Production of the Food Enzyme Acetolactate Decarboxylase (ALDC) from Bacillus subtilis ICA 56 Using Agro-Industrial Residues as Feedstock
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Strain and Inoculum Preparation
2.3. Culture Medium and Culture Condition in Shake Flasks
2.4. Extraction of the Enzyme α-Acetolactate Decarboxylase
2.5. Enzyme Activity
2.6. Protein Quantification
2.7. Electrophoresis SDS-PAGE (Sodium Dodecyl Sulphate-Polyacrylamide Gel)
2.8. Effect of Temperature and pH on ALDC Activity
2.9. Effect of Metal Ions on ALDC Activity
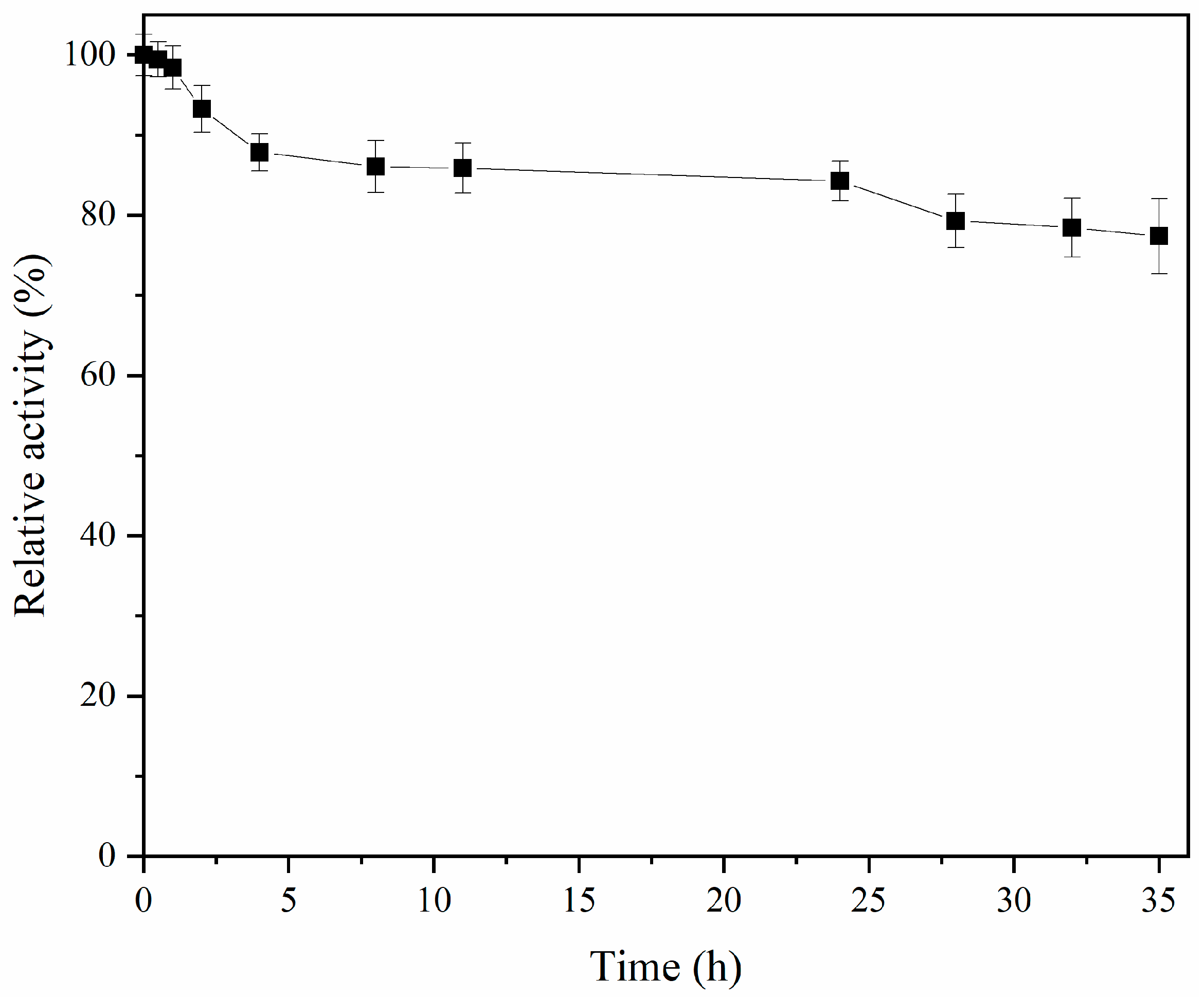
2.10. ALDC Thermal Stability
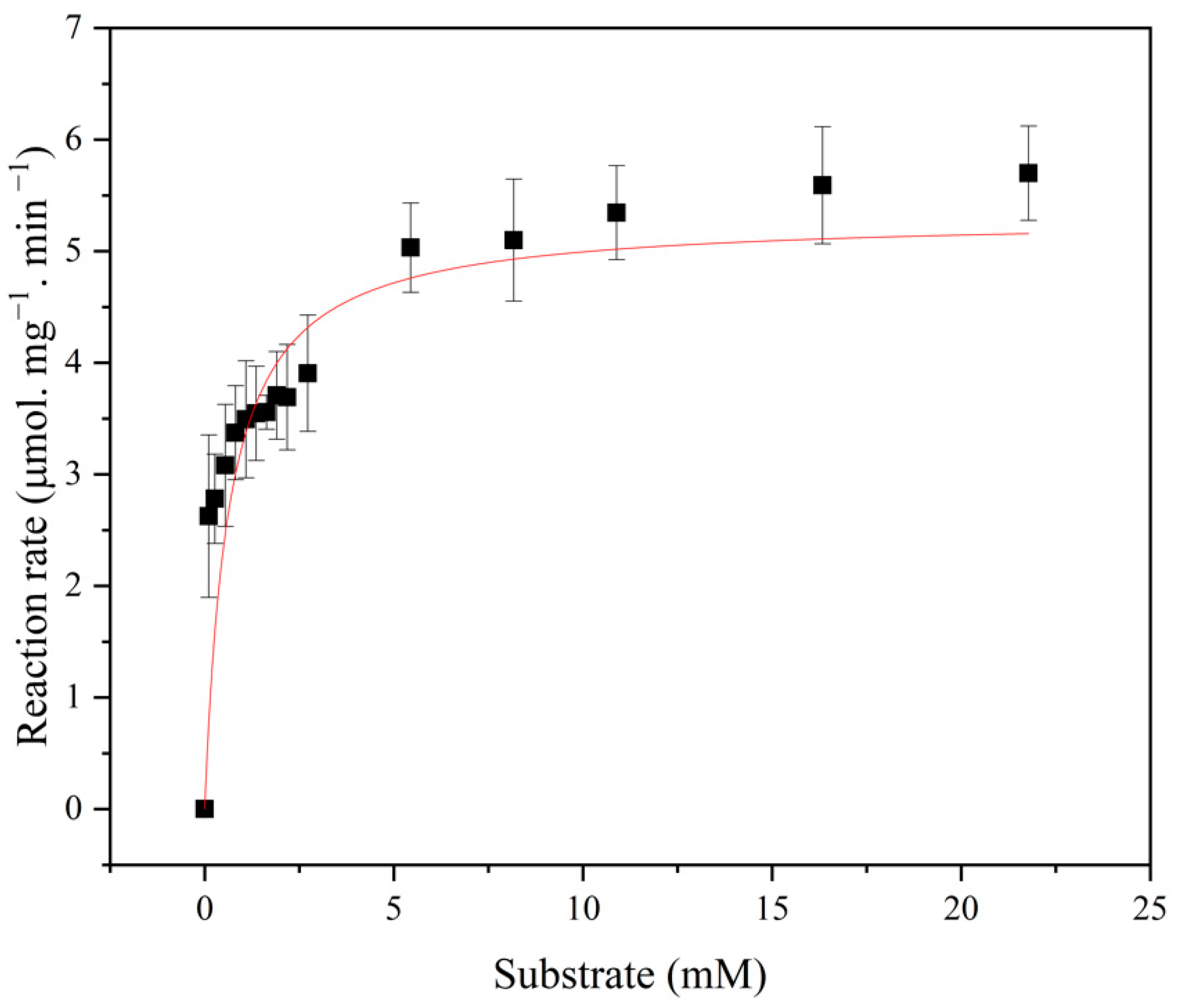
2.11. Determination of Kinetics Parameters
2.12. Optimization of Culture Medium
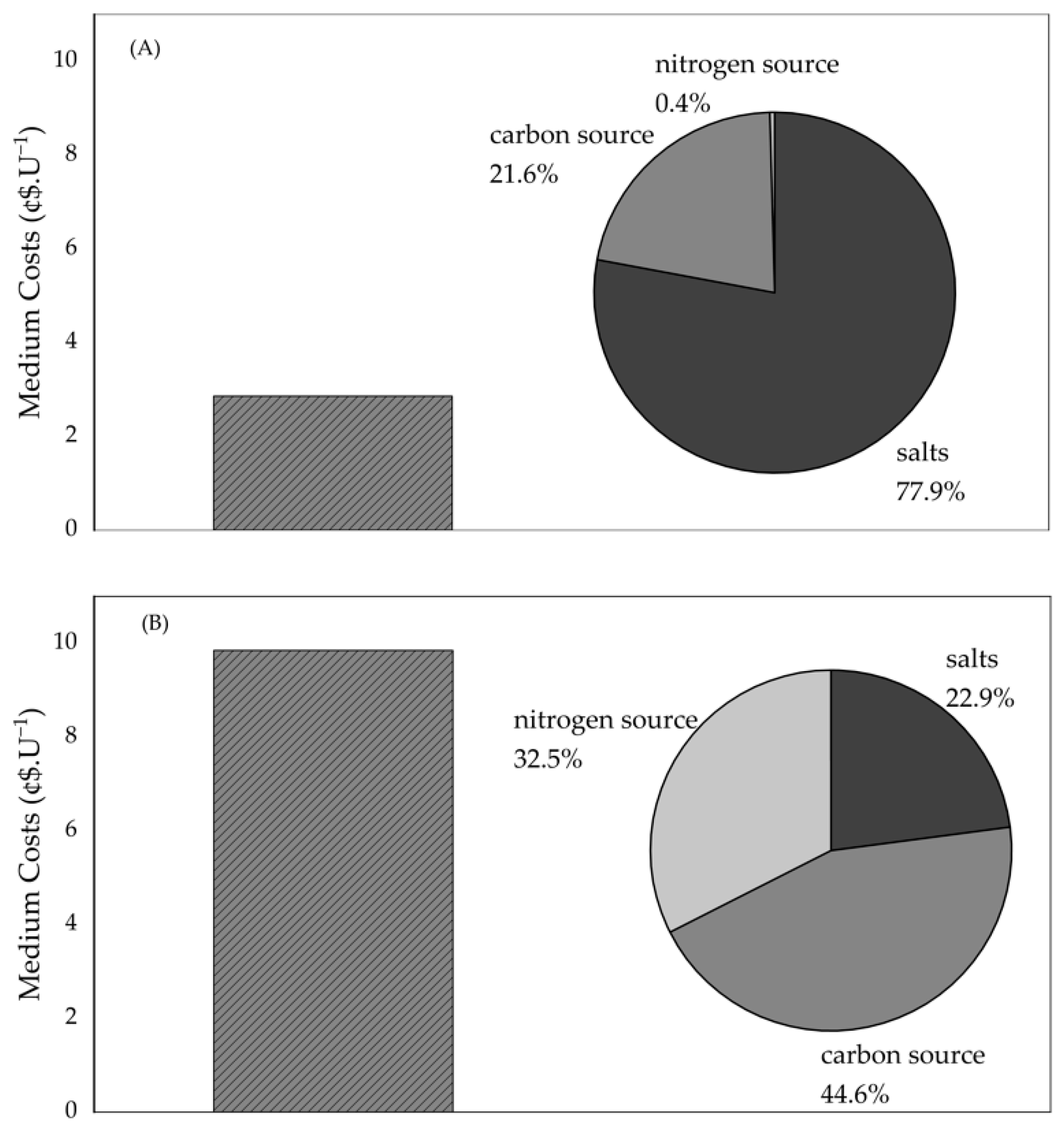
2.13. Medium Costs
3. Results and Discussion
3.1. Optimization of ALDC Production
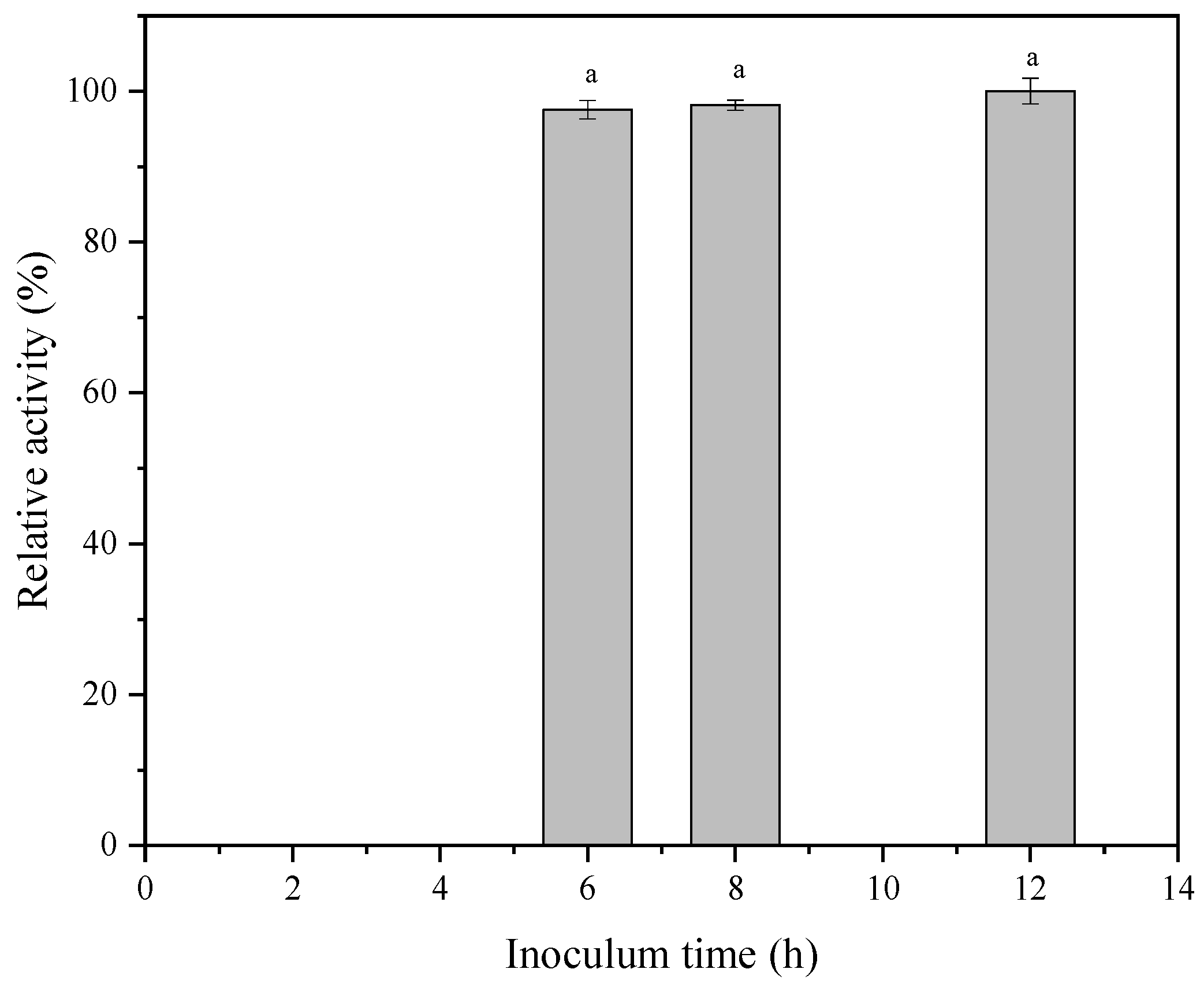
3.1.1. Effect of Inoculum Age on ALDC Activity
3.1.2. Analysis of Culture Medium
3.2. Characterization of ALDC Enzyme Obtained from B. subtilis ICA 56
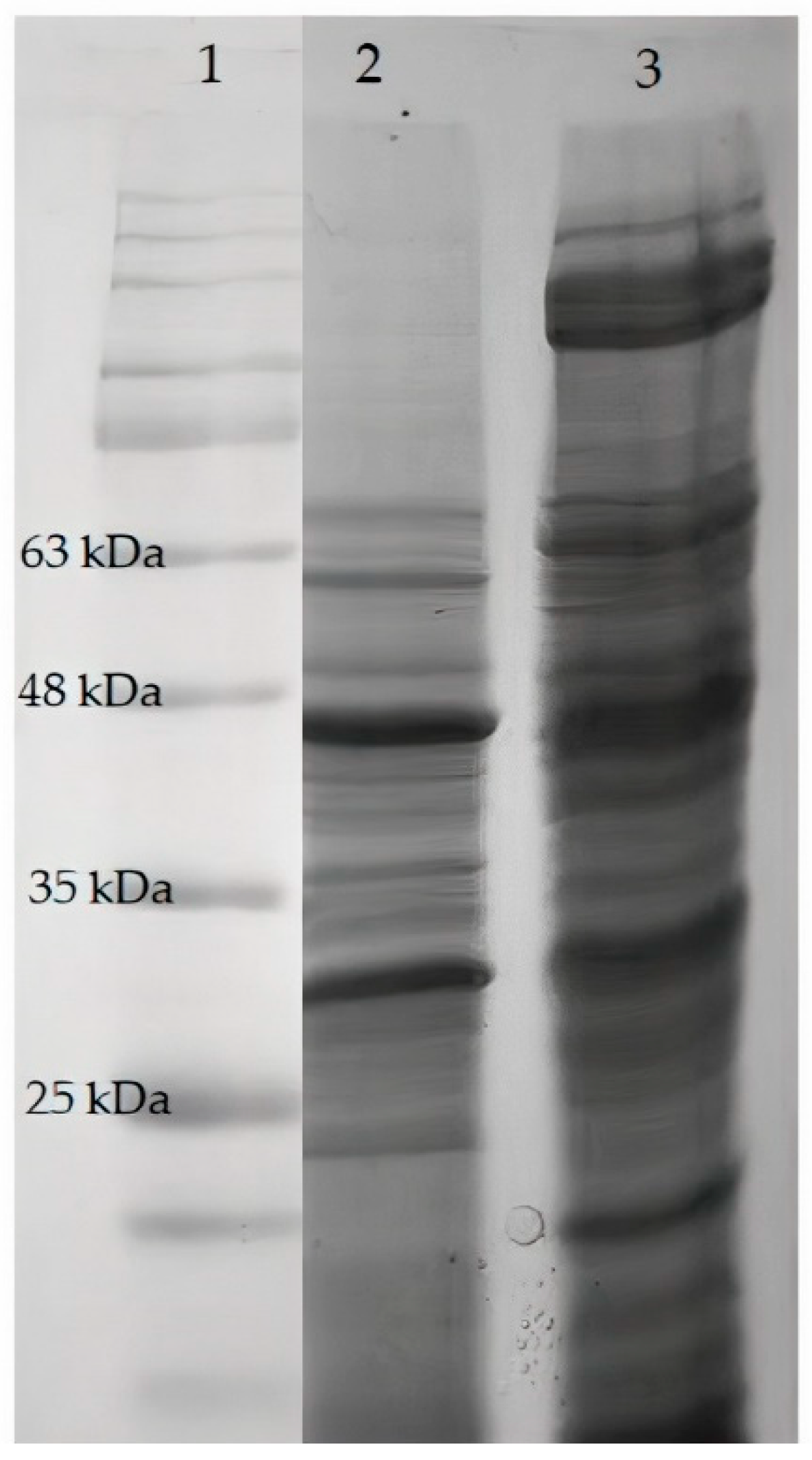
3.2.1. SDS-PAGE Analysis
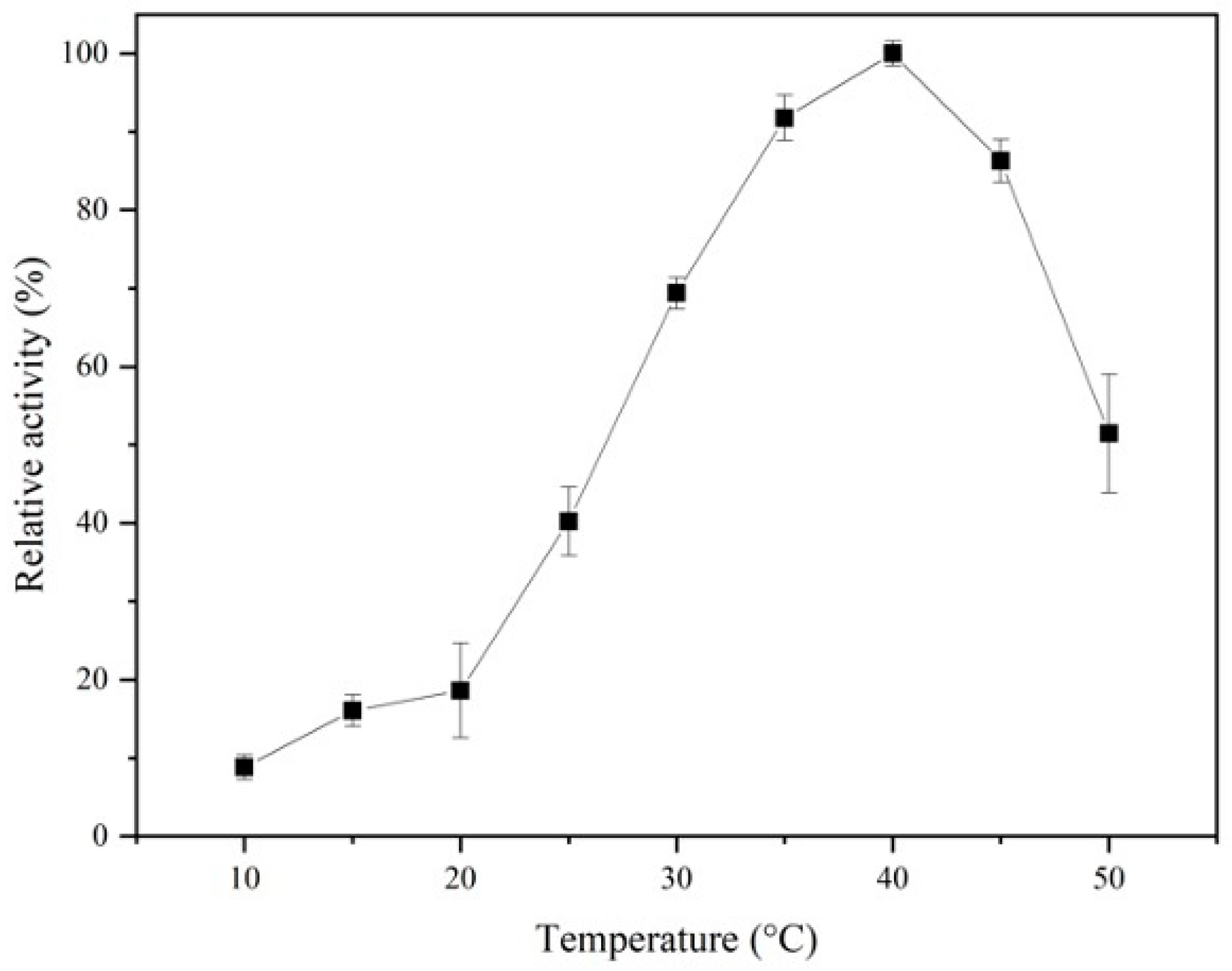
3.2.2. Effect of Temperature and pH on ALDC Activity
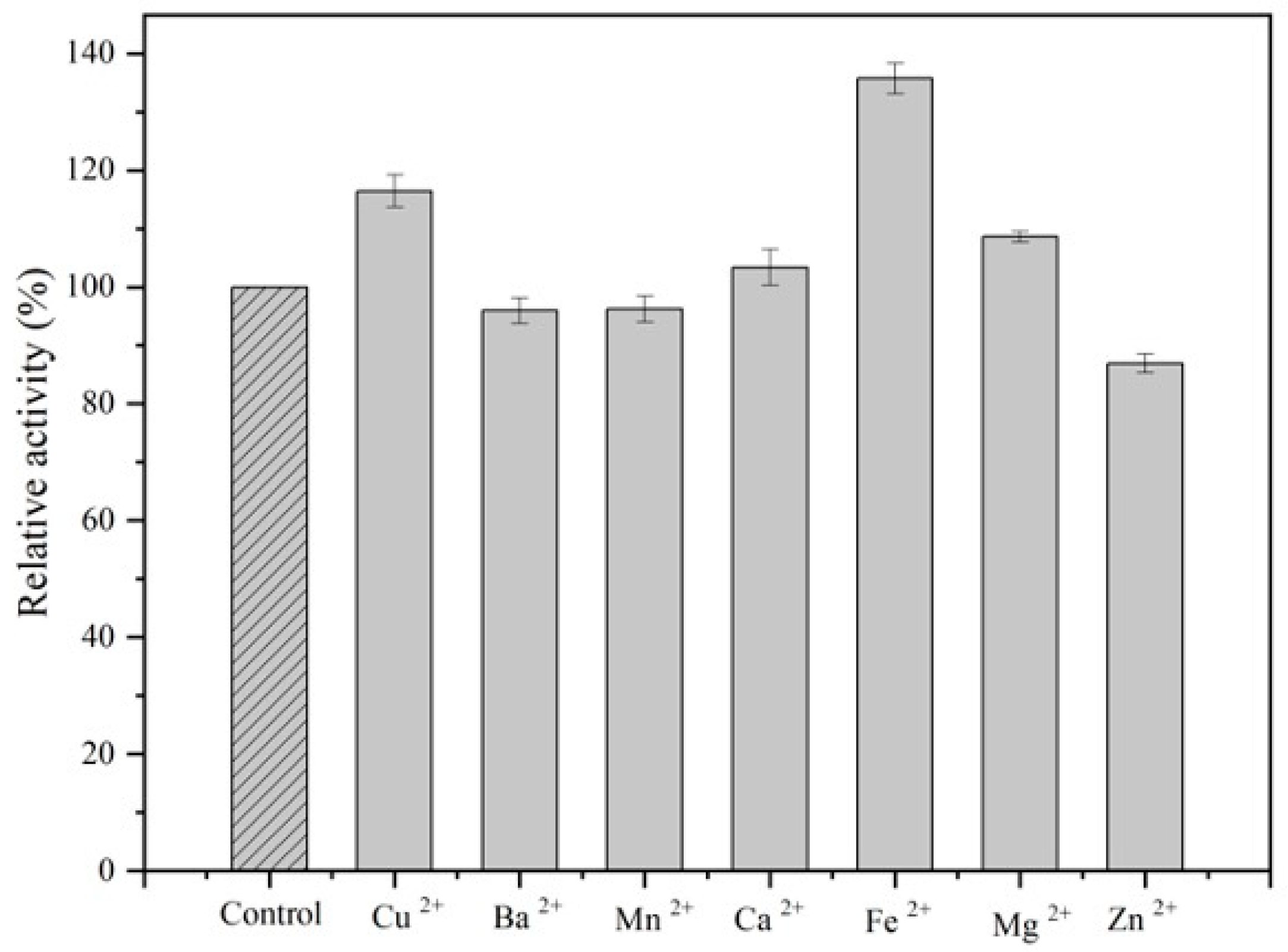
3.2.3. Effect of Metal Ions on ALDC Activity
3.2.4. ALDC Thermal Stability
3.2.5. Kinetic Parameters
3.3. Preliminary Economic Analysis of ALDC Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, C.-H.; Whitesides, G.M. Enzymes in Synthetic Organic Chemistry; Tetrahedron Organic Chemistry Series; Academic Press: Cambridge, MA, USA, 1994; Volume 12. [Google Scholar]
- Hao, W.; Ji, F.; Wang, J.; Wang, Y.; Zhang, Y.; Bao, Y. Metabolic Engineering of Bacillus sp. for Diacetyl Production. Process Biochem. 2017, 58, 69–77. [Google Scholar] [CrossRef]
- Fan, Y.; Tian, Y.; Zhao, X.; Zhang, J.; Liu, J. Isolation of Acetoin-Producing Bacillus Strains from Japanese Traditional Food-Natto. Prep. Biochem. Biotechnol. 2013, 43, 551–564. [Google Scholar] [CrossRef]
- Choi, E.J.; Ahn, H.W.; Kim, W.J. Effect of α-Acetolactate Decarboxylase on Diacetyl Content of Beer. Food Sci. Biotechnol. 2015, 24, 1373–1380. [Google Scholar] [CrossRef]
- Godtfredsen, S.E.; Ottesen, M. Maturation of Beer with Alpha-Acetolactate Decarboxylase. Carlsb. Res. Commun. 1982, 47, 93–102. [Google Scholar] [CrossRef]
