Abstract
In order to understand the influence of nitrogen and lipid nutrition on the aromatic quality of wines for cognac distillation, we developed a transdisciplinary approach that combined statistical modeling (experimental central composite design and response surface modeling) with metabolomic analysis. Three Saccharomyces cerevisiae strains that met the requirements of cognac appellation were tested at a laboratory scale (1 L) and a statistical analysis of covariance was performed to highlight the organoleptic profile (fermentative aromas, terpenes, alcohols and aldehydes) of each strain. The results showed that nitrogen and lipid nutrients had an impact on the aromatic quality of cognac wines: high lipid concentrations favored the production of organic acids, 1-octen-3-ol and terpenes and inhibited the synthesis of esters. Beyond this trend, each yeast strain displayed its own organoleptic characteristics but had identical responses to different nutritional conditions.
Keywords:
nutrients; central composite design; Saccharomyces cerevisiae; wine; strain effect; aromas 1. Introduction
Cognac is a French protected designation of origin (PDO) whose quality is recognized worldwide. This wine spirit is obtained after a double distillation using almost exclusively the Ugni blanc grape variety. The geographical area of production is divided into six crus around the city of Cognac, approximately 75,000 hectares, encircling the Charente River. Despite this, cognac spirits can be distinguished by very different and specific characteristics. Indeed, the quality of the final product not only depends on the terroir but also on viticultural and oenological practices, including blending and aging [1]. The character, delicacy and roundness that the Charente eaux-de-vie will acquire are mainly due to its aromas resulting from a great variety of volatile substances. Fermentative aromas (higher alcohols, esters, aldehydes, acetyls, fatty acids, etc.) are synthesized by yeast during alcoholic fermentation [2] while varietal aromas, such as terpenes or aliphatic aldehydes, originate from the grape berries. For both these aromatic classes, the proportions found in wines are influenced, modified or even disturbed during alcoholic fermentation by different environmental parameters [3]. Thus, yeast assimilable nitrogen (YAN) and lipids, which are the main nutrients in grape musts [4], can have a significant impact on the production of volatile compounds.
During recent years, many studies have focused on the impact of YAN must supplementations on the production of volatile compounds [3,5,6,7,8,9,10,11,12]. The results are partly contradictory because they vary depending on the type of must (synthetic or natural), the nature of the added assimilable nitrogen (mineral and/or organic) and the yeast strain used for fermentation. Nevertheless, global trends can be considered. Generally, the concentration of higher alcohols goes through a maximum as a function of must YAN concentration [5,8,13,14] while that of esters increases continuously [5,11,13,15]. The effect of lipid nutrition on aroma synthesis has been less well-studied. Lipids can be synthesized by yeast in the presence of oxygen; however, this mechanism is limited during oenological fermentation [16]. Therefore, yeasts primarily use the lipids (phytosterols) present in the solid particles of the must [17]. Most studies have concluded that phytosterols have an overall positive effect on higher alcohol synthesis and a negative impact on ester synthesis [9,18,19,20].
In this study, an experimental design was implemented to understand the influence of YAN and lipid nutrients, and to model their effect on aroma production. Compared to previous studies [7,9,13], this work has several specificities: (1) the alcoholic fermentations were carried out with natural musts containing very high lipid concentrations due to the high turbidities encountered in cognac (500 to 2000 NTU compared to 100 to 150 NTU in conventional oenology); (2) a metabolomic analysis including several families of volatile compounds was carried out in order to obtain a general overview of aroma production, not limited to a specific category of molecules; (3) three yeast strains of Saccharomyces cerevisiae commonly used in the cognac appellation area were included in the statistical modeling design to compare their metabolic response to nutrients and identify their specificities in terms of aromatic profiles.
2. Materials and Methods
2.1. Fermentations
Fermentations were performed in 1.2 L cylindrical glass fermenters containing 1 L of must with continuous magnetic stirring (150 rpm) at 23 °C. CO2 release was measured by an accurate, automatic online monitoring of weight loss every 20 min [21]. The fermenters were inoculated with 20 g/hL of active dry yeast (Lalvin FC9®, Lallemand SA, Montreal, QC, Canada; Fermivin 7013® and Fermivin SM102®, Erbslöh S.A.S., Servian, France) previously rehydrated for 30 min at 37 °C in a 50 g/L glucose solution. The must (Ugni blanc grape variety) was harvested in the Cognac region of France. The must characteristics were as follows: 183 g/L total sugars, 114 mg/L assimilable nitrogen, pH 3.32 and a turbidity of 100 NTU. Ugni blanc grapes were pressed, and the must was settled for 24 h at 4 °C in the presence of 3 mL/hL enzymes (MYZYM SPIRIT, Institut Oenologique de Champagne, Mardeuil, France). A final turbidity of 100 NTU was achieved for the must and the sludge was collected separately. A correlation between the sludge concentration and turbidity was previously performed (R2 = 0.999) by adding different amounts of solid particles to final volumes (100 mL) of must:
with the concentration of added solid particles ([solid particles]) in % (v/v) and the turbidity in NTU.
Turbidity was measured with a 2100 N turbidimeter (Hach®, Lognes, France).
Based on the determined relationship (Equation (1)) between turbidity and the percentage of solid particles, 59, 100, 200, 300 and 340 mL of sludge were added to a final volume of 1 L of must, and turbidities of 500, 820, 1600, 2380 and 2700 NTU, respectively, were obtained. Each corresponding volume of must was removed from the fermenter to be substituted by the sludge. Assimilable nitrogen was adjusted to 115, 140, 200, 260 or 285 mg Nass/L with a solution of amino acids and NH4Cl, respecting the proportions of 30% mineral nitrogen (NH4+) and 70% organic nitrogen (a mix of amino acids) found in the initial Ugni blanc must. The free amino acid content of the initial Ugni blanc must was determined by cation-exchange chromatography (see Section 2.3). The composition of the amino acid solution added was as follows (in g/L): tyrosine,1.77; tryptophan, 1.63; isoleucine, 0.74; aspartate, 16.48; glutamate, 17.2; arginine, 46.65; leucine, 1.81; threonine, 4.11; glycine, 0.23; glutamine, 14.18; alanine, 7.17; valine, 4.17; methionine, 0.44; phenylalanine, 3.59; serine, 6.42; histidine, 1.60; lysine, 0.53; asparagine, 0.82; and proline, 42.98. A solution of NH4Cl (80.24 g/L) was used as an ammonium source. To obtain 115, 140, 200, 260 and 285 mg/L of assimilable nitrogen in the must, 0.03, 0.76, 2.51, 4.27 and 5 mL of the amino acid stock solution and 0.04, 0.91, 3.02, 5.12 and 6 mL of an NH4Cl solution were added to 1 L of must, respectively.
2.2. Quantification of Sterols and Fatty Acids in Grape Solids
2.2.1. Dry Matter
A total of 200 mL of must were centrifuged for 10 min at 10,000 rpm to concentrate the grape solids. The supernatant was removed and the grape solids were washed consecutively three times with an NaCl solution (10 mM) to remove sugars. The final pellet was freeze-dried overnight to recover the dry matter (DM).
