Abstract
This study investigated the effects of mulberry branch and leaves (MBL) silage on milk yield, ruminal fermentation, and bacteria composition in dairy cows. Thirty-six mid-lactation cows were selected and randomly allocated into three groups. The control group (C) was fed on a total mixed ratio (TMR) diet, while the experimental groups were fed on TMR supplemented with 5% (L) and 10% (H) MBL silage. The experiment lasted for eight weeks, including two weeks of adaption. The results showed that Group H had an increased milk yield, milk fat content (p < 0.05), and 4% feed conversion ratio (p = 0.10). In addition, rumen propionic acid was significantly increased (p < 0.05), while acetate/propionate was significantly decreased (p < 0.05) in the high MBL silage group. The microbiome analysis showed that Bacteroides, Firmicutes, and Proteobacteria were the predominant phyla. Compared with Group C, the abundance of Bacteroides was significantly decreased (p < 0.01), while the Firmicutes and Proteobacteria were increased but not significantly different in Groups L and H. Prevotella was significantly decreased (p < 0.05) in the MBL silage groups, and Succinivibrionaceae_UCG-001 was increased in Group H. The correlation analysis showed that eight bacterial species belonging to Firmicutes were positively correlated with propionic acid. However, four bacterial species belonging to the Bacteroides group were negatively correlated with propionic acid. In conclusion, feed supplementation with about 5–10% of MBL silage could modulate the rumen microbiota and fermentation, and increase the abundance of fiber-digesting, propionic acid synthesis and milk fat-related microorganisms, thus improving milk yield in dairy cows.
1. Introduction
There is an increased shortage of forage in China due to increased animal production. Therefore, there is an urgent need to look for inexpensive, local feed sources [1]. Mulberry trees are fast-growing deciduous plants of the Moraceae family that are widely distributed in China [2]. Mulberry trees are multipurpose, and their fruits are used as food and for making wine, while the leaves are used as fodder due to the high biomass, crude protein content, and digestibility [3]. A previous study has shown that the tract and rumen digestibility of mulberry trees were in some cases comparable to alfalfa hay [4]. Meanwhile, the addition of mulberry leaves to feeds of ruminant animals reduces the need for expensive protein supplements [5,6]. Therefore, research is needed to evaluate the effects of feeding animals with mulberry.
The composition of gastrointestinal bacteria is relevant to the production performance of host animals [7]. Previous studies also showed that providing ensiled mulberry silage to dairy cows improved the immune and antioxidant function [8,9]. On the other hand, the application of mulberry leaves silage has shown that it could be used in finishing steer diets without affecting the production performance [10]. Dietary changes usually affect the rumen microbiome. The rumen microbiome in dairy cows plays an essential role in animal health and performance [11,12]. Hence, we hypothesize that the partial replacement of forage and concentrate with 5% or 10% mulberry branch and leaves silage (MBL) affects the ruminal microbiome. Therefore, this study evaluated the effects of diets supplemented with MBL on the milk yield, rumen fermentation characteristics, and microbial community in dairy cows.
2. Materials and Methods
2.1. Diet and Animal Management
Mulberry trees were cultivated in the fields of Yinxiang Weiye Group Co., Ltd. (Shandong, China). The mulberry leaves and branches (MBL, GuiSang 62) were harvested at 0.8–1.0 m height (late growing period) and ensiled using 10% maize flour after chopping them to 2~3 cm by CLAAS (JAGUAR 960, Germany). After that, they were sprayed with a fermenting agent (Lactobacillus ≥ 2.0 × 1010 CFU/g, Inner Mongolia Heimei Kesheng Biotechnology Co., Ltd., Inner Mongolia, China). The MBL silage was fermented for 45 days before being used for the feeding experiment.
