Pretreatment in Vortex Layer Apparatus Boosts Dark Fermentative Hydrogen Production from Cheese Whey
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum
2.2. Pretreatment of CW in VLA
2.3. Biochemical Hydrogen Potential Test
2.4. Analytical Methods and Data Analysis
2.5. Statistical Analysis
3. Results and Discussion
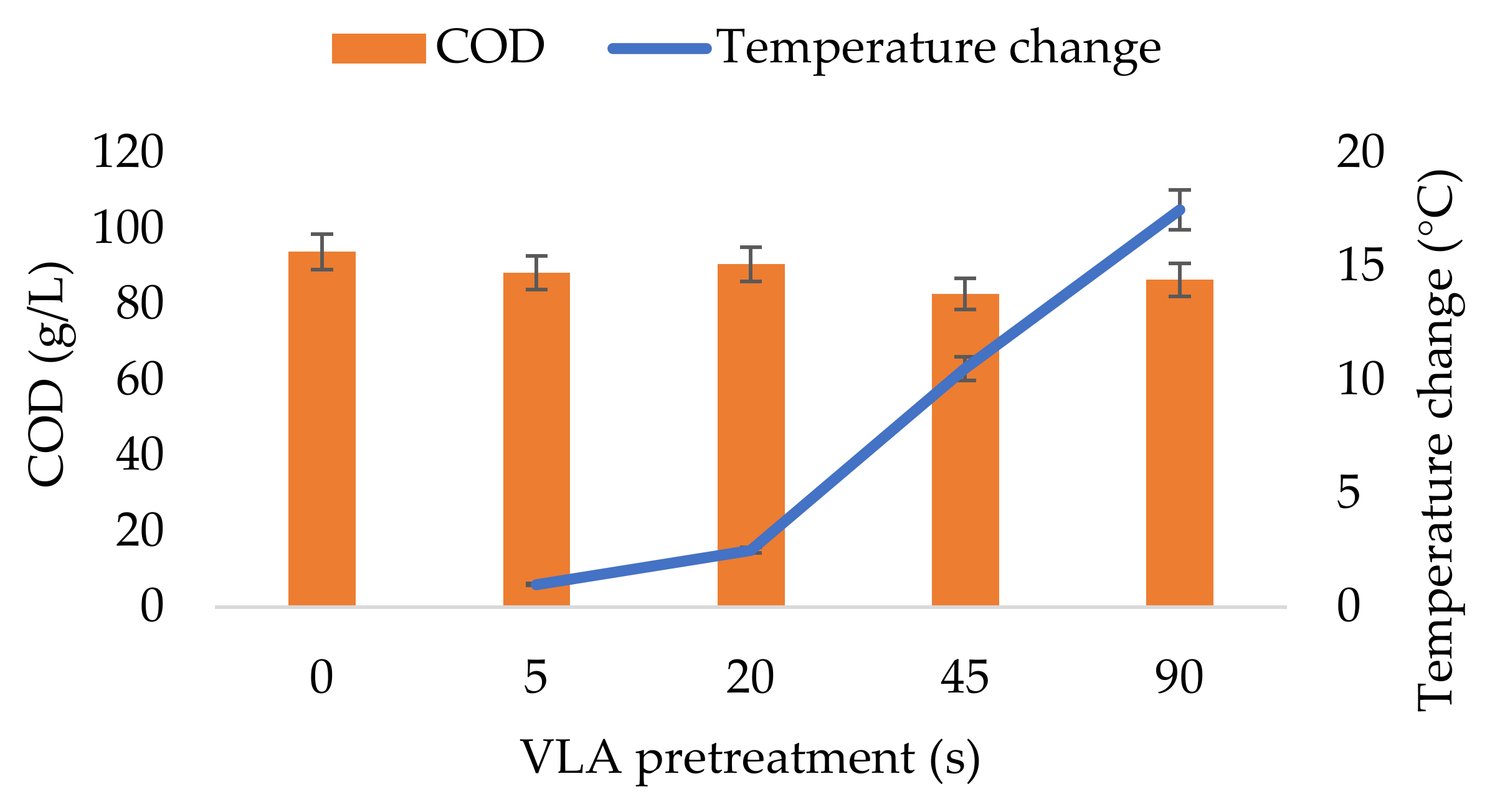
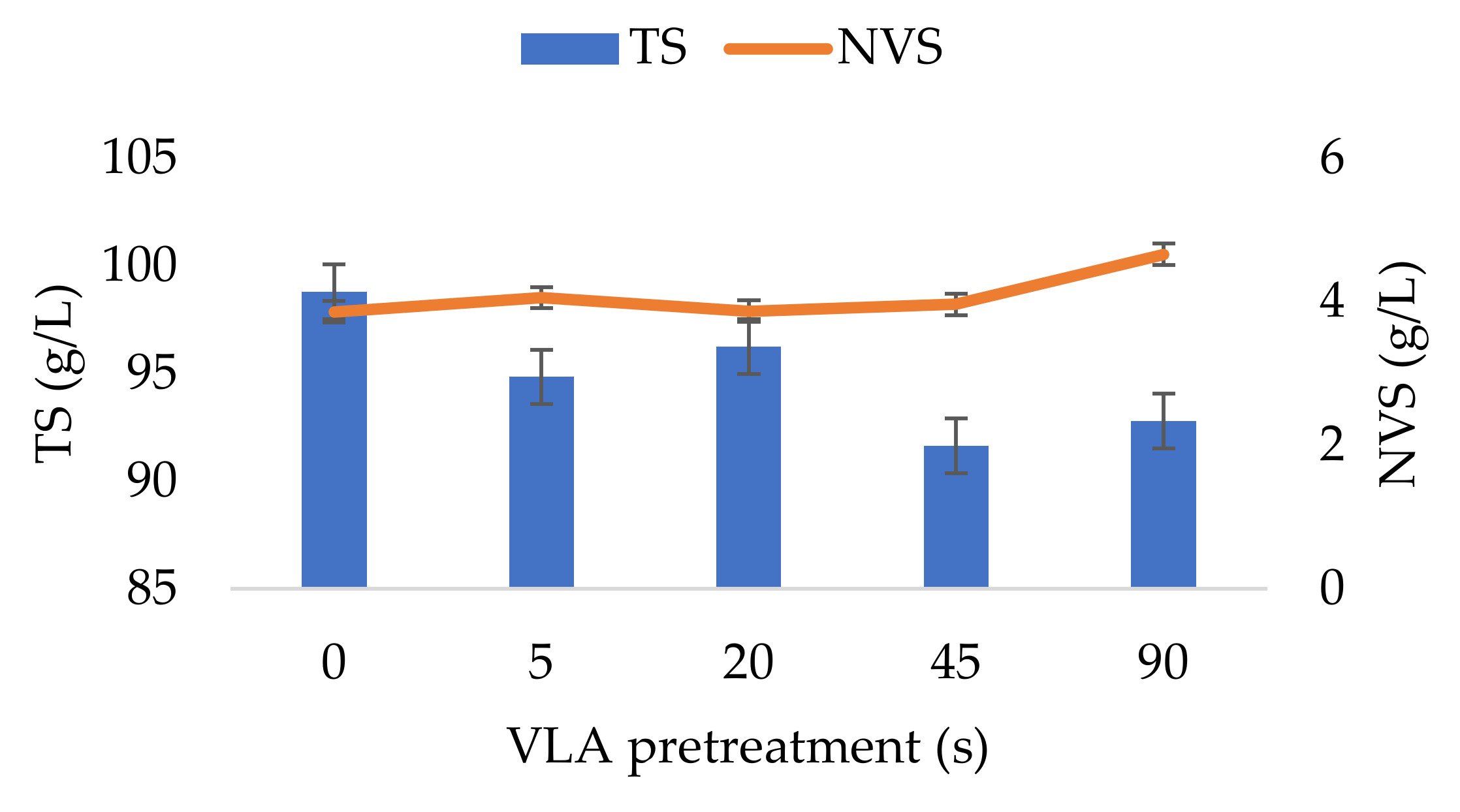
3.1. Changes in Physical and Chemical Properties of the Pretreated CW
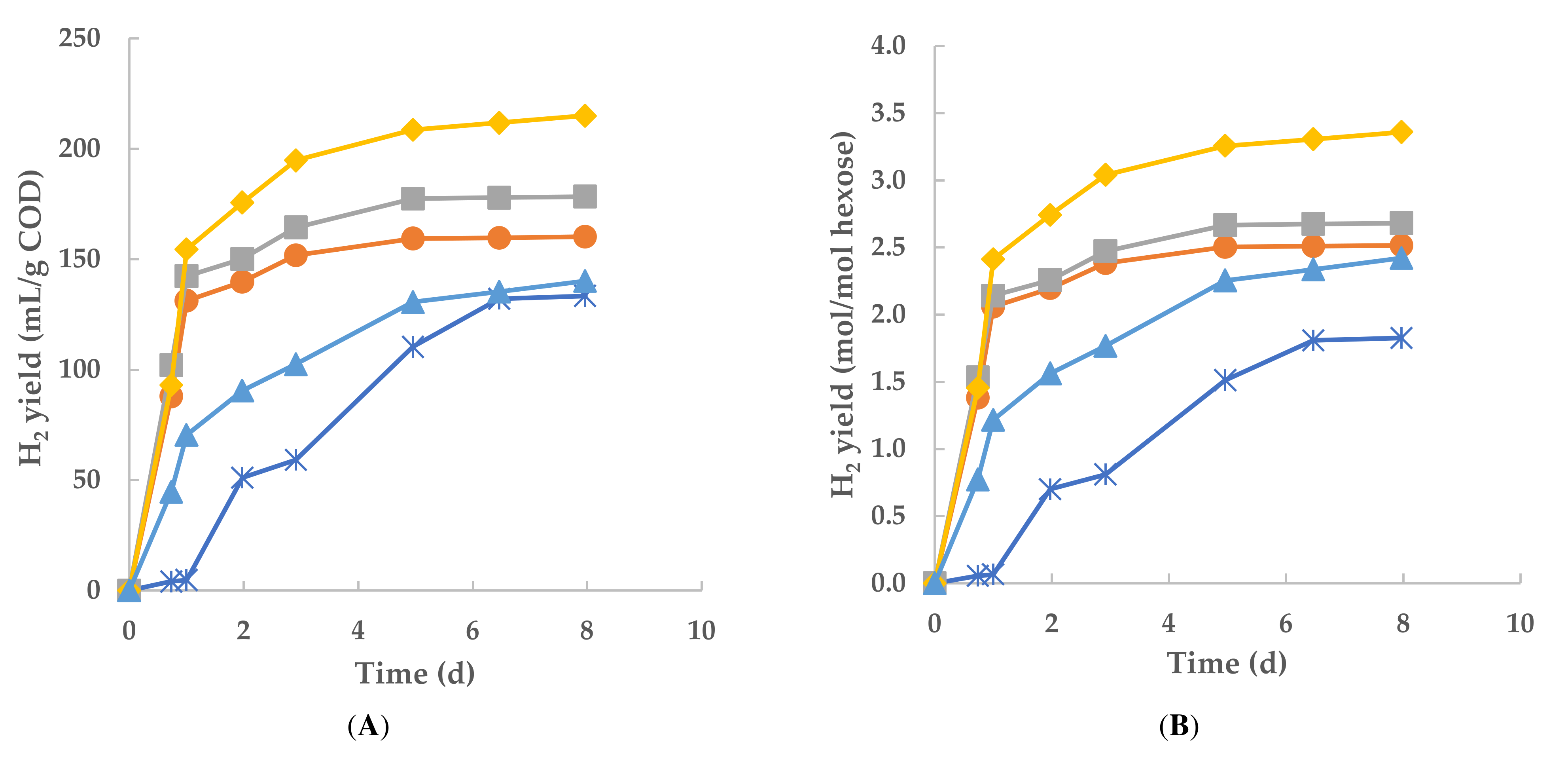
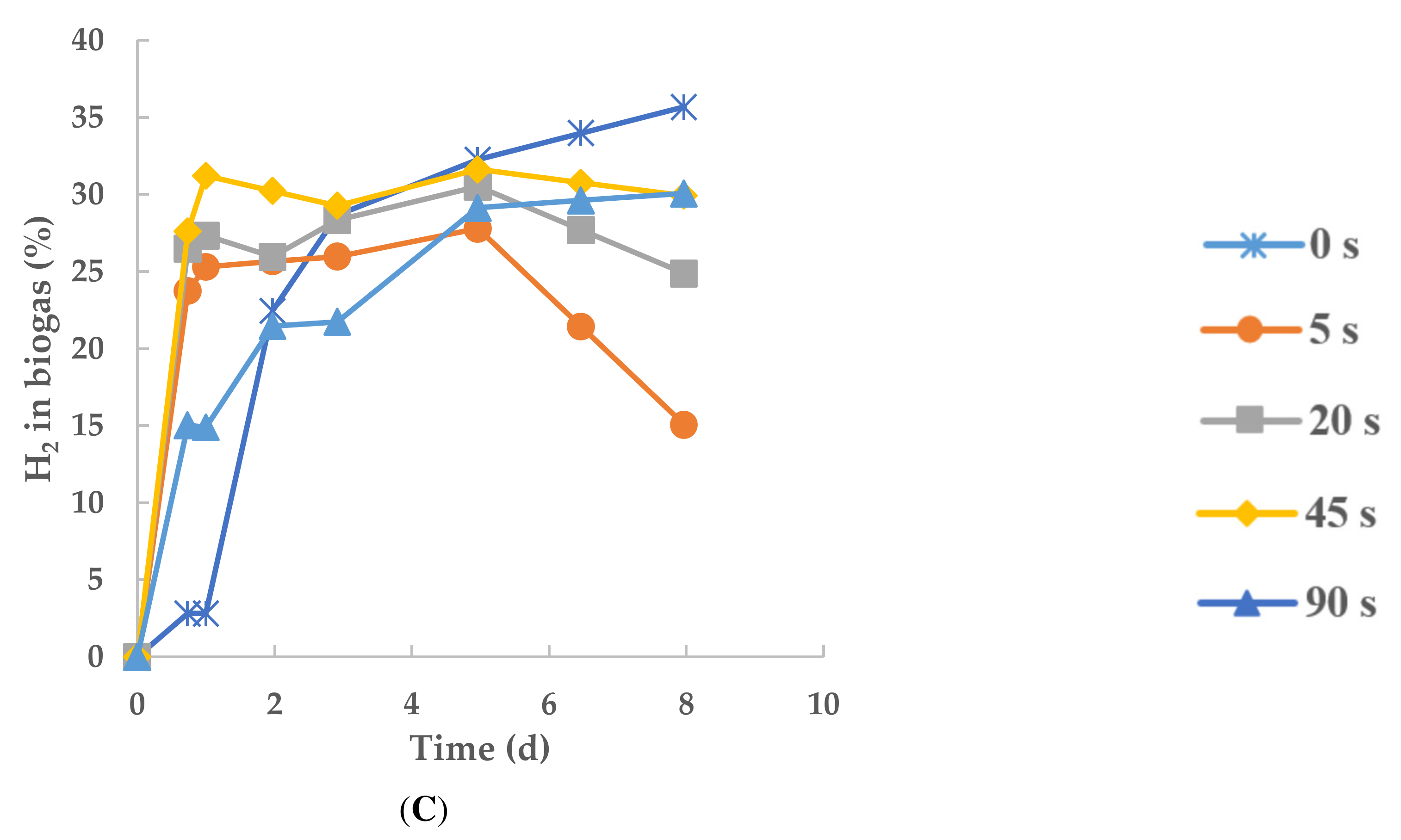
3.2. Dynamics of Biohydrogen Production
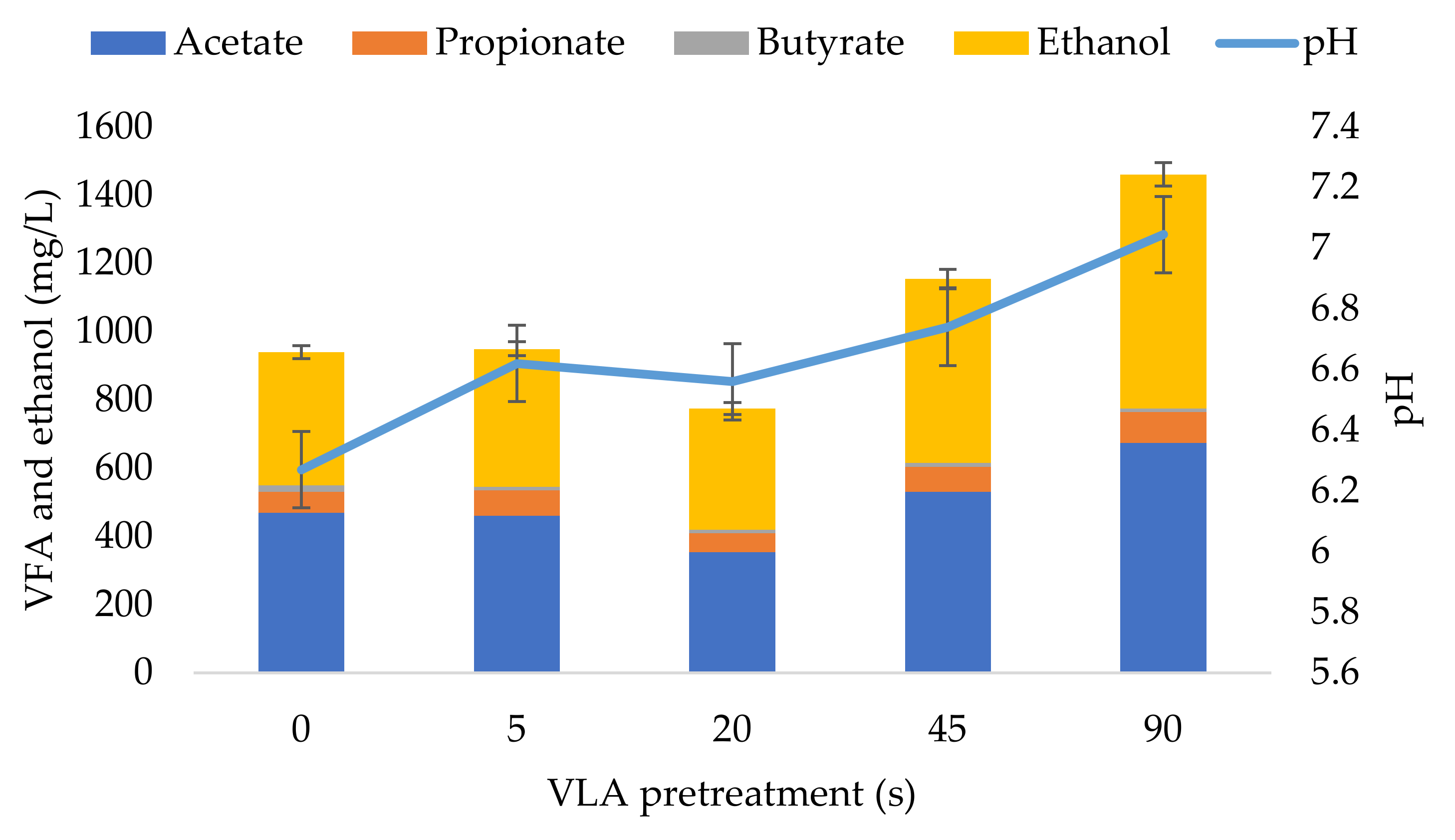
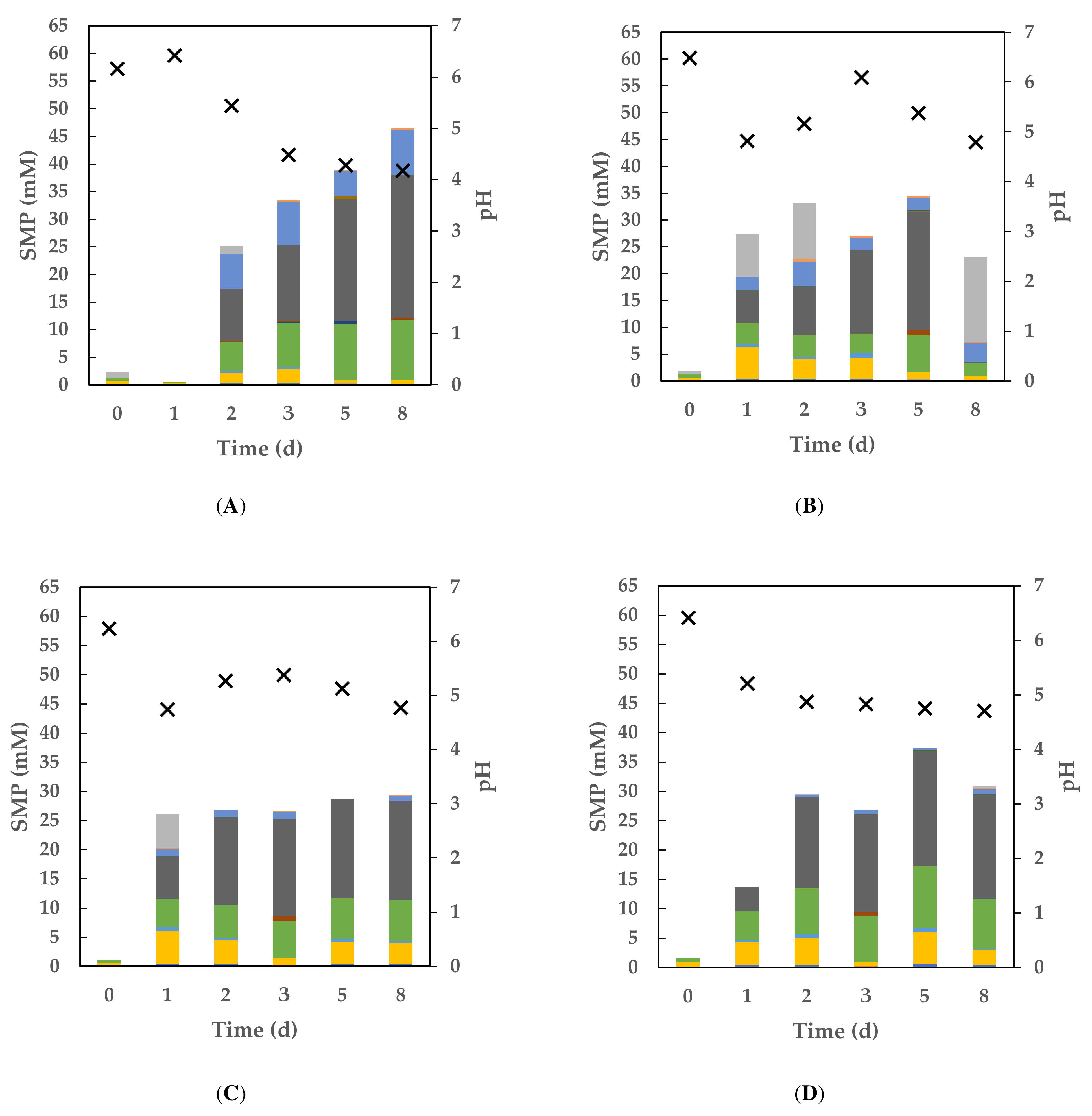
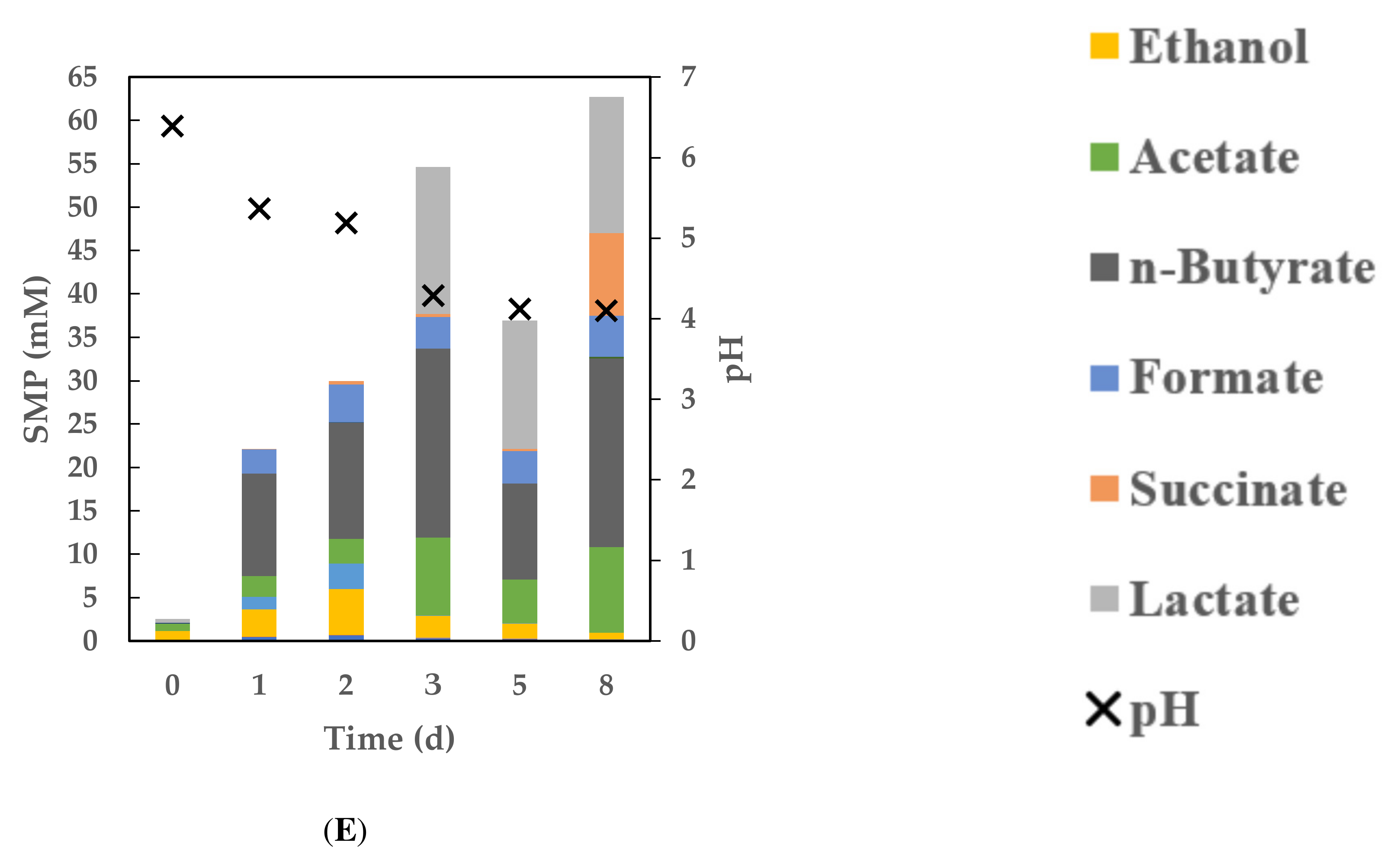
3.3. Dynamics of Soluble Metabolite Products
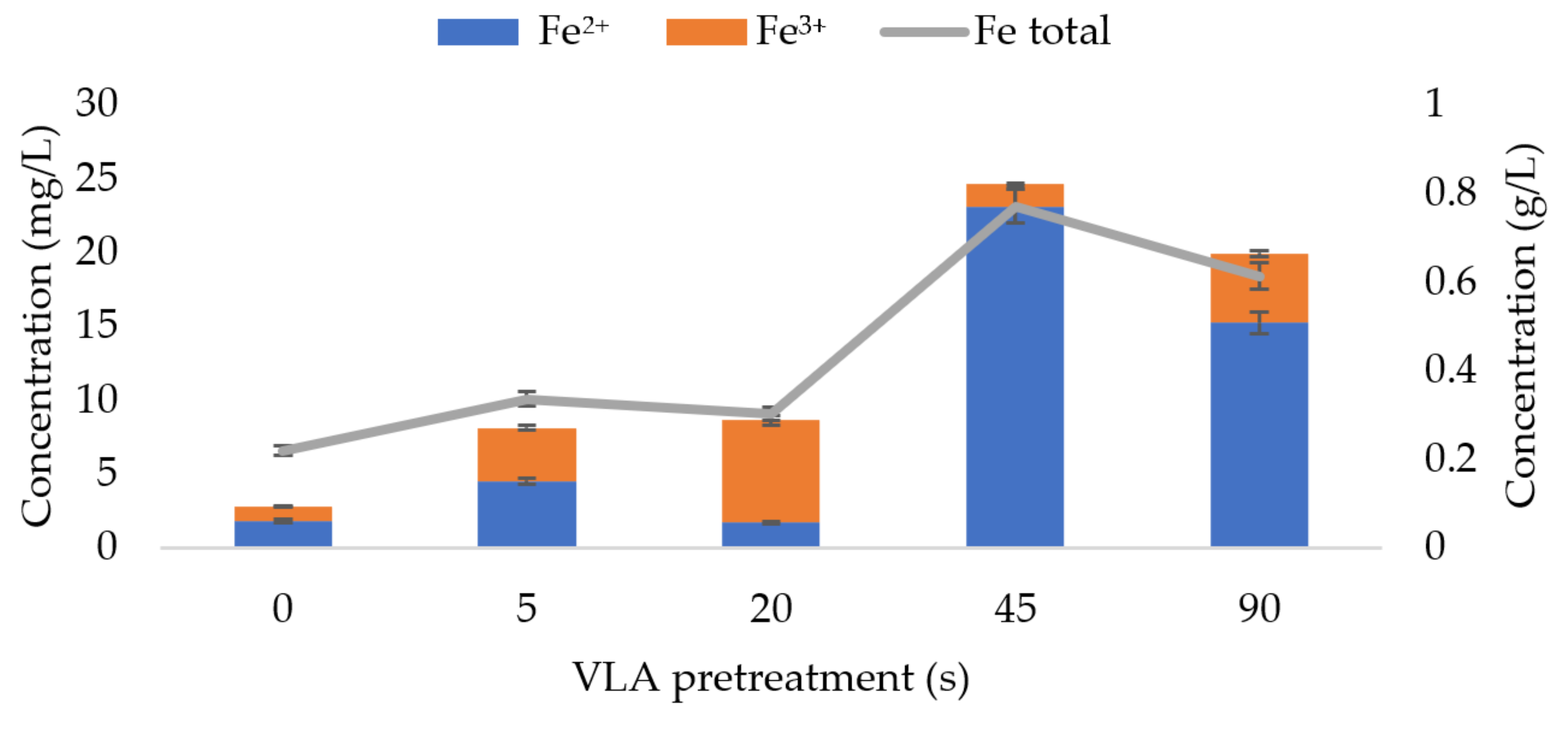
3.4. Soluble Forms of Iron
3.5. Kinetics of H2 Production and Correlation Analysis
3.6. Recent Progress in Various Pretreatment Methods: Comparison with VLA and Limitations
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Litti, Y.V.; Potekhina, M.A.; Zhuravleva, E.A.; Vishnyakova, A.V.; Gruzdev, D.S.; Kovalev, A.A.; Kovalev, D.A.; Katraeva, I.V.; Parshina, S.N. Dark fermentative hydrogen production from simple sugars and various wastewaters by a newly isolated thermoanaerobacterium thermosaccharolyticum SP-H2. Int. J. Hydrogen Energy 2022, 47, 24310–24327. [Google Scholar] [CrossRef]
- Mikheeva, E.R.; Katraeva, I.V.; Kovalev, A.A.; Kovalev, D.A.; Nozhevnikova, A.N.; Panchenko, V.; Fiore, U.; Litti, Y.V. The start-up of continuous biohydrogen production from cheese whey: Comparison of inoculum pretreatment methods and reactors with moving and fixed polyurethane carriers. Appl. Sci. 2021, 11, 510. [Google Scholar] [CrossRef]
- Mikheeva, E.R.; Katraeva, I.V.; Vorozhtsov, D.L.; Kovalev, D.A.; Kovalev, A.A.; Grigoriev, V.S.; Litti, Y.V. Dark fermentative biohydrogen production from confectionery wastewater in continuous-flow reactors. Int. J. Hydrogen Energy 2022, 47, 22348–22358. [Google Scholar] [CrossRef]
- Kovalev, A.A.; Kovalev, D.A.; Nozhevnikova, A.N.; Zhuravleva, E.A.; Katraeva, I.V.; Grigoriev, V.S.; Litti, Y.V. Effect of low digestate recirculation ratio on biofuel and bioenergy recovery in a two-stage anaerobic digestion process. Int. J. Hydrogen Energy 2021, 46, 39688–39699. [Google Scholar] [CrossRef]
- Florio, C.; Pirozzi, D.; Ausiello, A.; Micoli, L.; Pasquale, V.; Toscano, G.; Turco, M.; Dumontet, S. Effect of inoculum/substrate ratio on dark fermentation for biohydrogen production from organic fraction of municipal solid waste. Chem. Eng. Trans. 2017, 57, 175–180. [Google Scholar] [CrossRef]
- Ausiello, A.; Micoli, L.; Turco, M.; Toscano, G.; Florio, C.; Pirozzi, D. Biohydrogen production by dark fermentation of Arundo donax using a new methodology for selection of H2-producing bacteria. Int. J. Hydrogen Energy 2017, 42, 30599–30612. [Google Scholar] [CrossRef]