2.2.2. Lipid Composition
Total lipids were extracted from lyophilized grape solids (aliquot of 1 g) overnight with methanol/chloroform (2:1, v/v), and the solid residue was then extracted for 2 h with methanol/chloroform/water (2:1:0.8, v/v/v). The organic extracts were dried over Na2SO4 and concentrated to dryness using a rotatory evaporator. Phytosterols (campesterol, stigmasterol and β-sistosterol) and main fatty acids were determined in the remaining solids according to the protocol used by Grison et al. (2015), as adapted by Casalta et al. (2019). Total fatty acid concentration was 37.28 mg/g in the DM (Table 1) C18 unsaturated acids represented approximately 48% of the total fatty acid content, and the most abundant saturated fatty acid was palmitic acid (22%). The concentration of phytosterols was 8.43 mg/g in the DM (Table 2), i.e., within the limits of the concentrations described for other grape varieties [17]. The main phytosterol was β-sitosterol (83%), while campesterol and stigmasterol accounted for approximately 11% of the total phytosterols.

Table 1.
Fatty acid composition of Ugni blanc grape solids.

Table 2.
Phytosterols composition of Ugni blanc grape solids.
Using the lipid composition of the solid particles from the dry matter (Table 1 and Table 2) and Equation (1), we calculated turbidities of 500, 820, 1600, 2380 and 2700 NTU corresponding to 12.9, 21.9, 43.8, 65.6 and 74.5 mg/L sterols, respectively. These turbidities (500, 820, 1600, 2380 and 2700 NTU) also corresponded to fatty acid concentrations of 57.1, 96.8, 193.5, 290.3 and 329.9 mg/L, respectively.
2.3. Analytical Methods
Ammonium concentration was determined enzymatically (R-Biopharm AG™, Darmstadt, Germany). The free amino acid content of the must was measured by cation-exchange chromatography with post-column ninhydrin derivatization (Biochrom 30, Biochrom™, Cambridge, UK), as described by [22].
Ethanol, glucose, fructose, glycerol, succinic acid, alpha-ketoglutaric acid and acetic acid concentrations were determined by High Performance Liquid Chromatography (HPLC) (HPLC 1260 Infinity, Agilent™ Technologies, Santa Clara, CA, USA) on a Phenomenex Rezex ROA column (Phenomenex™, Le Pecq, France) at 60 °C. The column was eluted with 0.005 N H2SO4 at a flow rate of 0.6 mL/min. Organic acids were analyzed with a UV detector (Agilent™ Technologies, Santa Clara, CA, USA) at 210 nm; the concentrations of the other compounds were quantified with a refractive index detector (Agilent™ Technologies, Santa Clara, CA, USA). The analysis was carried out with the Agilent™ OpenLab CDS 2.x software package (Santa Clara, CA, USA).
The concentrations of ethyl acetate, ethyl propanoate, ethyl 2-methylpropanoate, ethyl butanoate, ethyl 2-methylbutanoate, ethyl 3-methylbutanoate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, ethyl dodecanoate, ethyl lactate, diethyl succinate, 2-methylpropyl acetate, 2-methylbutyl acetate, 3-methyl butyl acetate, 2-phenylethyl acetate, 2-methylpropanol, 2-methylbutanol, 3-methylbutanol, hexanol, 2-phenylethanol, propanoic acid, butanoic acid, 2-methylpropanoic acid, 2-methylbutanoic acid, 3-methylbutanoic acid, hexanoic acid, octanoic acid, decanoic acid and dodecanoic acid were measured in the liquid phase after sample pretreatment by double liquid-liquid extraction with dichloromethane in the presence of deuterated standards (ethyl butanoate-D5, ethyl decanoate-D5, ethyl hexanoate-D5, ethyl octanoate-D5 and phenylethanol-D4) [9]. The samples were analyzed with a Hewlett Packard (Agilent™ Technologies, Santa Clara, CA, USA) 6890 gas chromatograph equipped with a CTC Combi PAL autosampler AOC-5000 (Shimadzu™, Columbia, SC, USA) and coupled to a Hewlett Packard 5973 mass spectrometry detector (Agilent™ Technologies, Santa Clara, CA, USA).
The total liquid concentration of acetaldehyde was precisely measured using a commercial enzymatic test kit (Ref. 984347, ThermoFischer Scientific™, Waltham, MA, USA) and a Thermo Scientific™ Gallery™ automated photometric analyzer.
The metabolomic analysis was realized by solid-phase microextraction (SPME).
Before SPME analysis, 5 mL of wine were placed into a 20 mL headspace amber glass vial and diluted with 5 mL of ultra-pure water. The samples were spiked with 100 µL of 2-octanol (internal standard solution at 1 mg/L in ethanol) and saturated with sodium chloride before closure with a PTFE-faced silicone septum-aluminum crimp cap. A 50/30 μm DVB/CAR/PDMS fiber (divinylbenzene/carboxene/polydimethylsiloxane; Supelco, Bellefonte, PA, USA) was used for volatile extraction. Before the analysis, the fiber was conditioned into the GC injector at 270 °C for 30 min to prevent contamination. The sample was pre-incubated for 5 min at 40 °C. Adsorption lasted for 30 min at 40 °C with stirring at 250 rpm (10 s ON and 1 s OFF). Next, desorption took place in the injector in splitless mode for 600 s at 250 °C. All analyses were made in triplicate. The injections were fully automated using an MPS robotic autosampler (Gerstel) operated by Maestro software (Gerstel). An Agilent 8890 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA), coupled to an Agilent 5977B mass spectrometer were used. The sample was analyzed on a DB-HeavyWAX column (60 m × 0.25 mm i.d., 0.25 μm film thickness, Agilent Technologies). The injector and MSD transfer line temperatures were fixed at 250 °C. The oven temperature was held at 40 °C for 10 min, raised to 240 °C at a rate of 3 °C per minute and then held for 5 min. The column carrier gas was helium at a constant flow rate of 1 mL/min. The splitless injection mode was used. The temperature of the ion source was set at 230 °C while the electron impact mass spectra were recorded at 70 eV ionization energy. The GC-MS analysis was carried out in the scanning mode (SCAN); the mass range was from 35 to 350 m/z.
2.4. Statistical Analysis
2.4.1. Experimental Central Composite Design
The statistical analysis was performed with R software, version 3.6.2 (R Development Core Team 2012), and the rsm library [23].
In order to study and understand the impact of yeast nutrition in cognac, we used a central composite design (CCD), the most common design used to fit quadratic models, described by [24]. CCD combines a two-level factorial design with axial points (star points) and at least one point at the center of the experimental region in order to fit quadratic polynomials. The center points are usually repeated to obtain a good estimate of the experimental error (pure error). If the distance from the center of the design space to a factorial point is ±1 unit for each factor, the distance from the center of the design space to a star point is ±α, where |α| > 1. The star points represent new extreme values (low and high) for each factor in this design. The variables included the concentration of assimilable nitrogen and turbidity coded levels, −α, −1, 0, +1, +α (the value of α is 1.41 rotatable design), and are shown in Table 3 as well as the actual levels of the variables in the CCD experiments.

Table 3.
The experimental plan of fermentation conditions for each yeast used in spirit production.
The effect of the two independent variables on each volatile compound (Y) was modelled by a polynomial response surface:
where and represent the coded values of the initial nitrogen content and turbidity, respectively, is the predicted response, is the intercept term, is the linear coefficient, is the quadratic coefficient and is the interaction coefficient. When necessary, a simplified model was fitted for some compounds by suppressing the interactive terms of the equation according to validity criteria.
In addition, the normality of residual distributions and the homogeneity of variance were studied with standard diagnostic graphs; no violation of the assumptions was detected.