A total of 36 mid-lactation Holstein dairy cows within the initial days in milk (DIM) = 159.41 ± 34.5 d, milk yield = 38.26 ± 3.18 kg, body weight = 665.17 ± 65.39 kg were randomly allocated into three groups (n = 12). The control group (Group C) was fed on a total mixed ratio (TMR), while the other two treatment groups were fed on TMR supplemented with 5% (Group L) and 10% (Group H) MBL silage. The three different TMRs were adjusted as isonitrogenous and isoenergetic diets, as shown in Table 1. Feeding and milking were carried out three times per day (07:00, 14:00 and 22:00). The experiment lasted eight weeks, including the first two weeks of adaptation and six weeks of the formal period.

Table 1.
The ingredients and chemical composition of the experimental diets.
2.2. Samples Collection and Chemical Analysis
The dry matter intake (DMI) was calculated daily by the TMR and refusals every day. The dietary samples of MBL silage, TMRs, and residual samples were collected every two weeks during the experimental period. The samples were dried in an oven at 65 °C for 48 h and kept at room temperature for 24 h. After that, the samples were ground to pass through a 40-mesh sieve. Composite samples of forages and concentrates were analyzed for dry matter (DM), crude protein (CP), and ether extract (EE) [13]. The acid detergent fiber (ADF) and neutral detergent fiber (NDF) contents were determined using Ankom A200 apparatus (Ankom Technology, Macedon, NY, USA) with heat-stable amylase and sodium sulfite (Fisher Scientific, Waltham, MA, USA). They were expressed inclusive of residual ash [14].
The chemical composition and fermentation quality of MBL silage were determined before feeding and are shown in Table 2. The milk samples (50 mL) were collected from each cow every sixth day of each week. The milk samples were collected three times per day during the milking time at a ratio of 4:3:3, as previously described (Wang et al., 2014). Potassium dichromate 0.6 mg/mL was added to the milk samples for preservation. After that, the milk samples were stored at 4 °C while awaiting further analysis. The milk components, including protein, fat, lactose, somatic cell counts (SCC), and milk urea nitrogen (MUN), were determined using an automatic ultrasonic milk composition analyzer (Bentley Instruments, Chaska, MN, USA).

Table 2.
Chemical composition and fermentation quality of the mulberry branch and leaves silage.
An oral stomach tube was used to collect the rumen fluid 3 h after morning feeding on the last day of the experiment [15]. The initial 150 mL rumen fluid was discarded. The pH value was determined immediately. Samples were collected from all cows. Every sample was aliquoted into three tubes. Liquid nitrogen was immediately added into one tube and stored for DNA extraction. The other sample was kept for the determination of NH3-N, while the other sample was used for the determination of VFA [16,17]. The DNA extraction and sequencing of rumen fluid were determined according to our previous study [12].
2.3. Statistical Analysis
The ruminal fermentation characteristics were analyzed using SAS software (version 9.2, mixed model). The least squares means were calculated and separated by the PDIFF option in SAS. p-value ≤ 0.05 was considered statistically significant. Further, trends were declared significant at 0.05 < p ≤ 0.10.
The partial least squares discriminant analysis (PLS-DA), principal component analysis (PCA), and metabolic pathways spreading and enrichment analysis were analyzed using MetaboAnalyst 4.0 (https://www.metaboanalyst.ca/, accessed on 12 October 2020). Correlation analyses between ruminal fermentation parameters and the top 25 genera of microbiota were determined using Spearman’s correlation, and connections with p < 0.05 and r > 0.54 were retained.
3. Results
3.1. Milk Yield and Composition
As shown in Table 3, the milk yield and fat content were significantly higher in Group H than in Groups C and L (p < 0.05). In addition, the lactose yield was significantly higher in Groups C and H than in Group L (p < 0.05). Further, Group C had a significantly lower level of MUN than Groups L and H (p < 0.05). There were no differences in the dry matter intake (DMI), protein, and SCC content between the groups. However, the 4% feed conversion ratio (FCR) was increased in Group H (p = 0.10).

Table 3.
Effects of feeding mulberry silage on lactation performance of dairy cows.