- Ji, F.; Li, M.; Feng, Y.; Wu, S.; Wang, T.; Pu, Z.; Wang, J.; Yang, Y.; Xue, S.; Bao, Y. Structural and Enzymatic Characterization of Acetolactate Decarboxylase from Bacillus Subtilis. Appl. Microbiol. Biotechnol. 2018, 102, 6479–6491. [Google Scholar] [CrossRef]
- Marlow, V.A.; Rea, D.; Najmudin, S.; Wills, M.; Fülöp, V. Structure and Mechanism of Acetolactate Decarboxylase. ACS Chem. Biol. 2013, 8, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Su, H.; Liu, Y. Catalytic Mechanism of Acetolactate Decarboxylase from: Brevibacillus Brevis towards Both Enantiomers of α-Acetolactate. RSC Adv. 2016, 6, 80621–80629. [Google Scholar] [CrossRef]
- Ji, F.; Feng, Y.; Li, M.; Long, F.; Yang, Y.; Wang, T.; Wang, J.; Bao, Y.; Xue, S. Structure and Catalytic Mechanistic Insight into Enterobacter Aerogenes Acetolactate Decarboxylase. Enzyme Microb. Technol. 2019, 126, 9–17. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, S.J.; Kim, J.W.; Park, Y.C.; Seo, J.H. Molecular Cloning and Expression of Enterobacter Aerogenes α-Acetolactate Decarboxylase in Pyruvate Decarboxylase-Deficient Saccharomyces Cerevisiae for Efficient 2,3-Butanediol Production. Process Biochem. 2016, 51, 170–176. [Google Scholar] [CrossRef]
- Ji, F.; Feng, Y.; Li, M.; Yang, Y.; Wang, T.; Wang, J.; Bao, Y.; Xue, S. Studies on Structure-Function Relationships of Acetolactate Decarboxylase from: Enterobacter Cloacae. RSC Adv. 2018, 8, 39066–39073. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhao, Q.; Che, S.; Jia, H.; Liang, H.; Zhang, H.; Liu, R.; Zhang, Q.; Bartlam, M. Structural Characterization of an Acetolactate Decarboxylase from Klebsiella Pneumoniae. Biochem. Biophys. Res. Commun. 2019, 509, 154–160. [Google Scholar] [CrossRef]
- Yamano, S.; Tomizuka, K.; Tanaka, J.; Inoue, T. High Level Expression of α-Acetolactate Decarboxylase Gene from Acetobacter Aceti Subsp. Xylinum in Brewing. J. Biotechnol. 1994, 37, 45–48. [Google Scholar] [CrossRef]
- Zheng, Z.Q.; Luo, C.Y.; Chen, H.; Sun, H.; Hui, X.; Chen, Z.D.; Gao, W.Y.; Li, H. Characterization of Acetolactate Decarboxylase of Streptococcus Thermophilus and Its Stereoselectivity in Decarboxylation of α-Hydroxy-β-Ketoacids. Bioorg. Chem. 2022, 122, 105719. [Google Scholar] [CrossRef] [PubMed]