The accuracy and general ability of each polynomial model described above was evaluated by a lack of fit test, the Fisher test and the adjusted coefficient of determination (adjR2).
The reliability of the fitted models was very good overall: each polynomial model produced a non-significant lack of fit test at a 0.05 threshold, a significant Fisher test at a 0.05 threshold and a range of adjR2 between 35% and 99%.
A CCD was performed for each strain independently.
For graphical representations of the surface responses, a custom version of the persp() function was used.
2.4.2. Analysis of Covariance (ANCOVA)
ANCOVA is a general linear model with a continuous outcome variable and two or more predictor variables, where at least one is continuous (covariate) and at least one is categorical (factor). In addition to studying the impacts of nitrogen and lipid nutrition on the synthesis of fermentative aromas, an analysis of covariance was performed in order to demonstrate a difference in production between strains, which we will call the “strain effect”. This analysis did not allow us to determine which strain was different but evidenced any overall difference.
2.4.3. Analysis of Correlation Tree and PCA
The statistical evaluations of the metabolomic analysis were performed using the JMP (SAS Institute JMP, Brie Comte Robert, France) software, version 15.0.0.
3. Results and Discussion
The impacts of YAN and turbidity on the (1) kinetic parameters, (2) metabolites of central carbon metabolism and (3) production of volatile compounds (fermentative aromas, aldehydes and terpenes) were studied. A central composite design was used to minimize the number of factor combinations required to evaluate the effects of these two factors. Twelve fermentations were performed for each of the three strains with five different assimilable nitrogen concentrations (from 115 to 285 mgN/L) and five different turbidities (from 500 to 2700 NTU). The conditions are summarized in Table 3.
The central composite design enabled us to plot the concentrations of volatile compounds as a function of the two environmental parameters and to build an associated mathematical model. With this model, a “weight” was assigned to each parameter (assimilable nitrogen and turbidity); thus, a classification of these two parameters according to their influence on the synthesis of each volatile compound or on the fermentative parameters could be performed. The significance of this effect was determined from its expressed p-value (p). Based on the sign of the coefficients (Equation (2)) of the polynomial surface response, the positive or negative effect of the fermentative parameter on the production of the studied volatile compound was determined. The interactions between the parameters (i.e., the effect of one factor as a function of the value of another) were also studied, as well as the non-linear effects of these parameters, also called quadratic effects.
3.1. Fermentation Kinetics
In all the fermentations, the sugars were almost exhausted. The residual sugar content for all fermentations performed was lower than or equal to 5 g/L. The YAN was completely consumed at the end of the growth phase; therefore, assimilable nitrogen was the limiting nutrient in these experiments. The three yeast strains displayed similar fermentation kinetics (Figure 1), although the effects of the nutrients were slightly different. Under identical nutritional conditions, the maximum rates of CO2 production were identical for the three strains, while the fermentation durations were noticeably different: strain SM102® was 50 h faster at low nitrogen concentration than strain 7013® and 30 h faster than strain FC9® (Figure 1).
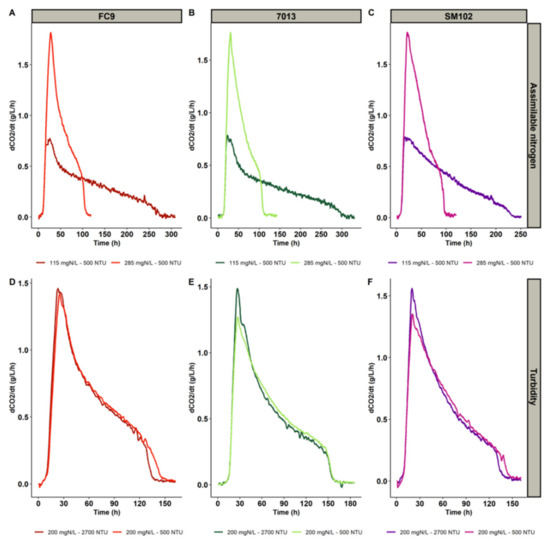
Figure 1.
The impact of assimilable nitrogen concentration in the must (A–C) and turbidity (D–F) on fermentation kinetics at 23 °C for three yeast strains: FC9® (A,D), 7013® (B,E) and SM102® (C,F).
For all strains, a very strong positive impact of assimilable nitrogen was noted on the maximum CO2 production rate, resulting in a shorter fermentation time, in agreement with previous works [25,26,27]. The maximum CO2 production rate was also positively impacted by turbidity, but to a much lesser extent (Table 4).

Table 4.
The effects of nutrient parameters on maximum CO2 production rate and central carbon metabolism (MCC) compounds for FC9®, 7013® and SM102® strains described by the CCP model with ANCOVA analysis to identify a “strain effect”.
3.2. Compounds of Central Carbon Metabolism
The amount of ethanol produced in all conditions was similar, whereas significant differences were observed in the concentrations of glycerol, succinic acid, acetic acid and α-ketoglutaric acid, which are metabolic markers that result from central carbon metabolism. The effects of the nutrients were different depending on the compound, although succinate and α-ketoglutarate generally reacted in the same way (Table 4). Firstly, only very small quantities of acetic acid (<0.015 g/L) were found, compared to the concentrations of 0.34 to 1.21 g/L generally present in wine [3]. This observation might be explained by the fact that our working range of phytosterol concentration is much higher (from 13 to 75 mg/L) than the range commonly used in previous studies [9,28,29]; indeed, the production of acetic acid during alcoholic fermentation is low for high concentrations of phytosterols [28,30,31]. Therefore, there was almost no demand for acetyl-CoA, the precursor of lipid biosynthesis. In these conditions, the pool of cytoplasmic acetyl-CoA not used to produce lipids can be converted to citrate by the citrate synthase Cit2p. Then, citrate can be transported to the mitochondria and participate in succinic acid synthesis via the TCA cycle [9]. Therefore, the synthesis of α-ketoglutaric and succinic acids is promoted by the intake of lipids (Table 4). On the other hand, and consistent with bibliographic data, the concentrations of α-ketoglutaric and succinic acids decreased when the YAN supply increased [5,7,9,11]. Meanwhile, the turbidity and assimilable nitrogen positively impacted glycerol production (Table 4). When nitrogen is the limiting nutrient face to lipids, as was the case in this work, glycerol production increases with lipid content [30]. This can be explained by the important role of glycerol in maintaining the redox balance of the cell. When the production of organic acids is increased after lipid addition, an excess of NADH will be synthesized that will then be oxidized by the glycerol synthesis pathway [32]. In this study, the glycerol concentration was also favored by YAN supply (Table 4). This quite surprising result differs from the literature [9,14,32,33]; however, it is still consistent with our previous study [7]. Finally, a highly significant strain effect was observed for all central metabolism compounds. Indeed, strain FC9® produced less organic acids and glycerol in all conditions, with average concentrations of 0.09 ± 0.03; 0.81 ± 0.08 and 6.81 ± 0.33 g/L of α-ketoglutaric acid, succinic acid and glycerol, respectively, whereas the other two strains (7013® and SM102®) produced 0.12 ± 0.05 and 0.14 ± 0.04 g/L of α-ketoglutaric acid, respectively; 1.08 ± 0.07 and 1.09 ± 0.1 g/L of succinic acid, respectively; and 8.18 ± 0.42 and 8.14 ± 0.37 g/L of glycerol, respectively.