3.2. Rumen Fermentation Characteristics
Table 4 shows the rumen fermentation characteristics. There were no differences in the pH, ammonia nitrogen, total volatile acid, acetic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid concentrations in the rumen fluid. The concentration of acetic acid showed a decreasing trend (p = 0.08) with an increase in the MBL silage concentration. However, the propionic acid concentration increased significantly (p < 0.05) with an increase in the MBL silage concentration. In addition, the acetate/propionate ratio was significantly lower in Groups L and H than in Group C (p < 0.05).

Table 4.
Effects of feeding MBL silage on the rumen fermentation characteristics in dairy cows.
3.3. Ruminal Bacterial Communities
A total of 2115 OTUs were identified in the three groups. Among them, 1548 OTUs were found in all groups, accounting for 73.19% of the total OTUs (Figure 1A). Based on the PCoA graph (Figure 1B), the clouds derived from the three groups were separated from each other. There were no significant differences in all groups based on Sobs, ACE, Chao, Simpson, Shannon, and Coverage (Table 5). Besides, six bacterial phyla (Bacteroidota, Firmicutes, Proteobacteria, Actinobacteria, Patescibacteria, and Spirochaetes) were identified in the rumen fluid with a relatively high abundance (>1%) (Figure 2A). Further, a total of 247 bacterial taxa were identified at the genus level. Prevotella, Succinivibrionaceae_UCG-001, norank_f_Muribaculaceae, NK4A214_group, Succiniclasticum, Lachnospiraceae_NK3A20_group, Ruminococcus, norank_f_norank_o_Clostridia_UCG-014, Rikenellaceae_RC9_gut_group, and Christensenellaceae_R-7_group were the top ten most abundant bacteria at the genus level (Figure 2B).
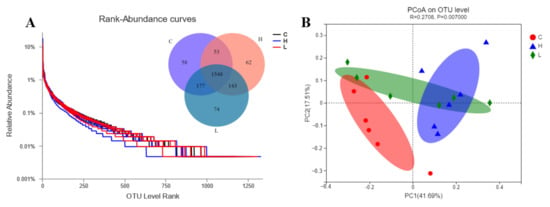
Figure 1.
The rank-abundance curves are derived from the microbial OTU level. (A) The Venn graph illustrates the overlap of microbial OTUs among the treatment groups at a 3% dissimilarity level. (B) PCoA analysis of taxonomical classifications in the control (C) and mulberry silage (L, H) groups.

Table 5.
The alpha diversity of the ruminal bacteria community in dairy cows.
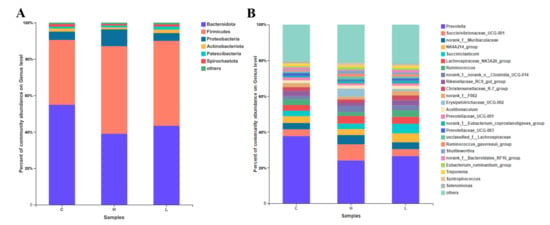
Figure 2.
The distribution of rumen fluid bacterial taxa under (A) phylum and (B) genus levels between the treatments.
A total of 30 clades were identified as biomarkers among the three groups, of which nine were in Group C, 17 were in Group H, and four were in Group L (Figure 3). The nine clades identified in Group C included four genera belonging to Bacteroidota (Prevotellaceae_UCG_003, Prevotellaceae_UCG_004, norank_f_251_o5, and Rikenellaceae_U29_B03), two genera belonging to Proteobacteria (Succinimonas and Anaerobiospirillum), and one genus belonging to Elusimicrobiota (Endomicrobium), Actinobacteriota (Corynebacterium), and Desulfobacterota (Mailhella). The 17 clades identified in Group H included two genera belonging to Bacteroidota (Rikenellaceae_SP3_e08 and unclassified_o_Bacteroidales), 14 genera belonging to Firmicutes (Lachnoclostridium, Shuttleworthia, Syntrophococcus, unclassified_f_Lachnospiraceae, Ruminococcus_gauvreauii_group, Eubacterium_ruminantium_group, norank_f _Selenomonadaceae, Dialister, Eubacterium_eligens_group, Coprococcus, Monoglobus, Lactobacillus, Catonella, and Lachnospiraceae_XPB1014_group) and one genus belonging to the Desulfobacterota (Desulfovibrio). The four clades identified in Group L included three genera belonging to Firmicutes (Saccharofermentans, unclassified_p_Firmicutes, and Lachnospiraceae_FE2018_group) and one genus belonging to Actinobacteriota (unclassified_f_Atopobiaceae).