- Goupil-Feuillerat, N.; Cocaign-Bousquet, M.; Godon, J.J.; Ehrlich, S.D.; Renault, P. Dual Role of α-Acetolactate Decarboxylase in Lactococcus lactis subsp. lactis. J. Bacteriol. 1997, 179, 6285–6293. [Google Scholar] [CrossRef]
- Xiao, Z.; Lu, J.R. Generation of Acetoin and Its Derivatives in Foods. J. Agric. Food Chem. 2014, 62, 6487–6497. [Google Scholar] [CrossRef]
- de Freitas, M.; Cavalcante, L.S.; Gudiña, E.J.; Silvério, S.C.; Rodrigues, S.; Rodrigues, L.R.; Gonçalves, L.R.B. Sustainable Lipase Production by Diutina Rugosa NRRL Y-95 Through a Combined Use of Agro-Industrial Residues as Feedstock. Appl. Biochem. Biotechnol. 2021, 193, 589–605. [Google Scholar] [CrossRef]
- Raina, D.; Kumar, V.; Saran, S. A Critical Review on Exploitation of Agro-Industrial Biomass as Substrates for the Therapeutic Microbial Enzymes Production and Implemented Protein Purification Techniques. Chemosphere 2022, 294, 133712. [Google Scholar] [CrossRef] [PubMed]
- Tuly, J.A.; Zabed, H.M.; Nizami, A.S.; Mehedi Hassan, M.; Roknul Azam, S.M.; Kumar Awasthi, M.; Janet, Q.; Chen, G.; Dzidzorgbe Kwaku Akpabli-Tsigbe, N.; Ma, H. Bioconversion of Agro-Food Industrial Wastes into Value-Added Peptides by a Bacillus sp. Mutant through Solid-State Fermentation. Bioresour. Technol. 2022, 346, 126513. [Google Scholar] [CrossRef]
- Joulak, I.; Concórdio-Reis, P.; Torres, C.A.V.; Sevrin, C.; Grandfils, C.; Attia, H.; Freitas, F.; Reis, M.A.M.; Azabou, S. Sustainable Use of Agro-Industrial Wastes as Potential Feedstocks for Exopolysaccharide Production by Selected Halomonas Strains. Environ. Sci. Pollut. Res. 2022, 29, 22043–22055. [Google Scholar] [CrossRef]
- Gaur, V.K.; Sharma, P.; Sirohi, R.; Varjani, S.; Taherzadeh, M.J.; Chang, J.S.; Yong Ng, H.; Wong, J.W.C.; Kim, S.H. Production of Biosurfactants from Agro-Industrial Waste and Waste Cooking Oil in a Circular Bioeconomy: An Overview. Bioresour. Technol. 2022, 343, 126059. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, A.; Pirozzi, D. Prospect of Agro-Industrial Residues as Feedstock of Biodiesel. In Proceedings of the 1st International Conference on the Developments in Renewable Energy Technology, ICDRET 2009, Dhaka, Bangladesh, 17–19 December 2009; pp. 115–119. [Google Scholar]
- Sanders, J.P.M.; Clark, J.H.; Harmsen, G.J.; Heeres, H.J.; Heijnen, J.J.; Kersten, S.R.A.; van Swaaij, W.P.M.; Moulijn, J.A. Process Intensification in the Future Production of Base Chemicals from Biomass. Chem. Eng. Process. Process Intensif. 2012, 51, 117–136. [Google Scholar] [CrossRef]