3.3. Aromas
Volatile compounds present in distilled beverages have been reported in the literature [34]. Aroma compounds involved in olfactory perception belong to different chemical classes, such as alcohols, esters, aldehydes, norisoprenoids and terpenes. In this work, a metabolomic analysis was applied to the study of the impact of YAN and lipids on the production of fermentative aromas (higher alcohols and esters), terpenes, aldehydes (acetaldehyde) and more atypical alcohols present in wines intended for charentaise batch distillation.
3.3.1. Fermentative Aromas
Since all the higher alcohols (isobutanol, isoamyl alcohol, hexanol, methionol and 2-phenylethanol) and acetate esters (isobutyl acetate, 2-methylbutyl acetate, isoamyl acetate, hexyl acetate and 2-phenylethyl acetate) reacted in the same way to the nutrients, we considered them as sums and by compound families (Table 5).

Table 5.
The effects of nutrient parameters on fermentative aromas: sum of higher alcohols and acetate esters for FC9®, 7013® and SM102® strains described by the CCP model with ANCOVA analysis to identify a “strain effect”.
For the three yeast strains, the production trend of higher alcohols decreased with an increasing supply of assimilable nitrogen (p-value < 0.001) but was promoted by enhanced turbidity (p-value < 0.001), whereas opposite effects of the nutrients were observed for acetate esters (Table 5 and Figure 2A,B). These data are consistent with previous findings [7]. The observed effects can be explained by the regulation of acetyltransferases that catalyze the conversion of higher alcohols into their corresponding esters. The positive impact of nitrogen on acetate ester [7,9] production can be explained by the higher expression of genes coding for alcohol acetyltransferases during nitrogen supplementation of the must [10,35]. In this case, the alcohol/ester balance changes in favor of ester accumulation with increasing doses of YAN in the medium. The effect of turbidity is, however, quite surprising because there is no metabolic connection between the anabolic pathways of alcohol production and the lipid pathway. This effect can also be explained by the activity of alcohol acetyl transferases, which decreases in the presence of lipids. Indeed, the expression of ATF1 (the major gene encoding this enzymatic activity) is repressed by lipids [36,37]. In the presence of increased lipids, the repression of acetate ester synthesis is to the benefit of the accumulation of higher alcohols. Although the strains responded similarly to the nutrients in the synthesis of higher alcohols and acetate esters, their production levels were significantly different (Table 5), indicating a probable strain effect on the contribution to the wine’s fruity and floral character. YAN supply resulted in complex effects on the synthesis of ethyl esters depending on the involved Saccharomyces cerevisiae strain for fermentations (Table 6).
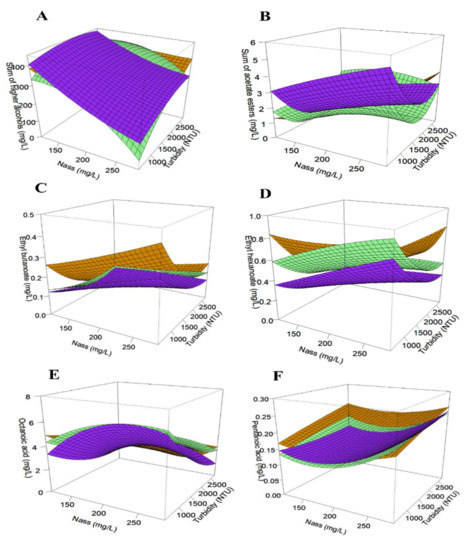
Figure 2.
Response surfaces of fermentative aromas: sum of higher alcohols (A), sum of acetate esters (B), ethyl esters (ethyl butanoate (C) and ethyl hexanoate (D)) and acids (octanoic acid (E) and pentanoic acid (F)) for all three strains: FC9® (―), 7013® (―) and SM102® (―).

Table 6.
The effects of nutrient parameters on fermentative aromas: ethyl esters for FC9®, 7013® and SM102® strains described by the CCP model with ANCOVA analysis to identify a “strain effect”.
Ethyl butanoate increased with assimilable nitrogen in 7013® and SM102® strains, while only 7013® reacted positively to this nutrient for the synthesis of ethyl hexanoate and ethyl decanoate. The production of these previous esters was not impacted by nitrogen addition for strain FC9®. Lastly, an opposing behavior was noticed with a decrease in ethyl isobutanoate production induced by nitrogen supply (FC9® and 7013®).
This complex YAN effect has already been observed in several studies, certainly due to the fact that the synthesis pathways of these esters are not linked to nitrogen metabolism [7,9,12,14] and depend on the yeast strain used [38]. More generic trends were noticed for the impact of turbidity, with a positive effect on the synthesis of ethyl isobutanoate (7013® and SM102®) and a negative effect on almost all other ethyl esters (Table 6). In addition to this negative impact, a positive quadratic effect (i.e., the bending of the curve) was also visible for ethyl butanoate and hexanoate (anise seed, apple-like aroma), where a minimum production of these flavors was obtained close to 1500 NTU (Figure 2C,D). Observing a similar effect of phytosterols on ethyl esters for the three strains is rather logical because these compounds are produced from the lipid metabolism whose regulation appears to be relatively conserved within the Saccharomyces cerevisiae species. Moreover, the negative effect of this nutrient is in accordance with the data obtained by [19]), showing that the addition of unsaturated fatty acids to the fermentation medium results in a global decrease in ethyl ester production. As for ethyl esters, the acids did not all respond in the same way to assimilable nitrogen (Table 7).

Table 7.
The effects of nutrient parameters on fermentative aromas: acids for FC9®, 7013® and SM102® strains described by the CCP model with ANCOVA analysis to identify a “strain effect”.
The production of C:6, C:8 and C:10 acids increased with YAN addition (7013® and SM102®). The production of theses acids is favored by the contribution of assimilable nitrogen in the must and passes through a maximum of concentration (quadratic effect) at 200 mg/L of nitrogen (Figure 2E), as previously observed by [7,39]. An opposing effect was observed for propionic acid, C:3 (only for SM102®) and pentanoic acid, C:5 (all three strains), with decreased synthesis when the nitrogen concentration was increased and a minimum concentration (quadratic effect) at 200 mg/L of nitrogen (Figure 2F). As observed for ethyl esters, lipid nutrition provided a more consensual response in acid production with a globally decreased synthesis (Table 7). First, the impact of nitrogen on acid synthesis can vary depending on the Saccharomyces cerevisiae strain, as do the intrinsic production levels [39,40]. Second, the pairing of the production of esters from their acid precursors was not systematic and not all of the pairs of compounds reacted in the same way to nutrient changes. This latter observation indicates that, for ethyl esters, the key factor is not only the availability of the precursors (fatty acids) but also the enzymatic activity (Eht1p and Eeb1p) responsible for this bioconversion [41].
3.3.2. Terpenes
While the contribution of terpenoids has been extensively studied in grapes and wines [42,43], some significant works have also been specially dedicated to mono- and sesquiterpenes in brandies and cognac [1,44,45,46]. Indeed, terpenes are some of the compounds that impart a fruity and floral character to cognac [47]. Terpenes are generally known and described as varietal compounds occurring in free as well as bound forms, depending on the odor active molecule (aglicone) that may or may not be bound to a sugar moiety. The majority of free monoterpenes are found in the grape berry skin as well as in the pulp, while the forms combined with sugar are mainly found in the juice [48]. Bound forms are commonly known as aroma precursors since they undergo hydrolysis easily, generating the active odor molecule and free sugar. Studies have shown that β-glucosidase activity is low or non-existent in Saccharomyces cerevisiae strains [49,50] because the fermentation conditions (acidic pH and high sugar concentration) are unfavorable for the activity of this enzyme.