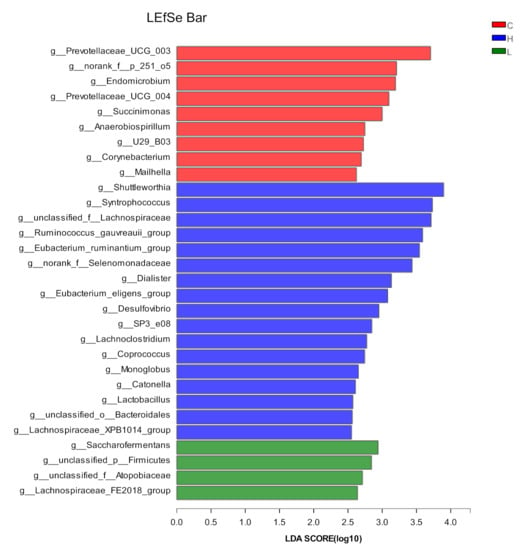
Figure 3.
The abundance of rumen fluid bacteria in the control (C) and mulberry silage (L, H) groups. Red, blue and green bars represent the bacterial community of the control group and Groups L and H with a significantly higher abundance compared with that in other groups, respectively.
3.4. Correlation Analysis between the Ruminal Microbiome and Fermentation Parameters
The bacterial communities were mainly correlated with the rumen propionate concentration and the acetate/propionate ratio (Figure 4). In detail, Ruminococcus, norank_f_Bacteroidales_RF16_group, NK4A214_group, Prevotellaceae_UCG-003, and Rikenellaceae_RC9_gut_group were positively correlated with the acetate/propionate ratio (p < 0.05). However, Shuttleworthia, Erysipelotrichaceae_UCG-002, Syntrophococcus, norank_f_norank_o_Clostridia_UCG-014, Eubacterium_ruminantium_group, unclassified_f_Lachnospiraceae, Ruminococcus_gauvreauii_group, and Succinivibrionaceae_UCG-001 were negatively correlated with the acetate/propionate ratio (p < 0.05). In addition, Succinivibrionaceae_UCG-001, norank_f_norank_o_Clostridia_UCG-014, Erysipelotrichaceae_UCG-002, Shuttleworthia, unclassified_f_Lachnospiraceae, Ruminococcus_gauvreauii_group, Eubacterium_ruminantium_group, and Syntrophococcus were positively correlated with propionate (p < 0.05). However, Rikenellaceae_RC9_gut_group, norank_f_Bacteroidales_RF16_group and Prevotellaceae_UCG-003 were negatively correlated with propionate (p < 0.05).
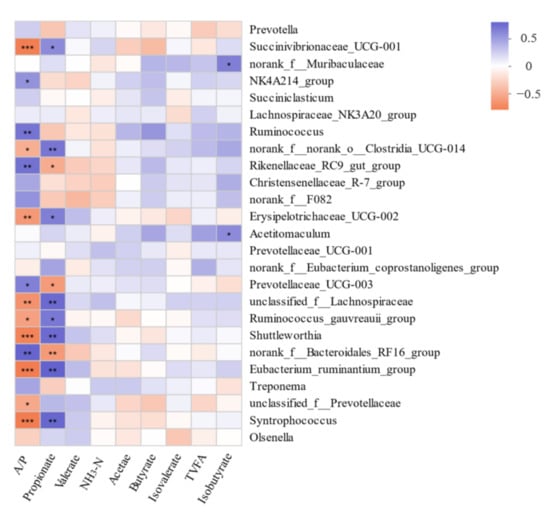
Figure 4.