- Ding, J.; You, S.; Ba, W.; Zhang, H.; Chang, H.; Qi, W.; Su, R.; He, Z. Bifunctional Utilization of Whey Powder as a Substrate and Inducer for β-Farnesene Production in an Engineered Escherichia coli. Bioresour. Technol. 2021, 341, 125739. [Google Scholar] [CrossRef]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fagioli, L.; Fusaro, I.; Biagi, G.; Formigoni, A.; Mammi, L. Short Communication: Characterization of Molasses Chemical Composition. J. Dairy Sci. 2020, 103, 6244–6249. [Google Scholar] [CrossRef]
- Hofer, A.; Hauer, S.; Kroll, P.; Fricke, J.; Herwig, C. In-Depth Characterization of the Raw Material Corn Steep Liquor and Its Bioavailability in Bioprocesses of Penicillium Chrysogenum. Process Biochem. 2018, 70, 20–28. [Google Scholar] [CrossRef]
- Hull, S.R.; Yang, B.Y.; Venzke, D.; Kulhavy, K.; Montgomery, R. Composition of Corn Steep Water during Steeping. J. Agric. Food Chem. 1996, 44, 1857–1863. [Google Scholar] [CrossRef]
- De França, Í.W.L.; Lima, A.P.; Lemos, J.A.M.; Lemos, C.G.F.; Melo, V.M.M.; De Sant’ana, H.B.; Gonçalves, L.R.B. Production of a Biosurfactant by Bacillus Subtilis ICA56 Aiming Bioremediation of Impacted Soils. Catal. Today 2015, 255, 10–15. [Google Scholar] [CrossRef]
- Olsen, F.; Aunstrup, K. Alpha-Acetolactate Decarboxylase Enzyme and Preparation Thereof. US Patent 4,617,273, 14 October 1986. [Google Scholar]
- Stormer, F.C. 2,3-Butanediol Biosynthetic System in Aerobacter Aerogenes. Methods Enzymol. 1975, 41, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Diogo, M.M.; Silva, S.; Cabral, J.M.S.; Queiroz, J.A. Hydrophobic Interaction Chromatography of Chromobacterium Viscosum Lipase on Polypropylene Glycol Immobilised on Sepharose. J. Chromatogr. A 1999, 849, 413–419. [Google Scholar] [CrossRef]
- Maldonado, R.R.; Burkert, J.F.M.; Aguiar-Oliveira, E.; Durrant, L.; Mazutti, M.A.; Filho, F.M.; Rodrigues, M.I. Elucidation of the Effects of Inoculum Size and Age on Lipase Production by Geotrichum Candidum. Biotecnol. Apl. 2014, 31, 216–221. [Google Scholar]
- Stanbury, P.F.; Whitaker, A.; Hall, S.J. Culture Preservation and Inoculum Development. In Principles of Fermentation Technology; Butterworth-Heinemann: Cambridge, MA, USA, 2017; pp. 335–399. [Google Scholar]