Therefore, the terpenes studied are considered to be the free forms when initially found in the musts [48] and the glycolized forms as those released during wine distillation [51]. In wines made from Ugni blanc grapes, the monoterpenes found include trans-nerolidol (rose aroma), citronellol (shade of lemongrass), α-terpineol (lily of the valley aroma) and linalool (floral aroma). Firstly, it should be noted that increasing the concentrations of assimilable nitrogen reduced wine terpene levels (trans-nerolidol, citronellol and linalool) for all strains (Table 8).

Table 8.
Effects of assimilable nitrogen and turbidity on varietal flavors, including terpenes and ketone compounds for FC9®, 7013® and SM102® strains described by the CCP model with ANCOVA analysis.
These results are partly comparable to the ones presented by [3], who reported that nitrogen supplementation (DAP) significantly decreased citronellol and nerol concentrations, while linalool was present in higher concentrations at moderate DAP concentrations (350 mgN/L) [52]. Secondly, the changes in nitrogen content had no effect on the synthesis of α-terpineol (Table 5A), likely suggesting that the low pH of the medium led to its formation through the chemical transformation of linalool, rather than through the involvement of a cyclase enzyme, as also demonstrated by [52]. Thus, the effect of nitrogen on terpenes is difficult to interpret and further studies will be necessary.
On the other hand, we observed that turbidity favors the presence of citronellol and linalool in wines (Table 8). This result can be explained by the fact that increasing turbidity results in the addition of important quantities of solid particles (from 6 to 34%), composed of the skin and pulp of grapes [17], that are important sources of free monoterpenes, such as citronellol or linalool [17]. Moreover, it has been proposed that terpene and sterol biosynthesis are related [53] and that terpenes can also be synthesized by Saccharomyces cerevisiae [52,54,55]. Anaerobic conditions were suggested to inhibit several essential steps in ergosterol biosynthesis, including squalene epoxidation and the oxidative demethylation/dehydrogenation of lanosterol, which are essential steps in the formation of ergosterol [56]. In this study, a large excess of phytosterols (13 to 75 mg/L) likely favored terpene biosynthesis from geranyl pyrophosphate (GPP) by the yeast. Finally, a strain effect was identified on terpene synthesis (Table 8). Strain SM102® produced more linalool while the 7013® strain synthesized more citronellol (Figure 3A). Although the link between the varietal aromas and the impact of fermentation on their production remains unclear, it has previously been shown that linalool production can vary depending on the Saccharomyces cerevisiae strain used, because the presence of polyphenolic and aromatic fractions in grapes exerts a strong influence on yeast metabolism [57]. In our case, the yeast strains’ metabolism did not always favor the production of linalool, nerolidol or citronellol from the precursor geranyl PP (GPP) [52]. In the FC9® strain, linalool synthesis (followed by α-terpineol) was very low. These two aromas only accounted for 5% of the terpenes produced, instead of about 25% for the other two strains (Figure 3). These data confirm that, in the case of wines dedicated to cognac distillation, the proportion of terpenes of varietal origin is essential; however, the proportion linked to yeast metabolism is also important. In fact, high turbidities provide the geraniol precursor in large quantities, and yeast becomes a tool to modulate the terpene-linked aromatic fraction of wines.
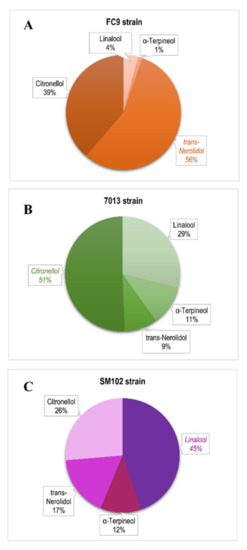
Figure 3.
The distribution of terpene synthesis for strain (A) FC9® (―), (B) 7013® (―) and (C) SM102® (―), including all fermentative conditions.
3.3.3. 1-octen-3-ol
1-octen-3-ol is a natural chemical derived from linoleic acid, which was first isolated from the matsutake pine mushroom and thereafter from plants and other fungi. This alcohol is a well-known compound associated with fresh mushroom odors in grapes and wines [58] and is associated with rotten grapes, particularly due to Botrytis cinerea [59]. 1-octen-3-ol has a relatively low perception threshold and, because its odor persists after alcoholic fermentation [60], it may be responsible for defects in wine. As shown in Table 9, the presence of 1-octen-3-ol seemed to be favored by the presence of solid particles in high quantities.

Table 9.
Effects of assimilable nitrogen and turbidity on other alcohols for FC9®, 7013® and SM102® strains described by the CCP model with ANCOVA analysis.
However, the vineyard from which the must was obtained was considered as healthy and was not affected by Botrytis cinerea. In our case, 1-octen-3-ol biosynthesis resulted from the aerobic oxidation of linoleic acid through a specific enzymatic reaction [61]. In the elaboration of cognac, the collection of musts as well as the pre-fermentation stages are carried out in the total absence of SO2. Oxidative phenomena, such as the oxidation of linoleic acid, are therefore favored. 1-octen-3-ol is thus a marker of this oxidative phenomenon, which was all the more marked as the musts were rich in solid particles and therefore in lipids.
3.3.4. Acetaldehyde
Acetaldehyde is a key compound in yeast metabolism and an intermediate of glycolysis that is produced during alcoholic fermentation, where pyruvate can be converted to acetaldehyde and carbon dioxide by pyruvate decarboxylase (PDC) and the acetaldehyde can then be reduced to ethanol through the action of alcohol dehydrogenase (ADH). In addition, acetaldehyde is a powerful aromatic compound that can be found in many food matrices [62]; however, excess acetaldehyde in cognac is not desired as it is responsible for a poor aromatic profile [47]. During the aging of brandies, acetaldehyde reacts with ethanol to form diethyl acetal, which can impart a strong apple smell when present in too high a concentration. In wine, free acetaldehyde can form more or less stable combinations with other molecules; we can then speak of combined or bound acetaldehyde, the sum of the two forms being the total acetaldehyde amount. In this study, only total acetaldehyde was discussed. The final acetaldehyde concentration appeared to be favored by a high concentration of assimilable nitrogen (Table 10).

Table 10.
Effects of assimilable nitrogen and turbidity on aliphatic aldehydes for FC9®, 7013® and SM102® strains described by the CCP model with ANCOVA analysis.
A high ammonium consumption can be associated with an increased concentration of final acetaldehyde [5], whereas a high concentration of amino acids in the must significantly decreases the acetaldehyde content [63]. A strain effect is superimposed on this nitrogen effect (mineral versus organic): in some strains, the residual acetaldehyde at the end of fermentation will be lower for a high nitrogen concentration, while for others the residual acetaldehyde will be higher [39]. The strain effect on the production of this molecule was also highlighted in this study (Table 9), which was in agreement with the fact that Saccharomyces cerevisiae strains have a different potential to produce this aroma [64]. Unlike nitrogen, an increase in lipid content in the musts reduced acetaldehyde synthesis. Presumably, when there is an excess of lipids, the yeast does not need to produce acetyl-CoA for lipid biosynthesis; therefore, the yeast does not need to overproduce and therefore accumulate acetaldehyde to switch to the acetate (lipid precursor) metabolism pathway. Nevertheless, the impact of sterols on acetaldehyde has been poorly studied and makes comparisons difficult. One study showed that the most turbid musts had significantly higher concentrations of acetaldehyde during the first 4 days of fermentation; however, at the end, the difference was statistically non-significant [65]. Therefore, the impact of turbidity remains difficult to analyze and is not well understood at this time [65].