- Castelló, E.; Ferraz-Junior, A.D.N.; Andreani, C.; Anzola-Rojas, M.D.P.; Borzacconi, L.; Buitrón, G.; Carrillo-Reyes, J.; Gomes, S.D.; Maintinguer, S.I.; Moreno-Andrade, I.; et al. Stability problems in the hydrogen production by dark fermentation: Possible causes and solutions. Renew. Sustain. Energy Rev. 2019, 119, 109602. [Google Scholar] [CrossRef]
- Kovalev, A.; Mikheeva, E.; Katraeva, I.; Kozlov, A.; Yu, V.L. Bioenergy recovery from two-stage mesophilic-thermophilic anaerobic digestion of cheese whey. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Banu, J.R.; Merrylin, J.; Usman, T.M.; Kannah, R.Y.; Gunasekaran, M.; Kim, S.-H.; Kumar, G. Impact of pretreatment on food waste for biohydrogen production: A review. Int. J. Hydrogen Energy 2019, 45, 18211–18225. [Google Scholar] [CrossRef]
- Moodley, P.; Trois, C. Lignocellulosic Biorefineries: The Path Forward; Academic Press: Cambridge, MA, USA, 2021; pp. 21–42. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Ishola, M.M.; Richards, T.; Taherzadeh, M.J. An overview of existing individual unit operations. In Biorefineries: Integrated Biochemical Processes for Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–36. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Sewage sludge for hydrogen production. In Green Energy and Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 339–433. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Sambusiti, C.; Bellucci, M.; Zabaniotou, A.; Beneduce, L.; Monlau, F. Algae as promising feedstocks for fermentative biohydrogen production according to a biorefinery approach: A comprehensive review. Renew. Sustain. Energy Rev. 2015, 44, 20–36. [Google Scholar] [CrossRef]
- Prabakar, D.; Manimudi, V.T.; Suvetha, S.S.; Sampath, S.; Mahapatra, D.M.; Rajendran, K.; Pugazhendhi, A. Advanced biohydrogen production using pretreated industrial waste: Outlook and prospects. Renew. Sustain. Energy Rev. 2018, 96, 306–324. [Google Scholar] [CrossRef]
- Balasundaram, G.; Vidyarthi, P.K.; Gahlot, P.; Arora, P.; Kumar, V.; Kumar, M.; Kazmi, A.; Tyagi, V.K. Energy feasibility and life cycle assessment of sludge pretreatment methods for advanced anaerobic digestion. Bioresour. Technol. 2022, 357, 127345. [Google Scholar] [CrossRef]
- Mikheeva, E.; Katraeva, I.; Kovalev, A.; Litti, Y.V. Effects of pretreatment in a vortex layer apparatus on the properties of confectionery wastewater and its dark fermentation. Int. J. Hydrogen Energy 2022, 47, 23165–23174. [Google Scholar] [CrossRef]
- Kovalev, A.A.; Kovalev, D.A.; Litti, Y.V.; Katraeva, I.V. The synergistic effect of the thickened digestate treatment in the vortex layer apparatus prior to its recirculation into the reactor on the characteristics of anaerobic bioconversion of organic waste. J. Phys.: Conf. Ser. 2020, 1652, 012014. [Google Scholar] [CrossRef]
- Mikheeva, E.R.; Katraeva, I.V.; Vorozhtsov, D.L.; Litti, Y.V.; Nozhevnikova, A.N. Efficiency of two-phase anaerobic fermentation and the physicochemical properties of the organic fraction of municipal solid waste processed in a vortex-layer apparatus. Appl. Biochem. Microbiol. 2020, 56, 736–742. [Google Scholar] [CrossRef]