- Stulke, J.; Hillen, W. Regulation of Carbon Catabolism in Bacillus Species. Annu. Rev. Microbiol. 2000, 54, 849–880. [Google Scholar] [CrossRef]
- Deshmukh, A.N.; Mistry, S.R.; Yewale, T.B.; Mahajan, D.M.; Jain, R. Production of 2,3-Butanediol from Sugarcane Molasses Using Bacillus Subtilis. Int. J. Adv. Biotechnol. Res. 2015, 6, 976–2612. [Google Scholar]
- Jenilarani, D.; Parthasarathy, N.; Dhasarathan, P.; Yuvashree, R. Production of Extra Cellular Enzymes by Microbial Strains in Molasses and Additives Supplemented Fermentation Media. Afr. J. Microbiol. Res. 2015, 9, 771–775. [Google Scholar] [CrossRef][Green Version]
- Luna, C.L.; Mariano, R.L.R.; Souto-Maior, A.M. Production of a Biocontrol Agent for Crucifers Black Rot Disease. Braz. J. Chem. Eng. 2002, 19, 133–140. [Google Scholar] [CrossRef]
- Bajaj, B.K.; Singh, S.; Khullar, M.; Singh, K.; Bhardwaj, S. Optimization of Fibrinolytic Protease Production from Bacillus Subtilis I-2 Using Agro-Residues. Braz. Arch. Biol. Technol. 2014, 57, 653–662. [Google Scholar] [CrossRef]
- Maleki-Kakelar, M.; Azarhoosh, M.J.; Golmohammadi Senji, S.; Aghaeinejad-Meybodi, A. Urease Production Using Corn Steep Liquor as a Low-Cost Nutrient Source by Sporosarcina Pasteurii: Biocementation and Process Optimization via Artificial Intelligence Approaches. Environ. Sci. Pollut. Res. 2022, 29, 13767–13781. [Google Scholar] [CrossRef] [PubMed]
- Ja’afar, J.N.; Shitu, A. Utilization of Lignocellulosic Agro-Waste as an Alternative Carbon Source for Industrial Enzyme Production. In Waste Management, Processing and Valorisation; Springer: Singapore, 2022; pp. 221–233. [Google Scholar]
- Sopanen, T.; Laurière, C. Release and Activity of Bound β-Amylase in a Germinating Barley Grain. Plant Physiol. 1989, 89, 244–249. [Google Scholar] [CrossRef]
- Hardie, D.G. Control of Carbohydrase Formation by Gibberellic Acid in Barley Endosperm. Phytochemistry 1975, 14, 1719–1722. [Google Scholar] [CrossRef]
- Sahoo, A.; Mahanty, B.; Daverey, A.; Dutta, K. Nattokinase production from Bacillus subtilis using cheese whey: Effect of nitrogen supplementation and dynamic modelling. J. Water Process Eng. 2020, 38, 101533. [Google Scholar] [CrossRef]
- Ferreyra, R.; Lorda, G.; Balatti, A. Production of Alpha-Amylase in Acid Cheese Whey Culture Media with Automatic PH Control. Rev. Microbiol. 1998, 29, 259–264. [Google Scholar] [CrossRef]