The other compounds mentioned, benzaldehyde, 2-nonanol and acetophenone, are recognized for their importance to the aromatic quality of cognac [1].
3.4. Aromatic Typicity of the Strains
Regardless of the yeast strain, the synthesis of aroma compounds is similarly affected by the main fermentation parameters, such as YAN and lipids. However, notable differences were obtained in the production levels for the three strains tested. A fruity wine or, on the contrary, a softer or rounder wine, can be obtained by prior management of nutritional parameters that will impact aroma synthesis, and also by the choice of yeast strain. A strong interaction between these two control levers of fermentation was demonstrated here: the FC9® strain displayed a greater synthesis of fruity aromas, such as esters (Figure 4A).
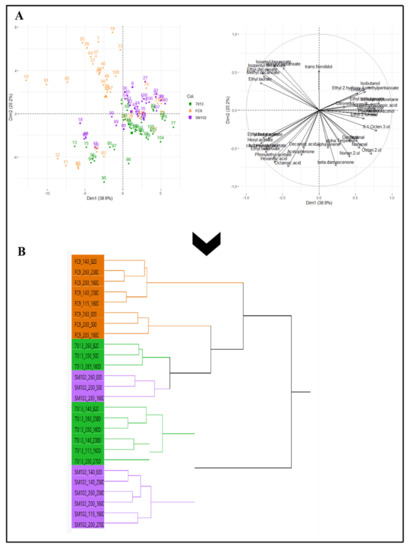
Figure 4.
Principal component analysis of aroma production (fermentative aromas, terpenes, aldehydes, noriseprenoids and alcohols) for the three strains (FC9® (―), 7013® (―) and SM102® (―) under all conditions of fermentation (A) and its correlation with the hierarchical classification of the aromatic composition of wines (B).
The other two strains, 7013® and SM102®, seemed to be quite similar, although they each had their own aromatic character. This result was also supported by the decision tree, which demonstrated the aromatic typicity of strain FC9® (Figure 4B). The other strains had a similar strong interaction effect with the fermentation parameters: the same aroma fingerprint for SM102® and 7013® was correlated to the same levels of YAN and lipid nutrition parameters. Beyond the classic aromas resulting from the well-known fermentation metabolism, the Saccharomyces cerevisiae strains differed in their terpene fraction through the distribution of monoterpene production (Figure 3). Strain FC9® contributed a rose aroma note (trans-nerolidol), strain 7013® had lemon grass nuances (citronellol) and strain SM102® produced a flower aroma (linalool). Under high lipid level conditions, the metabolic pathway of terpene biosynthesis is activated and should not be neglected compared to the varietal source.
4. Conclusions
In conclusion, this study allowed us to analyze the impact of the two main fermentation parameters, nitrogen and turbidity, on the production of fermentation aromas and also on a wider range of aromas through a metabolomic analysis. First, it appeared that the results for the CCM metabolites were consistent with the literature: at a high initial lipid content, the acetate synthesis pathway was repressed while that of glycerol was favored. Moreover, in the working range of assimilable nitrogen in this study, the production of higher alcohols decreased, while their synthesis was favored by the presence of phytosterols and fatty acids. The effects of these nutrients on acetate esters were the complete opposite, indicating that the key element in the regulation of their synthesis is not the availability of their precursor. Finally, the metabolism of ethyl esters and their corresponding acids remained more complex to understand, although the lipid concentration was decreased. This study also supported the fact that terpenes are not only aromas of varietal origin, and an important part of their production originates from yeast metabolism under conditions of excess lipids in the medium, and moreover, in a different way, depending on the strains used. In addition, acetaldehyde, which plays a central role in Saccharomyces cerevisiae metabolism, seemed to be strongly impacted by the nitrogen and lipid supply. A forthcoming study will detail the chronology of its synthesis based on data obtained from an online monitoring system.
Author Contributions
Conceptualization, X.P., J.-R.M. and V.F.; methodology, C.G., F.M. and A.B.; formal analysis, C.G., I.S. and A.B.; investigation, C.G., A.B. and V.F.; writing—original draft preparation, C.G., I.S., A.B. and V.F.; writing—review and editing, C.G., X.P., J.-R.M., J.-M.S. and V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We would like to thank the aroma analysis platform (PTV, UMR SPO, INRAE Montpellier) for their training, patience and availability for the analysis of aromas and the ChemoSens platform (CSGA, INRAE Dijon) for the analysis of lipids. Finally, we thank Thérèse Marlin for the analysis of amino acids (ADEL Team, UMR SPO, INRAE Montpellier) and Mélanie Veyret (UE Pech Rouge, INRAE) for her help with the acetaldehyde assays.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Ferrari, G.; Lablanquie, O.; Cantagrel, R.; Ledauphin, J.; Payot, T.; Fournier, N.; Guichard, E. Determination of Key Odorant Compounds in Freshly Distilled Cognac Using GC-O, GC-MS, and Sensory Evaluation. J. Agric. Food Chem. 2004, 52, 7. [Google Scholar] [CrossRef] [PubMed]
- Lurton, L.; Snakkers, G.; Roulland, C.; Galy, B.; Versavaud, A. Influence of the fermentation yeast strain on the composition of wine spirits. J. Sci. Food Agric. 1995, 67, 485–491. [Google Scholar] [CrossRef]
- Vilanova, M.; Siebert, T.E.; Varela, C.; Pretorius, I.S.; Henschke, P.A. Effect of ammonium nitrogen supplementation of grape juice on wine volatiles and non-volatiles composition of the aromatic grape variety Albariño. Food Chem. 2012, 133, 124–131. [Google Scholar] [CrossRef]
- Casalta, E.; Cervi, M.F.; Salmon, J.M.; Sablayrolles, J.M. White wine fermentation: Interaction of assimilable nitrogen and grape solids: Interaction of assimilable nitrogen and grape solids on alcoholic fermentation under oenological conditions. Aust. J. Grape Wine Res. 2013, 19, 47–52. [Google Scholar] [CrossRef]
- Beltran, G.; Esteve-Zarzoso, B.; Rozès, N.; Mas, A.; Guillamón, J.M. Influence of the timing of nitrogen additions during synthetic grape must fermentations on fermentation kinetics and nitrogen consumption. J. Agric. Food Chem. 2005, 53, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Gobert, A.; Tourdot-Maréchal, R.; Sparrow, C.; Morge, C.; Alexandre, H. Influence of nitrogen status in wine alcoholic fermentation. Food Microbiol. 2019, 83, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Guittin, C.; Maçna, F.; Sanchez, I.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. Impact of high lipid contents on the production of fermentative aromas during white wine fermentation. Appl. Microbiol. Biotechnol. 2021, 105, 6435–6449. [Google Scholar] [CrossRef]