- Kovalev, A.A.; Kovalev, D.A.; Grigoriev, V.S.; Litti, Y.V. The vortex layer apparatus as a source of low-grade heat in the process of pretreatment of the substrate before anaerobic digestion. IOP Conf. Ser. Earth Environ. Sci. 2021, 938, 012004. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Li, Y.-Y.; Oh, Y.-K.; Kim, M.-S.; Noike, T. Effect of iron concentration on continuous H2 production using membrane bioreactor. Int. J. Hydrogen Energy 2009, 34, 1244–1252. [Google Scholar] [CrossRef]
- Jiang, D.; Fang, Z.; Chin, S.-X.; Tian, X.; Su, T.-C. Biohydrogen production from hydrolysates of selected tropical biomass wastes with clostridium butyricum. Sci. Rep. 2016, 6, 27205. [Google Scholar] [CrossRef]
- Salama, E.-S.; Saha, S.; Kurade, M.B.; Dev, S.; Chang, S.W.; Jeon, B.-H. Recent trends in anaerobic co-digestion: Fat, oil, and grease (FOG) for enhanced biomethanation. Prog. Energy Combust. Sci. 2018, 70, 22–42. [Google Scholar] [CrossRef]
- Thakur, H.; Dhar, A.; Powar, S. Biogas production from anaerobic co-digestion of sewage sludge and food waste in continuously stirred tank reactor. Results Eng. 2022, 16, 100617. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Peterson, W.H.; Fred, E.B.; Anderson, J.A. The fermentation of glucose, galactose, and mannose by lactobacillus pentoaceticus, n. sp. J. Biol. Chem. 1920, 42, 273–287. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A review of the enhancement of bio-hydrogen generation by chemicals addition. Catalysts 2019, 9, 353. [Google Scholar] [CrossRef]
- Montecchio, D.; Yuan, Y.; Malpei, F. Hydrogen production dynamic during cheese whey dark fermentation: New insights from modelization. Int. J. Hydrogen Energy 2018, 43, 17588–17601. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, S.-H.; Yoon, J.-J.; Kim, S.-H.; Park, H.-D. Predominance of cluster I clostridium in hydrogen fermentation of galactose seeded with various heat-treated anaerobic sludges. Bioresour. Technol. 2014, 157, 98–106. [Google Scholar] [CrossRef]
- Pan, C.-M.; Fan, Y.-T.; Zhao, P.; Hou, H.-W. Fermentative hydrogen production by the newly isolated Clostridium beijerinckii Fanp3. Int. J. Hydrogen Energy 2008, 33, 5383–5391. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Ding, L.; Lin, R.; Song, W.; Zhou, J.; Cen, K. Enhancement of energy production efficiency from mixed biomass of Chlorella pyrenoidosa and cassava starch through combined hydrogen fermentation and methanogenesis. Appl. Energy 2014, 120, 23–30. [Google Scholar] [CrossRef]
- Xia, A.; Cheng, J.; Ding, L.; Lin, R.; Song, W.; Su, H.; Zhou, J.; Cen, K. Substrate consumption and hydrogen production via co-fermentation of monomers derived from carbohydrates and proteins in biomass wastes. Appl. Energy 2015, 139, 9–16. [Google Scholar] [CrossRef]