Correlation analyses between the fermentation characteristics and top 25 rumen bacterial genera. The blue squares show the positive correlations, while orange squares show the negative correlations. * p < 0.05; ** p < 0.01; *** p < 0.001. NH3-N = ammonia nitrogen, A/P = the ratio of acetate to propionate, TVFA = total volatile fatty acid.
4. Discussion
Milk production in dairy cows mainly depends on the feed intake and quality, with the quality of roughage, green feed and dietary protein being the main contributing factors. In this study, although the CP and lactic acid of MBL silage was relatively lower than that of mulberry leaves silage, the quality was comparable to corn silage [9]. This means that it could be an available resource for dairy cows. There were no significant differences in the dry matter intake between the groups. However, Group H showed an increased milk production. A previous study showed that fermented mulberry leaves had significantly increased levels of bioactive components, CP and EE, and that the crude fiber content and anti-nutritional factors were significantly decreased [18]. Ensiling MBL could reduce the levels of lignin in the branches and increase the levels of soluble reducing sugars, leading to a higher rate of degradation in the rumen. Therefore, we hypothesized that the improved milk production was due to the enhanced digestibility of MBL in the rumen.
Milk fat can be synthesized de novo from acetic acid and β-hydroxybutyric acid produced during rumen fermentation (40–45%) or could be directly obtained from feeds (55–60%) [19,20]. Previous studies showed that plant flavonoids could promote the development of mammary glands, improve milk quality, and modulate the ruminal microbiome and fermentation [21,22]. In addition, fatty acids such as palmitic acid and octadecenoic acid could promote the synthesis of milk fat [23]. The present study showed that increasing the concentration of MBL silage resulted in a significant increase in the milk fat yield, attributed to the effect of flavonoids in MBL silage on rumen microorganisms [22].
Bacteroidota, Firmicutes and Proteobacteria were shown to be the dominant phyla in the rumen, consistent with a previous study [24]. Bacteria of the Bacteroidota phylum are mainly considered degraders of protein and nonstructural polysaccharides [25]. Prevotella belongs to the phylum Bacteroidetes, which can degrade and utilize structural polysaccharides in plants such as pectin, starch and xylan. However, they have to be co-cultivated with cellulolytic bacterium [26]. Rumenococcus and Laurespirillum are Firmicutes bacteria that degrade fiber. The decomposition of fiber can affect the function of carbohydrate enzymes [27]. In addition, Proteobacteria are involved in the formation of biofilms and the digestion of soluble carbohydrates [28]. In this study, there were no significant differences in the composition of the dominant bacteria phyla between the groups, and this was consistent with a previous study on finishing steers [10]. However, the proportions of the different bacteria phyla between the groups showed large differences. There was a significant decrease in the abundance of Bacteroides and a significant increase in the abundance of Proteobacteria in the group fed on high MBL silage.