- De Oliveira, D.W.F.; Cara, A.B.; Lechuga-Villena, M.; García-Román, M.; Melo, V.M.M.; Gonçalves, L.R.B.; Vaz, D.A. Aquatic Toxicity and Biodegradability of a Surfactant Produced by Bacillus Subtilis ICA56. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2017, 52, 174–181. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Y.; Han, Y. A Thermophilic Cell-Free Cascade Enzymatic Reaction for Acetoin Synthesis from Pyruvate. Sci. Rep. 2017, 7, 4333. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pan, D.; Ding, H.; Wu, Z.; Sun, Y.; Zeng, X. Purification and Characterization of α-Acetolactate Decarboxylase (ALDC) from Newly Isolated Lactococcus lactis DX. J. Sci. Food Agric. 2015, 95, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Ohshiro, T.; Aisaka, K.; Uwajima, T. Purification and Characterization of α-Acetolactate Decarboxylase from Brevibacterium Acetylicum. Agric. Biol. Chem. 1989, 53, 1913–1918. [Google Scholar] [CrossRef]
- Qian, S.; Wang, C.; Wang, H.; Yu, F.; Zhang, C.; Yu, H. Synthesis and Characterization of Surface-Functionalized Paramagnetic Nanoparticles and Their Application to Immobilization of α-Acetolactate Decarboxylase. Process Biochem. 2015, 50, 1388–1393. [Google Scholar] [CrossRef]
- Kisrieva, Y.S.; Serebrennikov, V.M.; Zagustina, N.A.; Bezborodov, A.M. Isolation and Purification of Acetolactate Synthase and Acetolactate Decarboxylase from the Culture of Lactococcus lactis. Appl. Biochem. Microbiol. 2000, 36, 109–114. [Google Scholar] [CrossRef]
- Costa, G.P.; Spolidoro, L.S.; Manfroi, V.; Rodrigues, R.C.; Hertz, P.F. α-Acetolactate Decarboxylase Immobilized in Chitosan: A Highly Stable Biocatalyst to Prevent Off-flavor in Beer. Biotechnol. Prog. 2022, 38, e3295. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.; Monduzzi, M. Not Only pH. Specific Buffer Effects in Biological Systems. Curr. Opin. Colloid Interface Sci. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Stoll, V.S.; Blanchard, J.S. Chapter 6 Buffers. Principles and Practice1. In Methods in Enzymology; Academic Press Inc.: Cambridge, MA, USA, 2009; Volume 463, pp. 43–56. [Google Scholar]