- Xiao, N.; Chen, Y.; Chen, A.; Feng, L. Enhanced Bio-hydrogen Production from Protein Wastewater by Altering Protein Structure and Amino Acids Acidification Type. Sci. Rep. 2014, 4, 3992. [Google Scholar] [CrossRef]
- Xia, A.; Jacob, A.; Herrmann, C.; Murphy, J.D. Fermentative bio-hydrogen production from galactose. Energy 2016, 96, 346–354. [Google Scholar] [CrossRef]
- Maru, B.T.; Bielen, A.A.M.; Constantí, M.; Medina, F.; Kengen, S.W.M. Glycerol fermentation to hydrogen by thermotoga maritima: Proposed pathway and bioenergetic considerations. Int. J. Hydrogen Energy 2013, 38, 5563–5572. [Google Scholar] [CrossRef]
- Oh, S.T.; Martin, A.D. Long chain fatty acids degradation in anaerobic digester: Thermodynamic equilibrium consideration. Process. Biochem. 2010, 45, 335–345. [Google Scholar] [CrossRef]
- Mainardis, M.; Flaibani, S.; Trigatti, M.; Goi, D. Techno-economic feasibility of anaerobic digestion of cheese whey in small Italian dairies and effect of ultrasound pre-treatment on methane yield. J. Environ. Manag. 2019, 246, 557–563. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Zieliński, M.; Bartkowska, I.; Dębowski, M. Effect of acid whey pretreatment using ultrasonic disintegration on the removal of organic compounds and anaerobic digestion efficiency. Int. J. Environ. Res. Public Health 2022, 19, 11362. [Google Scholar] [CrossRef]
- Gannoun, H.; Khelifi, E.; Bouallagui, H.; Touhami, Y.; Hamdi, M. Ecological clarification of cheese whey prior to anaerobic digestion in upflow anaerobic filter. Bioresour. Technol. 2008, 99, 6105–6111. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Microwave radiation influence on dairy waste anaerobic digestion in a multi-section hybrid anaerobic reactor (M-SHAR). Processes 2021, 9, 1772. [Google Scholar] [CrossRef]
- Skripsts, E.; Dubrovskis, V.; Jasko, J.; Zabarovskis, E.; Kotelenecs, V. Investigation of biogas production of cheese whey in processing with ozone before anaerobic digestion. Eng. Rural. Dev. 2011, 10, 377–381. [Google Scholar]
- Escalante, H.; Castro, L.; Amaya, M.P.; Jaimes, L.; Jaimes-Estévez, J. Anaerobic digestion of cheese whey: Energetic and nutritional potential for the dairy sector in developing countries. Waste Manag. 2018, 71, 711–718. [Google Scholar] [CrossRef]
- da Silva, A.N.; Macêdo, W.V.; Sakamoto, I.K.; Pereyra, D.D.L.A.D.; Mendes, C.O.; Maintinguer, S.I.; Filho, R.A.C.; Damianovic, M.; Varesche, M.B.A.; de Amorim, E.L.C. Biohydrogen production from dairy industry wastewater in an anaerobic fluidized-bed reactor. Biomass Bioenergy 2018, 120, 257–264. [Google Scholar] [CrossRef]
- Prapinagsorn, W.; Sittijunda, S.; Reungsang, A. Co-digestion of napier grass and its silage with cow dung for bio-hydrogen and methane production by two-stage anaerobic digestion process. Energies 2017, 11, 47. [Google Scholar] [CrossRef]
- Ghimire, A.; Luongo, V.; Frunzo, L.; Pirozzi, F.; Lens, P.N.; Esposito, G. Continuous biohydrogen production by thermophilic dark fermentation of cheese whey: Use of buffalo manure as buffering agent. Int. J. Hydrogen Energy 2017, 42, 4861–4869. [Google Scholar] [CrossRef]
