.D.; Pretorius, I.S. A breeding strategy to harness flavor diversity of Saccharomyces interspecific hybrids and minimize hydrogen sulfide production. FEMS Yeast Res. 2012, 12, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Gancedo, J.M. Control of pseudohyphae formation in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2001, 25, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Pierce, J. Absorption of Amino Acids from Wort by Yeast. J. Inst. Brew. 1964, 70, 307–315. [Google Scholar] [CrossRef]
- Esposito, R.E.; Klapholz, S. Meiosis and Ascospore Development. In The Molecular Biology of the Yeast Saccharomyces: Life Cycle and Inheritance; Strathern, J.N., Jones, E.W., Broach, E.R., Eds.; Cold Springs Harbor Laboratory Press: Cold Springs Harbor, NY, USA, 1981; pp. 211–287. [Google Scholar]
- Liti, G. The fascinating and secret wild life of the budding yeast S. cerevisiae. Elife 2015, 4, e05835. [Google Scholar] [CrossRef]
- Krogerus, K.; Magalhães, F.; Vidgren, V.; Gibson, B. Novel brewing yeast hybrids: Creation and application. Appl. Microbiol. Biotechnol. 2017, 101, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Gunge, N.; Nakatomi, Y. Genetic mechanisms of rare matings of the yeast saccharomyces cerevisiae heterozygous for mating type. Genetics 1972, 70, 41–58. [Google Scholar] [CrossRef]
- Ferenczy, L.; Maráz, A. Transfer of mitochondria by protoplast fusion in Saccharomyces cerevisiae. Nature 1977, 268, 524–525. [Google Scholar] [CrossRef]
- Ogata, T.; Okumura, Y.; Tadenuma, M.; Tamura, G. Improving Transformation Method for Industrial Yeasts: Construction of ADH1-APT2 Gene and Using Electroporation. J. Gen. Appl. Microbiol. 1993, 294, 285–294. [Google Scholar] [CrossRef][Green Version]
- Alexander, W.G.; Peris, D.; Pfannenstiel, B.T.; Opulente, D.A.; Kuang, M.; Hittinger, C.T. Efficient engineering of marker-free synthetic allotetraploids of Saccharomyces. Fungal Genet. Biol. 2016, 89, 10–17. [Google Scholar] [CrossRef]
- Fukuda, N.; Kaishima, M.; Ishii, J.; Kondo, A.; Honda, S. Continuous crossbreeding of sake yeasts using growth selection systems for a-type and α-type cells. AMB Express 2016, 6, 45. [Google Scholar] [CrossRef]
- Krogerus, K.; Fletcher, E.; Rettberg, N.; Gibson, B.; Preiss, R. Efficient breeding of industrial brewing yeast strains using CRISPR/Cas9-aided mating-type switching. Appl. Microbiol. Biotechnol. 2021, 105, 8359–8376. [Google Scholar] [CrossRef]
- Strong, G.; England, K. Beer Style Guidelines—BJCP; Brewers Association: Boulder, CO, USA, 2021. [Google Scholar]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Mcmurrough, I.; Madigan, D.; Donnelly, D.; Hurley, J.; Doyle, A.; Hennigan, G.; McNulty, N. Control of Ferulic Acid and 4-Vinyl Guaiacol in Brewing. J. Inst. Brew. 1996, 102, 327–332. [Google Scholar] [CrossRef]