- Metrick, M.A.; Temple, J.E.; Macdonald, G. The Effects of Buffers and PH on the Thermal Stability, Unfolding and Substrate Binding of RecA. Biophys. Chem. 2013, 184, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bauduin, P.; Nohmie, F.; Touraud, D.; Neueder, R.; Kunz, W.; Ninham, B.W. Hofmeister Specific-Ion Effects on Enzyme Activity and Buffer PH: Horseradish Peroxidase in Citrate Buffer. J. Mol. Liq. 2006, 123, 14–19. [Google Scholar] [CrossRef]
- Salis, A.; Bilaničova, D.; Ninham, B.W.; Monduzzi, M. Hofmeister Effects in Enzymatic Activity: Weak and Strong Electrolyte Influences on the Activity of Candida Rugosa Lipase. J. Phys. Chem. B 2007, 111, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Kornecki, J.F.; Carballares, D.; Morellon-Sterling, R.; Siar, E.H.; Kashefi, S.; Chafiaa, M.; Arana-Peña, S.; Rios, N.S.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Influence of Phosphate Anions on the Stability of Immobilized Enzymes. Effect of Enzyme Nature, Immobilization Protocol and Inactivation Conditions. Process Biochem. 2020, 95, 288–296. [Google Scholar] [CrossRef]
- O’Sullivan, S.M.; Condon, S.; Cogan, T.M.; Sheehan, D. Purification and Characterisation of Acetolactate Decarboxylase from Leuconostoc Lactis NCW1. FEMS Microbiol. Lett. 2001, 194, 245–249. [Google Scholar] [CrossRef]
- Kennedy, M.; Krouse, D. Strategies for Improving Fermentation Medium Performance: A Review. J. Ind. Microbiol. Biotechnol. 1999, 23, 456–475. [Google Scholar] [CrossRef]


| Experiment | Medium | Carbon Source (10 g·L−1) | Nitrogen Source (3 g·L−1) |
|---|---|---|---|
| 01 | Standard | Glucose | Yeast extract |
| 02 | 02 | Fructose | Yeast extract |
| 03 | 03 | Fructose + Glucose | Yeast extract |
| 04 | 04 | Sucrose | Yeast extract |
| 05 | 05 | Lactose | Yeast extract |
| 06 | 06 | Glycerol | Yeast extract |
| 07 | 07 | Molasses | Yeast extract |
| 08 | 08 | Glucose | Corn steep solids |
| 09 | 09 | Glucose | Ammonium sulfate |
| 10 | 10 | Glucose | Sodium nitrate |
| 11 | 11 | Glucose | Malt powder |
| 12 | 12 | Glucose | Urea |
| 13 | Optimized | Molasses | Corn steep solids |
| Component | Cost (US¢·kg−1) | Concentrations for Each Experiment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