- Kovalev, A.A.; Kovalev, D.A.; Zhuravleva, E.A.; Katraeva, I.V.; Panchenko, V.; Fiore, U.; Litti, Y.V. Two-stage anaerobic digestion with direct electric stimulation of methanogenesis: The effect of a physical barrier to retain biomass on the surface of a carbon cloth-based biocathode. Renew. Energy 2021, 181, 966–977. [Google Scholar] [CrossRef]
- Bundhoo, M.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrogen Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Ferchichi, M.; Crabbe, E.; Gil, G.-H.; Hintz, W.; Almadidy, A. Influence of initial pH on hydrogen production from cheese whey. J. Biotechnol. 2005, 120, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Arenas, C.B.; González, R.; González, J.; Cara, J.; Papaharalabos, G.; Gómez, X.; Martínez, E.J. Assessment of electrooxidation as pre- and post-treatments for improving anaerobic digestion and stabilisation of waste activated sludge. J. Environ. Manag. 2021, 288, 112365. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.P.; Gogate, P.R. Cavitation-based pre-treatment of wastewater and waste sludge for improvement in the performance of biological processes: A review. J. Environ. Chem. Eng. 2020, 9, 104743. [Google Scholar] [CrossRef]
- Chen, Z.; Rao, Y.; Usman, M.; Chen, H.; Białowiec, A.; Zhang, S.; Luo, G. Anaerobic fermentation of hydrothermal liquefaction wastewater of dewatered sewage sludge for volatile fatty acids production with focuses on the degradation of organic components and microbial community compositions. Sci. Total Environ. 2021, 777, 146077. [Google Scholar] [CrossRef]
- Hitit, Z.Y.; Hallenbeck, P.C. Analytical procedures, data reporting and selected reference values for biological hydrogen production. Biomass Bioenergy 2021, 147, 106014. [Google Scholar] [CrossRef]
- Leaño, E.; Babel, S. Effects of pretreatment methods on cassava wastewater for biohydrogen production optimization. Renew. Energy 2012, 39, 339–346. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Enhancement of biohydrogen production from grass by ferrous ion and variation of microbial community. Fuel 2018, 233, 404–411. [Google Scholar] [CrossRef]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical conversion pathways for carbohydrates. Green Chem. 2014, 17, 40–71. [Google Scholar] [CrossRef]
- Dereli, R.K.; Loverdou, L.; van der Zee, F.P.; van Lier, J.B. A systematic study on the effect of substrate acidification degree and acidogenic biomass on sludge filterability. Water Res. 2015, 82, 94–103. [Google Scholar] [CrossRef]
- Lee, Y.J.; Miyahara, T.; Noike, T. Effect of iron concentration on hydrogen fermentation. Bioresour. Technol. 2001, 80, 227–231. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Effect of Fe2+ concentration on fermentative hydrogen production by mixed cultures. Int. J. Hydrogen Energy 2008, 33, 1215–1220. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, S.-H.; Ju, H.-J.; Kim, S.-H.; Yoon, J.-J.; Park, H.-D. Failure of biohydrogen production by low levels of substrate and lactic acid accumulation. Renew. Energy 2016, 86, 889–894. [Google Scholar] [CrossRef]