- Gibson, B.; Geertman, J.M.A.; Hittinger, C.T.; Krogerus, K.; Libkind, D.; Louis, E.J.; Magalhães, F.; Sampaio, J.P. New yeasts-new brews: Modern approaches to brewing yeast design and development. FEMS Yeast Res. 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Krogerus, K.; Preiss, R.; Gibson, B. A unique saccharomyces cerevisiae saccharomyces uvarum hybrid isolated from norwegian farmhouse beer: Characterization and reconstruction. Front. Microbiol. 2018, 9, 2253. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, F.; Krogerus, K.; Castillo, S.; Ortiz-Julien, A.; Dequin, S.; Gibson, B. Exploring the potential of Saccharomyces eubayanus as a parent for new interspecies hybrid strains in winemaking. FEMS Yeast Res. 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Giannakou, K.; Visinoni, F.; Zhang, P.; Nathoo, N.; Jones, P.; Cotterrell, M.; Vrhovsek, U.; Delneri, D. Biotechnological exploitation of Saccharomyces jurei and its hybrids in craft beer fermentation uncovers new aroma combinations. Food Microbiol. 2021, 100, 103838. [Google Scholar] [CrossRef]
- Gray, J.V.; Petsko, G.A.; Johnston, G.C.; Ringe, D.; Singer, R.A.; Werner-washburne, M. “Sleeping Beauty”: Quiescence in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2004, 68, 187–206. [Google Scholar] [CrossRef]
- Dashko, S.; Zhou, N.; Compagno, C.; Piškur, J. Why, when, and how did yeast evolve alcoholic fermentation? FEMS Yeast Res. 2014, 14, 826–832. [Google Scholar] [CrossRef]
- Fleming, T.H.; John Kress, W. A brief history of fruits and frugivores. Acta Oecologica 2011, 37, 521–530. [Google Scholar] [CrossRef]
- Hagman, A.; Sall, T.; Compagno, C.; Piskur, J. Yeast “Make-Accumulate-Consume ” Life Strategy Evolved as a Multi-Step Process That Predates the Whole Genome Duplication. PLoS ONE 2013, 8, e68734. [Google Scholar] [CrossRef]
- Christiaens, J.F.; Franco, L.M.; Cools, T.L.; de Meester, L.; Michiels, J.; Wenseleers, T.; Hassan, B.A.; Yaksi, E.; Verstrepen, K.J. The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep. 2014, 9, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, I.; Dapporto, L.; Berń, L.; Polsinelli, M.; Turillazzi, S.; Cavalieri, D. Social wasps are a saccharomyces mating nest. Proc. Natl. Acad. Sci. USA 2016, 113, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Stefanini, I.; Dapporto, L.; Legras, J.-L.; Calabretta, A.; Di Paola, M.; De Filippo, C.; Viola, R.; Capretti, P.; Polsinelli, M.; Turillazzi, S.; et al. Role of social wasps in Saccharomyces cerevisiae ecology and evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 13398–13403. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Bell, G.; Greig, D. Increased outbreeding in yeast in response to dispersal by an insect vector. Curr. Biol. 2007, 17, R81–R83. [Google Scholar] [CrossRef]