- Jiménez-Martí, E.; Aranda, A.; Mendes-Ferreira, A.; Mendes-Faia, A.; lí del Olmo, M. The nature of the nitrogen source added to nitrogen depleted vinifications conducted by a Saccharomyces cerevisiae strain in synthetic must affects gene expression and the levels of several volatile compounds. Antonie Leeuwenhoek 2007, 92, 61–75. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.-M.; Dequin, S.; Mouret, J.-R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef]
- Seguinot, P.; Rollero, S.; Sanchez, I.; Sablayrolles, J.-M.; Ortiz-Julien, A.; Camarasa, C.; Mouret, J.-R. Impact of the timing and the nature of nitrogen additions on the production kinetics of fermentative aromas by Saccharomyces cerevisiae during winemaking fermentation in synthetic media. Food Microbiol. 2018, 76, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Torrea, D.; Varela, C.; Ugliano, M.; Ancin-Azpilicueta, C.; Leigh Francis, I.; Henschke, P.A. Comparison of inorganic and organic nitrogen supplementation of grape juice—Effect on volatile composition and aroma profile of a Chardonnay wine fermented with Saccharomyces cerevisiae yeast. Food Chem. 2011, 127, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Torrea, D.; Henschke, P.A. Ammonium supplementation of grape juice-effect on the aroma profile of a Chardonnay wine. TechRev 2004, 150, 59–63. [Google Scholar]
- Mouret, J.R.; Camarasa, C.; Angenieux, M.; Aguera, E.; Perez, M.; Farines, V.; Sablayrolles, J.M. Kinetic analysis and gas–liquid balances of the production of fermentative aromas during winemaking fermentations: Effect of assimilable nitrogen and temperature. Food Res. Int. 2014, 62, 1–10. [Google Scholar] [CrossRef]
- Vilanova, M.; Ugliano, M.; Varela, C.; Siebert, T.; Pretorius, I.S.; Henschke, P.A. Assimilable nitrogen utilisation and production of volatile and non-volatile compounds in chemically defined medium by Saccharomyces cerevisiae wine yeasts. Appl. Microbiol. Biotechnol. 2007, 77, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Miller, A.C.; Wolff, S.R.; Bisson, L.F.; Ebeler, S.E. Yeast Strain and Nitrogen Supplementation: Dynamics of Volatile Ester Production in Chardonnay Juice Fermentations. Am. J. Enol. Vitic. 2007, 58, 470–483. [Google Scholar]
- Andreasen, A.A.; Stier, T.J.B. Anaerobic nutrition of Saccharomyces cerevisiae. I. Ergosterol requirement for growth in a defined medium. J. Cell. Physiol. 1953, 41, 23–36. [Google Scholar] [CrossRef]
- Casalta, E.; Salmon, J.-M.; Picou, C.; Sablayrolles, J.-M. Grape Solids: Lipid Composition and Role during Alcoholic Fermentation under Enological Conditions. Am. J. Enol. Vitic. 2019, 70, 147–154. [Google Scholar] [CrossRef]
- Rollero, S.; Mouret, J.-R.; Sanchez, I.; Camarasa, C.; Ortiz-Julien, A.; Sablayrolles, J.-M.; Dequin, S. Key role of lipid management in nitrogen and aroma metabolism in an evolved wine yeast strain. Microb. Cell Factories 2016, 15, 32. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.; Verstrepen, K.J.; Van Dijck, P.; Thevelein, J.M.; Delvaux, F.R. Parameters Affecting Ethyl Ester Production by Saccharomyces cerevisiae during Fermentation. Appl. Environ. Microbiol. 2008, 74, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Yunoki, K.; Yasui, Y.; Hirose, S.; Ohnishi, M. Fatty acids in must prepared from 11 grapes grown in Japan: Comparison with wine and effect on fatty acid ethyl ester formation. Lipids 2005, 40, 361–367. [Google Scholar] [CrossRef]
- Sablayrolles, J.M.; Barre, P.; Grenier, P. Design of a laboratory automatic system for studying alcoholic fermentations in anisothermal enological conditions. Biotechnol. Tech. 1987, 1, 181–184. [Google Scholar] [CrossRef]
- Crépin, L.; Nidelet, T.; Sanchez, I.; Dequin, S.; Camarasa, C. Sequential Use of Nitrogen Compounds by Saccharomyces cerevisiae during Wine Fermentation: A Model Based on Kinetic and Regulation Characteristics of Nitrogen Permeases. Appl. Environ. Microbiol. 2012, 78, 8102–8111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenth, R.V. Response-Surface Methods in R, Using rsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Box, G.E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B 1951, 13, 1–38. [Google Scholar] [CrossRef]
- Manginot, C.; Sablayrolles, J.M.; Roustan, J.L.; Barre, P. Use of constant rate alcoholic fermentations to compare the effectiveness of different nitrogen sources added during the stationnary phase. Enz. Microbiol. Tech. 1997, 20, 373–380. [Google Scholar] [CrossRef]
- Blateyron, L.; Sablayrolles, J. Stuck and Slow Fermentations in Enology: Statistical Study of Causes and Effectiveness of Combined Additions of Oxygen and Diammonium Phosphate. J. Biosci. Bioeng. 2001, 91, 184–189. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.M.; Barre, P. Description of Alcoholic Fermentation Kinetics: Its Variability and Significance. Am. J. Enol. Vitic. 1990, 41, 319–324. [Google Scholar]
- Ochando, T.; Mouret, J.-R.; Humbert-Goffard, A.; Sablayrolles, J.-M.; Farines, V. Impact of initial lipid content and oxygen supply on alcoholic fermentation in champagne-like musts. Food Res. Int. 2017, 98, 87–94. [Google Scholar] [CrossRef]
- Deroite, A.; Legras, J.-L.; Rigou, P.; Ortiz-Julien, A.; Dequin, S. Lipids modulate acetic acid and thiol final concentrations in wine during fermentation by Saccharomyces cerevisiae × Saccharomyces kudriavzevii hybrids. AMB Expr. 2018, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Luparia, V.; Soubeyrand, V.; Berges, T.; Julien, A.; Salmon, J.-M. Assimilation of grape phytosterols by Saccharomyces cerevisiae and their impact on enological fermentations. Appl. Microbiol. Biotechnol. 2004, 65, 25–32. [Google Scholar] [CrossRef]
- Rollero, S. Impact des paramètres environnementaux sur la synthèse des arômes fermentaires par Saccharomyces cerevisiae en fermentation oenologique. Ph.D. Thesis, Montpellier SupAgro, Montpellier, France, 2015. [Google Scholar]
- Albers, E.; Larsson, C.; Lidén, G.; Niklasson, C.; Gustafsson, L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microbiol. 1996, 62, 3187–3195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Remize, F.; Sablayrolles, J.M.; Dequin, S. Re-assessment of the influence of yeast strain and environmental factors on glycerol production in wine. J. Appl. Microbiol. 2000, 88, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Nykanen, I. Aroma of Beer, Wine and Distilled Alcoholic Beverages; Kluwer Academic Publishers: Berlin, Germany, 1983. [Google Scholar]