A previous study showed that MBL alkaloids had an inhibitory effect on α-glucosidase (the key enzyme involved in carbohydrate digestion) [29]. It is speculated that the alkaloids in MBL reduce the body’s absorption and utilization of carbohydrate. Thus, the available glycogen substrates for Bacillus and Prevotella are reduced, which in turn leads to a decrease of the abundance. In this study, the LDA graph showed that the abundance of Lachnospiraceae (Lachnospiraceae_NK3A20_group) and Ruminococcaceae (Ruminococcaceae_NK4A214_group) was significantly higher than in the control group, suggesting that the MBL silage led to an increase in the abundance of rumen fiber-degrading bacteria. The ratio of the Firmicutes and Bacteroides Desulfovibrio (Proteobacteria), Lactobacillus, Laospirillaceae, Eubacteria and Microbacteria (Firmicutes) were shown to be positively correlated with the milk fat yield. However, the abundance of Prevotella was shown to be negatively correlated with the milk fat yield [30]. In this study, the ratio of Firmicutes and Bacteroides was shown to be increased in the high MBL group. However, Prevotella was significantly decreased in the high MBL group. The LDA graph showed that the Lacetospiraceae, Eubacteria, Microbacteria, Lactobacillus and Desulfovibrio in Group H were significantly higher than in Group C. Therefore, the MBL silage significantly increased the abundance of rumen microorganisms related to milk fat synthesis, thereby increasing the milk fat yield in dairy cows.
In this study, Succinivibrionaceae_UCG-001, belonging to Proteobacteria, was the second most abundant bacteria out of all the samples. A previous study showed that the feed efficiency was positively correlated to the abundance of Succinivibrio and Enterococcus faecalis (Firmicutes). Succinivibrio can convert succinic acid into propionic acid, thus increasing the ruminal levels of propionic acid and consequently increasing the lactose and milk yield [31]. Succinivibrio and Enterococcus faecalis had a higher abundance in Group H than in Groups C and L. Further, the correlation analysis showed that Succinivibrionaceae_UCG-001 was positively correlated with propionic acid levels. This explains the increase in the ruminal propionic acid levels and feed efficiency.
The correlation analysis between rumen fermentation and microorganisms showed that propionic acid was positively correlated with Firmicutes bacteria (norank_f_norank_o_Clostridia_UCG-014, unclassified_f_Lachnospiraceae, Eubacterium_ruminantium_group, Shuttleworthia, Syntrophococcus, Succinivibrionaceae_UCG-001, Ruminococcus_gauvreauii_group and Erysipelotrichaceae_UCG-002) in the high MBL silage group. However, propionic acid was negatively correlated with Bacteroides bacteria (norank_f_Bacteroidales_RF16_group, Selenomonas, Rikenellaceae_RC9_gut_group and Prevotellaceae_UCG-003) in Group C. These results suggest that MBL silage was associated with an increased abundance of propionic acid synthesizing microorganisms of the phylum Firmicutes in the rumen.
5. Conclusions
MBL silage can be used as an alternative feed source in lactating dairy cows. Further, feed supplementation with about 5% to 10% of MBL silage could modulate the rumen microbiota and fermentation, and increase the abundance of fiber-digesting, propionic acid synthesis and milk fat-related microorganisms, consequently improving milk yield in dairy cows.
Author Contributions