- García-Depraect, O.; Castro-Muñoz, R.; Muñoz, R.; Rene, E.R.; León-Becerril, E.; Valdez-Vazquez, I.; Kumar, G.; Reyes-Alvarado, L.C.; Martínez-Mendoza, L.J.; Carrillo-Reyes, J.; et al. A review on the factors influencing biohydrogen production from lactate: The key to unlocking enhanced dark fermentative processes. Bioresour. Technol. 2020, 324, 124595. [Google Scholar] [CrossRef]
- Yin, Q.; Miao, J.; Li, B.; Wu, G. Enhancing electron transfer by ferroferric oxide during the anaerobic treatment of synthetic wastewater with mixed organic carbon. Int. Biodeterior. Biodegrad. 2017, 119, 104–110. [Google Scholar] [CrossRef]
- Zhuravleva, E.A.; Shekhurdina, S.V.; Kotova, I.B.; Loiko, N.G.; Popova, N.M.; Kryukov, E.; Kovalev, A.A.; Kovalev, D.A.; Litti, Y.V. Effects of various materials used to promote the direct interspecies electron transfer on anaerobic digestion of low-concentration swine manure. Sci. Total Environ. 2022, 839, 156073. [Google Scholar] [CrossRef]
- Gadhe, A.; Sonawane, S.S.; Varma, M.N. Influence of nickel and hematite nanoparticle powder on the production of biohydrogen from complex distillery wastewater in batch fermentation. Int. J. Hydrogen Energy 2015, 40, 10734–10743. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Li, Y.; Quan, X.; Zhao, Z. Comparing the mechanisms of ZVI and Fe3O4 for promoting waste-activated sludge digestion. Water Res. 2018, 144, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhao, X.; Zhao, Y.; Wang, H.; Yuan, X.; Zhu, W.; Cui, Z.; Wang, X. Optimization of Fe2+ supplement in anaerobic digestion accounting for the Fe-bioavailability. Bioresour. Technol. 2018, 250, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Ko, J.H.; Zhou, L.; Gao, X.; Liu, Y.; Shi, X.; Xu, Q. Iron oxide alleviates acids stress by facilitating syntrophic metabolism between syntrophomonas and methanogens. Chemosphere 2020, 247, 125866. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Sivagurunathan, P.; Lee, M.-K.; Yun, Y.-M.; Song, Y.-C.; Kim, D.-H. Enhanced hydrogen fermentation by zero valent iron addition. Int. J. Hydrogen Energy 2019, 44, 3387–3394. [Google Scholar] [CrossRef]
- Wei, J.; Hao, X.; van Loosdrecht, M.C.; Li, J. Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: A review. Renew. Sustain. Energy Rev. 2018, 89, 16–26. [Google Scholar] [CrossRef]
- Durán-Padilla, V.R.; Davila-Vazquez, G.; Chavez-Vela, N.A.; Tinoco-Valencia, J.R.; Jáuregui-Rincón, J. Iron effect on the fermentative metabolism of Clostridium acetobutylicum ATCC 824 using cheese whey as substrate. Biofuel Res. J. 2014, 1, 129–133. [Google Scholar] [CrossRef]
- Zhu, H.; Seto, P.; Parker, W.J. Enhanced dark fermentative hydrogen production under the effect of zero-valent iron shavings. Int. J. Hydrogen Energy 2014, 39, 19331–19336. [Google Scholar] [CrossRef]