- Tsai, I.J.; Bensasson, D.; Burt, A.; Koufopanou, V. Population genomics of the wild yeast Saccharomyces paradoxus: Quantifying the life cycle. Proc. Natl. Acad. Sci. USA 2008, 105, 4957–4962. [Google Scholar] [CrossRef]
- Ruderfer, D.M.; Pratt, S.C.; Seidel, H.S.; Kruglyak, L. Population genomic analysis of outcrossing and recombination in yeast. Nat. Genet. 2006, 38, 1077–1081. [Google Scholar] [CrossRef]
- James, T.Y.; Michelotti, L.A.; Glasco, A.D.; Clemons, R.A.; Powers, R.A.; James, E.S.; Simmons, D.R.; Bai, F.; Ge, S. Adaptation by Loss of Heterozygosity in Saccharomyces cerevisae Clones Under Divergent Selection. Genetics 2019, 213, 665–683. [Google Scholar] [CrossRef]
- Lancaster, S.M.; Payen, C.; Heil, C.S.; Dunham, M.J. Fitness benefits of loss of heterozygosity in Saccharomyces hybrids. Genome Res. 2019, 29, 1685–1692. [Google Scholar] [CrossRef]
- Smukowski Heil, C.S.; DeSevo, C.G.; Pai, D.A.; Tucker, C.M.; Hoang, M.L.; Dunham, M.J. Loss of Heterozygosity Drives Adaptation in Hybrid Yeast. Mol. Biol. Evol. 2017, 34, 1596–1612. [Google Scholar] [CrossRef]
- Smukowski Heil, C.S.; Large, C.R.L.; Patterson, K.; Hickey, A.S.M.; Yeh, C.L.C.; Dunham, M.J. Temperature preference can bias parental genome retention during hybrid evolution. PLoS Genet. 2019, 15, e1008383. [Google Scholar] [CrossRef] [PubMed]
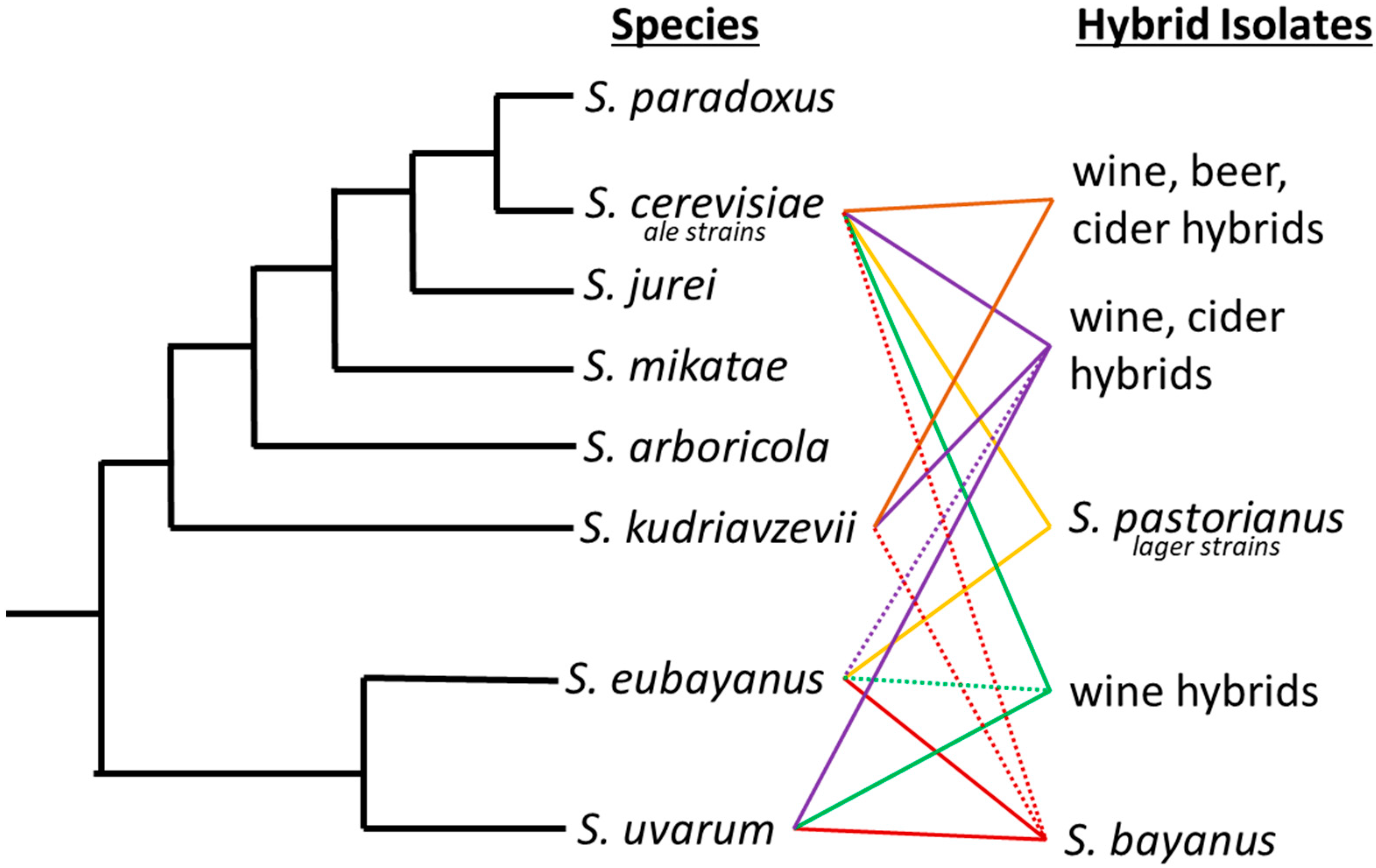
| Saccharomyces | Described | Substrate | Location | Reference |
|---|---|---|---|---|
| cerevisiae | 1838 | Beer | Germany | [4] |
| uvarum | 1898 | Ribes rubrum, redcurrant juice | South Holland, The Netherlands | [16,17] |
| paradoxus | 1914 | Tree sap | Russia | [15] |
| kudriavzevii | 1991 | Decayed leaf | Japan | [18] |
| mikatae | 1993 | Decayed leaf | Japan | [19] |
| arboricola | 2008 | Fagaceae spp. | West China | [6] |
| eubayanus | 2011 | Nothofagus spp. & parasitic fungi Cyttaria spp. | Andean, Patagonia | [11] |
| jurei | 2017 | Quercus robur | Saint Auban, France | [14] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winans, M.J. Yeast Hybrids in Brewing. Fermentation 2022, 8, 87. https://doi.org/10.3390/fermentation8020087
Winans MJ. Yeast Hybrids in Brewing. Fermentation. 2022; 8(2):87. https://doi.org/10.3390/fermentation8020087
Chicago/Turabian StyleWinans, Matthew J. 2022. "Yeast Hybrids in Brewing" Fermentation 8, no. 2: 87. https://doi.org/10.3390/fermentation8020087
APA StyleWinans, M. J. (2022). Yeast Hybrids in Brewing. Fermentation, 8(2), 87. https://doi.org/10.3390/fermentation8020087