Conceptualization, Y.L. and J.W.; methodology, J.W.; software, J.W.; validation, Y.L. and J.M.; data curation, J.M.; writing—original draft preparation, Y.L.; writing—review and editing, L.H. and H.L.; project administration, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System (No. CARS-36).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Animal Care Committee of Zhejiang University and was conducted according to the animal welfare practices and procedures (Protocol No. ZJU20160379, Hangzhou, China).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors appreciate the staff of Yinxiang Weiye Dairy Farm (Shandong, China) for their assistance in feeding, milking, and caring for the animals. We also acknowledge the members of the Institute of Dairy Science Zhejiang University for their assistance with rumen fluid sampling.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yao, J.; Yan, B.; Wang, X.Q.; Liu, J.X. Nutritional evaluation of mulberry leaves as feeds for ruminants. Livest. Res. Rural Dev. 2000, 12, 9–16. [Google Scholar]
- Sánchez, M. Mulberry: An exceptional forage available almost worldwide. World Rev. Anim. Prod. 2002, 93, 36–46. [Google Scholar]
- Hejcman, M.; Hejcmanová, P.; Pavlů, V.; Thorhallsdottir, A.G. Forage quality of leaf fodder from the main woody species in Iceland and its potential use for livestock in the past and present. Grass Forage Sci. 2016, 71, 649–658. [Google Scholar] [CrossRef]
- Doran, M.; Laca, E.; Sainz, R. Total tract and rumen digestibility of mulberry foliage (Morus alba), alfalfa hay and oat hay in sheep. Anim. Feed Sci. Technol. 2007, 138, 239–253. [Google Scholar] [CrossRef]
- Salinas-Chavira, J.; Castillo-Martínez, O.; Ramirez-Bribiesca, J.E.; Mellado, M. Effect of increasing levels of white mulberry leaves (Morus alba) on ruminal dry matter degradability in lambs. Trop. Anim. Health Prod. 2011, 43, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.C.; Verstegen, M.W.A.; Hendriks, W.H.; Pham, K.C. The Nutritive Value of Mulberry Leaves (Morus alba) and Partial Replacement of Cotton Seed in Rations on the Performance of Growing Vietnamese Cattle. Asian-Australas. J. Anim. Sci. 2011, 24, 1233–1242. [Google Scholar] [CrossRef]
- Cui, Z.; Meng, Q.; Ma, W.; Zhang, X.; Zhou, Z.; Ren, L. Diversity of the Intestinal Bacteria of Cattle Fed on Diets with Different Doses of Gelatinized Starch-Urea. Indian J. Microbiol. 2015, 55, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Si, B.; Tao, H.; Zhang, X.; Guo, J.; Cui, K.; Tu, Y.; Diao, Q. Effect of Broussonetia papyrifera L. (paper mulberry) silage on dry matter intake, milk composition, antioxidant capacity and milk fatty acid profile in dairy cows. Asian Australas. J. Anim. Sci. 2018, 31, 1259–1266. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, S.; Si, J.; Zhang, J.; Gaowa, N.; Sun, X.; Lv, J.; Liu, G.; He, Y.; Wang, W.; et al. Effects of Paper Mulberry Silage on the Milk Production, Apparent Digestibility, Antioxidant Capacity, and Fecal Bacteria Composition in Holstein Dairy Cows. Animals 2020, 10, 1152. [Google Scholar] [CrossRef]
- Niu, Y.; Meng, Q.; Li, S.; Ren, L.; Zhou, B.; Schonewille, T.; Zhou, Z. Effects of Diets Supplemented with Ensiled Mulberry Leaves and Sun-Dried Mulberry Fruit Pomace on the Ruminal Bacterial and Archaeal Community Composition of Finishing Steers. PLoS ONE 2016, 11, e0156836. [Google Scholar] [CrossRef]
- Li, Y.; Meng, Q.; Zhou, B.; Zhou, Z. Effect of ensiled mulberry leaves and sun-dried mulberry fruit pomace on the fecal bacterial community composition in finishing steers. BMC Microbiol. 2017, 17, 97. [Google Scholar] [CrossRef]
- Li, Y.; Lv, M.; Wang, J.; Tian, Z.; Yu, B.; Wang, B.; Liu, J.; Liu, H. Dandelion (Taraxacum mongolicum Hand.-Mazz.) Supplementation-Enhanced Rumen Fermentation through the Interaction between Ruminal Microbiome and Metabolome. Microorganisms 2020, 9, 83. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1990; Volume I. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Shen, J.; Chai, Z.; Song, L.; Liu, J.; Wu, Y. Insertion depth of oral stomach tubes may affect the fermentation parameters of ruminal fluid collected in dairy cows. J. Dairy Sci. 2012, 95, 5978–5984. [Google Scholar] [CrossRef]