- Taherdanak, M.; Zilouei, H.; Karimi, K. Investigating the effects of iron and nickel nanoparticles on dark hydrogen fermentation from starch using central composite design. Int. J. Hydrogen Energy 2015, 40, 12956–12963. [Google Scholar] [CrossRef]
- Mahata, C.; Dhar, S.; Ray, S.; Das, D. Effect of thermal pretreated organic wastes on the dark fermentative hydrogen production using mixed microbial consortia. Fuel 2020, 284, 119062. [Google Scholar] [CrossRef]
- Wang, Q.; Gong, Y.; Liu, S.; Wang, D.; Liu, R.; Zhou, X.; Nghiem, L.D.; Zhao, Y. Free ammonia pretreatment to improve bio-hydrogen production from anaerobic dark fermentation of microalgae. ACS Sustain. Chem. Eng. 2019, 7, 1642–1647. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhao, J.; Gao, Y.; Lu, C.; Luo, S.; Sun, Y.; Zhang, D. Enhanced hydrogen production from food waste dark fermentation by potassium ferrate pretreatment. Environ. Sci. Pollut. Res. 2020, 27, 18145–18156. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, D.; Xu, Q.; Liu, Y.; Wang, Q.; Ni, B.-J.; Yang, Q.; Li, X.; Yang, F. Calcium peroxide promotes hydrogen production from dark fermentation of waste activated sludge. Chem. Eng. J. 2019, 355, 22–32. [Google Scholar] [CrossRef]
- Singh, H.; Rout, S.; Das, D. Dark fermentative biohydrogen production using pretreated Scenedesmus obliquus biomass under an integrated paradigm of biorefinery. Int. J. Hydrogen Energy 2022, 47, 102–116. [Google Scholar] [CrossRef]
- Liu, X.; He, D.; Wu, Y.; Xu, Q.; Wang, D.; Yang, Q.; Liu, Y.; Ni, B.-J.; Wang, Q.; Li, X. Freezing in the presence of nitrite pretreatment enhances hydrogen production from dark fermentation of waste activated sludge. J. Clean. Prod. 2020, 248, 119305. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Hosseinzadeh-Bandbafha, H.; Shahbeik, H.; Tabatabaei, M. The role of sustainability assessment tools in realizing bioenergy and bioproduct systems. Biofuel Res. J. 2022, 9, 1697–1706. [Google Scholar] [CrossRef]
- Song, W.; Ding, L.; Liu, M.; Cheng, J.; Zhou, J.; Li, Y.-Y. Improving biohydrogen production through dark fermentation of steam-heated acid pretreated alternanthera philoxeroides by mutant enterobacter aerogenes ZJU1. Sci. Total Environ. 2020, 716, 134695. [Google Scholar] [CrossRef]

| Parameters | Value |
|---|---|
| COD, g/L | 93.8 ± 11.1 |
| pH | 6.3 ± 0.2 |
| Fat, mg/L | 20.7 ± 2.3 |
| Lactose, g/L | 46.6 ± 10.2 |
| Glucose, g/L | 6.4 ± 1.2 |
| TS, % | 9.9 ± 0.8 |
| NVS, %TS | 3.9 ± 0.8 |
| VS, % TS | 96.1 ± 0.8 |
| Fetotal, g/L | 0.22 ± 0.01 |
| Acetate, mg/L | 470 ± 25 |
| Propionate, mg/L | 60 ± 3 |
| Butyrate, mg/L | 20 ± 1 |
| Ethanol, mg/L | 390 ± 20 |
| Concentration, mg/L | Pretreatment Time, s | ||||
|---|---|---|---|---|---|
| 0 | 5 | 20 | 45 | 90 | |
| Lactose | 46.6 ± 10.2 | 38.5 ± 1.5 | 41.2 ± 0.2 | 36.2 ± 1.8 | 34.3 ± 3.6 |
| Glucose | 6.4 ± 1.2 | 4.6 ± 0.1 | 5.1 ± 1.5 | 4.5 ± 1.1 | 4.1 ± 0.7 |
| Pretreatment Time | Potential Hydrogen Yield γ, mL H2/g COD | Maximum Hydrogen Production Rate K, mL H2/g COD/d | Lag Phase 𝜆, d | R2 |