- Verstrepen, K.J.; Laere, S.D.M.V.; Vanderhaegen, B.M.P.; Derdelinckx, G.; Dufour, J.-P.; Pretorius, I.S.; Winderickx, J.; Thevelein, J.M.; Delvaux, F.R. Expression Levels of the Yeast Alcohol Acetyltransferase Genes ATF1, Lg-ATF1, and ATF2 Control the Formation of a Broad Range of Volatile Esters. Appl. Environ. Microbiol. 2003, 69, 5228–5237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujii, T.; Kobayashi, O.; Yoshimoto, H.; Furukawa, S.; Tamai, Y. Effect of aeration and unsaturated fatty acids on expression of the Saccharomyces cerevisiae alcohol acetyltransferase gene. Appl. Environ. Microbiol. 1997, 63, 910–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujiwara, D.; Yoshimoto, H.; Sone, H.; Harashima, S.; Tamai, Y. Transcriptional co-regulation of Saccharomyces cerevisiae alcohol acetyltransferase gene, ATF1 and Δ-9 fatty acid desaturase gene, OLE1 by unsaturated fatty acids. Yeast 1998, 14, 711–721. [Google Scholar] [CrossRef]
- Torrea, D. Production of volatile compounds in the fermentation of chardonnay musts inoculated with two strains of Saccharomyces cerevisiae with different nitrogen demands. Food Control 2003, 14, 565–571. [Google Scholar] [CrossRef]
- Ugliano, M.; Travis, B.; Francis, I.L.; Henschke, P.A. Volatile Composition and Sensory Properties of Shiraz Wines as Affected by Nitrogen Supplementation and Yeast Species: Rationalizing Nitrogen Modulation of Wine Aroma. J. Agric. Food Chem. 2010, 58, 12417–12425. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Torrea, D.; Schmidt, S.A.; Ancin-Azpilicueta, C.; Henschke, P.A. Effect of oxygen and lipid supplementation on the volatile composition of chemically defined medium and Chardonnay wine fermented with Saccharomyces cerevisiae. Food Chem. 2012, 135, 2863–2871. [Google Scholar] [CrossRef]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Black, C.A.; Parker, M.; Siebert, T.E.; Capone, D.L.; Francis, I.L. Terpenoids and their role in wine flavour: Recent advances: Terpenoids: Role in wine flavour. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Ribereau-Gayon, P.; Dubourdieu, D.; Glories, Y.; Maujean, A. Traitéd’oenologie: Chimie Du Vin, Stabilisation et Traitements; Dunod: Malakoff, France, 2017. [Google Scholar]
- Malfondet, N.; Gourrat, K.; Brunerie, P.; Le Quéré, J.-L. Aroma characterization of freshly-distilled French brandies; their specificity and variability within a limited geographic area: Aroma characterization of freshly-distilled French brandies. Flavour Fragr. J. 2016, 31, 361–376. [Google Scholar] [CrossRef]
- Schreier, P.; Drawert, F.; Winkler, F. Composition of neutral volatile constituents in grape brandies. J. Agric. Food Chem. 1979, 27, 365–372. [Google Scholar] [CrossRef]
- Thibaud, F.; Courregelongue, M.; Darriet, P. Contribution of Volatile Odorous Terpenoid Compounds to Aged Cognac Spirits Aroma in a Context of Multicomponent Odor Mixtures. J. Agric. Food Chem. 2020, 68, 13310–13318. [Google Scholar] [CrossRef] [PubMed]
- Eliseev, M.; Gribkova, I.; Kosareva, O.; Alexeyeva, O. Effect of organic compounds on cognac sensory profile. Foods Raw Mater. 2021, 244–253. [Google Scholar] [CrossRef]
- Wilson, B.; Strauss, C.R.; Williams, J. The Distribution of Free and Glycosidically-Bound Monoterpenes among Skin, Juice, and Pulp Fractions of Some White Grape Varieties. Am. J. Enol. Vitic. 1986, 37, 107–111. [Google Scholar]
- Charoenchai, C.; Fleet, G.H.; Henschke, P.A.; Todd, B.E.N.T. Screening of non-Saccharomyces wine yeasts for the presence of extracellular hydrolytic enzymes. Aust. J. Grape Wine Res. 1997, 3, 2–8. [Google Scholar] [CrossRef]
- Strauss, M.L.A.; Jolly, N.P.; Lambrechts, M.G.; van Rensburg, P. Screening for the production of extracellular hydrolytic enzymes by non-Saccharomyces wine yeasts. J. Appl. Microbiol. 2001, 91, 182–190. [Google Scholar] [CrossRef]
- Awad, P.; Athès, V.; Decloux, M.E.; Ferrari, G.; Snakkers, G.; Raguenaud, P.; Giampaoli, P. Evolution of Volatile Compounds during the Distillation of Cognac Spirit. J. Agric. Food Chem. 2017, 65, 7736–7748. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Boido, E.; Farina, L.; Gaggero, C.; Dellacassa, E.; Versini, G.; Henschke, P.A. De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol. Lett. 2005, 243, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynen, F. The pathway from activated acetic acid to the terpenes and fatty acids. Proc. R. Caroline Inst. 1964, 103–138. [Google Scholar]
- Gamero, A.; Manzanares, P.; Querol, A.; Belloch, C. Monoterpene alcohols release and bioconversion by Saccharomyces species and hybrids. Int. J. Food Microbiol. 2011, 145, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Steyer, D. Genetic analysis of geraniol metabolism during fermentation. Food Microbiol. 2013, 7, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Vaudano, E.; Moruno, E.G.; Stefano, R. Modulation of Geraniol Metabolism during Alcohol Fermentation. J. Inst. Brew. 2004, 110, 213–219. [Google Scholar] [CrossRef]
- Denat, M.; Pérez, D.; Heras, J.M.; Querol, A.; Ferreira, V. The effects of Saccharomyces cerevisiae strains carrying alcoholic fermentation on the fermentative and varietal aroma profiles of young and aged Tempranillo wines. Food Chem. X 2021, 9, 100116. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Güntert, M.; Engel, K.-H. (Eds.) Aroma Active Compounds in Foods: Chemistry and Sensory Properties; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2001; Volume 794, ISBN 978-0-8412-3694-3. [Google Scholar]
- Pallotta, U.; Castellari, M.; Piva, A.; Baumes, R.; Bayonove, C. Effects of Botrytis cinerea on must composition of three italian grape varieties. Wein-Wissenschaft 1998, 53, 32–36. [Google Scholar]
- La Guerche, S.; Dauphin, B.; Pons, M.; Blancard, D.; Darriet, P. Characterization of Some Mushroom and Earthy Off-Odors Microbially Induced by the Development of Rot on Grapes. J. Agric. Food Chem. 2006, 54, 9193–9200. [Google Scholar] [CrossRef]
- Wurzenberger, M.; Grosch, W. The formation of 1-octen-3-ol from the 10-hydroperoxide isomer of linoleic acid by a hydroperoxide lyase in mushrooms (Psalliota bispora). Biochim. Biophys. Acta Lipids Lipid Metab. 1984, 794, 25–30. [Google Scholar] [CrossRef]
- Liu, S.-Q.; Pilone, G.J. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbiological implications. Int. J. Food Sci. Technol. 2000, 35, 49–61. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. LWT Food Sci. Technol. 2008, 41, 501–510. [Google Scholar] [CrossRef]
- Wucherpfenning, K.; Semmler, G. Formation of acetaldehyde during fermentation in relation to pH-value and to individual vitamins. Z. Lebensm. Unters. Forsch. 1972, 148, 138–145. [Google Scholar]
- Bosso, A.; Guaita, M. Study of some factors involved in ethanal production during alcoholic fermentation. Eur. Food Res. Technol. 2008, 227, 911–917. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).