- Hu, W.-L.; Liu, J.-X.; Ye, J.-A.; Wu, Y.-M.; Guo, Y.-Q. Effect of tea saponin on rumen fermentation in vitro. Anim. Feed Sci. Technol. 2005, 120, 333–339. [Google Scholar] [CrossRef]
- Broderick, G.; Kang, J. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and In Vitro Media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Dong, Z.H.; Wang, S.; Zhao, J.; Li, J.; Shao, T. Effects of additives on the fermentation quality, in vitro digestibility and aerobic stability of mulberry (Morus alba L.) leaves silage. Asian-Australas. J. Anim. Sci. 2020, 33, 1292–1300. [Google Scholar] [CrossRef]
- Bauman, D.; Mellenberger, R.; Ingle, D. Metabolic Adaptations in Fatty Acid and Lactose Biosynthesis by Sheep Mammary Tissue during Cessation of Lactation. J. Dairy Sci. 1974, 57, 719–723. [Google Scholar] [CrossRef]
- Jenkins, T. Lipid Metabolism in the Rumen. J. Dairy Sci. 1993, 76, 3851–3863. [Google Scholar] [CrossRef]
- Zhan, J.; Liu, M.; Su, X.; Zhan, K.; Zhang, C.; Zhao, G. Effects of alfalfa flavonoids on the production performance, immune system, and ruminal fermentation of dairy cows. Asian-Australas. J. Anim. Sci. 2017, 30, 1416–1424. [Google Scholar] [CrossRef]
- Hassan, F.-U.; Arshad, M.A.; Li, M.; Rehman, M.S.-U.; Loor, J.J.; Huang, J. Potential of Mulberry Leaf Biomass and Its Flavonoids to Improve Production and Health in Ruminants: Mechanistic Insights and Prospects. Animals 2020, 10, 2076. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.; Mathews, A.; Lovett, J.; Haughey, N.; McFadden, J. Palmitic acid feeding increases ceramide supply in association with increased milk yield, circulating nonesterified fatty acids, and adipose tissue responsiveness to a glucose challenge. J. Dairy Sci. 2016, 99, 8817–8830. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Mizrahi, I. Composition and Similarity of Bovine Rumen Microbiota across Individual Animals. PLoS ONE 2012, 7, e33306. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hou, Y.; Yang, H.; Shi, R.; Wu, C.; Hou, Y.; Zhao, G. Effects of Forage Sources on Rumen Fermentation Characteristics, Performance, and Microbial Protein Synthesis in Midlactation Cows. Asian-Australas. J. Anim. Sci. 2014, 27, 667–673. [Google Scholar] [CrossRef][Green Version]
- Purushe, J.; The North American Consortium for Rumen Bacteria; Fouts, D.E.; Morrison, M.; White, B.A.; Mackie, R.I.; Coutinho, P.M.; Henrissat, B.; Nelson, K.E. Comparative Genome Analysis of Prevotella ruminicola and Prevotella bryantii: Insights into Their Environmental Niche. Microb. Ecol. 2010, 60, 721–729. [Google Scholar] [CrossRef]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of Novel Bovine Gastrointestinal Tract Treponema Isolates and Comparison with Bovine Digital Dermatitis Treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef]
- Pitta, D.W.; Pinchak, W.E.; Indugu, N.; Vecchiarelli, B.; Sinha, R.; Fulford, J.D. Metagenomic Analysis of the Rumen Microbiome of Steers with Wheat-Induced Frothy Bloat. Front. Microbiol. 2016, 7, 689. [Google Scholar] [CrossRef]
- Alam, F.; Shafique, Z.; Amjad, S.T.; Bin Asad, M.H.H. Enzymes inhibitors from natural sources with antidiabetic activity: A review. Phytother. Res. 2019, 33, 41–54. [Google Scholar] [CrossRef]
- Jami, E.; White, B.A.; Mizrahi, I. Potential Role of the Bovine Rumen Microbiome in Modulating Milk Composition and Feed Efficiency. PLoS ONE 2014, 9, e85423. [Google Scholar] [CrossRef]
- Indugu, N.; Vecchiarelli, B.; Baker, L.D.; Ferguson, J.D.; Vanamala, J.K.P.; Pitta, D.W. Comparison of rumen bacterial communities in dairy herds of different production. BMC Microbiol. 2017, 17, 190. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).