|---|---|---|---|---|
| 0 (Control) | 138.8 | 31.8 | 0.82 | 0.99 |
| 5 | 154.4 | 256.5 | 0.39 | 0.99 |
| 20 | 170.5 | 201.1 | 0.20 | 0.98 |
| 45 | 202.4 | 237.2 | 0.33 | 0.98 |
| 90 | 134.6 | 44.2 | 0.04 | 0.96 |
| Type | Pretreatment Method | Feedstock | DF Temperature, °C | Microorganism | H2 Production Improvements | Reference |
|---|---|---|---|---|---|---|
| Thermal | Heat | Starchy wastewater supplemented with groundnut de-oiled cake | 37.5 | Anaerobically digested sludge, dominated by Clostridium sp. and Eubacterium sp. | 20% higher H2 yield, twofold improvement in bioenergy recovery | [72] |
| Chemical | Free ammonia | Microalgae | 37 | Anaerobically digested sludge | Up to 21% increase in the potential H2 yield, up to 52% increase in the maximum H2 production rate | [73] |
| Potassium ferrate | Food waste | 35 | Sewage sludge | 2.2-fold increase in H2 yield, lag-phase shortened from 120 to 96 h | [74] | |
| Calcium peroxide | Waste activated sludge | 35 | Mixed consortia | 13.7 times higher maximum H2 yield | [75] | |
| Combined thermal and chemical | Acid and heat | Deoiled Scenedesmus obliquus biomass | 37 | Mixed consortia | 10 times higher maximum H2 yield | [76] |
| Steam and acid | Alternanthera philoxeroides | 37 | Enterobacter aerogenes ZJU1 mutagenized by 60Co-γ irradiation | 59.9% increase in H2 yield | [79] | |
| Freezing with nitrite | Waste activated sludge | 35 | Waste activated sludge | 5.5–13.4 times increase in H2 yield | [77] | |
| Physical | VLA | CW | 55 | T. thermosacharoliticum SP-H2 | Up to 45.8% increase in the potential H2 yield, up to 8.06 times the maximum H2 production rate, more than a twofold reduction in lag-phase | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikheeva, E.R.; Katraeva, I.V.; Kovalev, A.A.; Biryuchkova, P.D.; Zhuravleva, E.A.; Vishnyakova, A.V.; Litti, Y.V. Pretreatment in Vortex Layer Apparatus Boosts Dark Fermentative Hydrogen Production from Cheese Whey. Fermentation 2022, 8, 674. https://doi.org/10.3390/fermentation8120674
Mikheeva ER, Katraeva IV, Kovalev AA, Biryuchkova PD, Zhuravleva EA, Vishnyakova AV, Litti YV. Pretreatment in Vortex Layer Apparatus Boosts Dark Fermentative Hydrogen Production from Cheese Whey. Fermentation. 2022; 8(12):674. https://doi.org/10.3390/fermentation8120674
Chicago/Turabian StyleMikheeva, Elza R., Inna V. Katraeva, Andrey A. Kovalev, Polina D. Biryuchkova, Elena A. Zhuravleva, Anastasia V. Vishnyakova, and Yuriy V. Litti. 2022. "Pretreatment in Vortex Layer Apparatus Boosts Dark Fermentative Hydrogen Production from Cheese Whey" Fermentation 8, no. 12: 674. https://doi.org/10.3390/fermentation8120674
APA StyleMikheeva, E. R., Katraeva, I. V., Kovalev, A. A., Biryuchkova, P. D., Zhuravleva, E. A., Vishnyakova, A. V., & Litti, Y. V. (2022). Pretreatment in Vortex Layer Apparatus Boosts Dark Fermentative Hydrogen Production from Cheese Whey. Fermentation, 8(12), 674. https://doi.org/10.3390/fermentation8120674