| KH2PO4 (g L−1) | 120 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Na2HPO4 (g L−1) | 200 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| (NH4)2SO4 (g L−1) | 13 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 5.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| NaCl (g L−1) | 6 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| MgSO4 7H2O (g L−1) | 30 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| FeCl3 6H2O (g L−1) | 12 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 |
| MnSO4 H2O (mg L−1) | 50 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Glucose (g L−1) | 70 | 10 | - | 5 | - | - | - | - | - | - | - | - | - | - |
| Fructose (g L−1) | 170 | - | 10 | 5 | - | - | - | - | - | - | - | - | - | - |
| Sucrose (g L−1) | 25.0 | - | - | - | 10 | - | - | - | - | - | - | - | - | - |
| Lactose (g L−1) | 200 | - | - | - | - | 10 | - | - | - | - | - | - | - | - |
| Glycerol (g L−1) | 100 | - | - | - | - | - | 10 | - | - | - | - | - | - | - |
| Molasses (g L−1) | 9.8 | - | - | - | - | - | - | 10 | - | - | - | - | - | 10 |
| Yeast extract (g L−1) | 170 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | - | - | - | - | - | - |
| CSS (g L−1) | 0.5 | - | - | - | - | - | - | - | 3 | - | - | - | - | 3 |
| NaNO3 (g L−1) | 43.0 | - | - | - | - | - | - | - | - | - | 3 | - | - | - |
| Malt powder (g L−1) | 150 | - | - | - | - | - | - | - | - | - | - | 3 | - | - |
| Urea (g L−1) | 48.0 | - | - | - | - | - | - | - | - | - | - | - | 3 | - |
| Culture Medium | Parameters | |||||
|---|---|---|---|---|---|---|
| Carbon Source | Lag Phase (h) | Log Phase (h) | μmax (h−1) | Biomass (g·L−1) | ALDC Activity (U·g−1) | |
| Standard | Glucose | 0–3 | 3–12 | 0.30 ± 0.02 | 9.19 ± 0.21 | 17.36 ± 0.19 |
| 02 | Fructose | 0–4 | 4–12 | 0.31 ± 0.06 | 9.64 ± 0.19 | 19.50 ± 0.37 |
| 03 | Fructose + Glucose | 0–3 | 3–10 | 0.65 ± 0.02 | 8.29 ± 0.11 | 20.69 ± 0.45 |
| 04 | Sucrose | 0–3 | 3–11 | 0.39 ± 0.03 | 10.99 ± 0.41 | 16.67 ± 0.41 |
| 05 | Lactose | 0–8 | 8–12 | 0.25 ± 0.02 | 9.44 ± 0.35 | 0.04 ± 0.03 |
| 06 | Glycerol | 0–8 | 8–12 | 0.20 ± 0.06 | 8.62 ± 0.17 | 1.16 ± 0.18 |
| 07 | Molasses | 0–2 | 2–10 | 0.57 ± 0.03 | 7.99 ± 0.13 | 23.94 ± 0.92 |
| Experiment | Medium | At (U·mL−1) | Pr (mg·mL−1) | Sp Act (U·mg−1) |
|---|---|---|---|---|
| 01 | Standard | 3.99 ± 0.04 d | 5.57 ± 0.05 | 0.72 ± 0.10 |
| 02 | 02 | 4.70 ± 0.07 a | 6.48 ± 0.18 | 0.73 ± 0.25 |
| 03 | 03 | 4.29 ± 0.05 c | 5.68 ± 0.03 | 0.76 ± 0.08 |
| 04 | 04 | 4.58 ± 0.17 a,b | 4.77 ± 0.06 | 0.96 ± 0.23 |
| 05 | 05 | 0.01 ± 0.01 g | 0.76 ± 0.01 | 0.01 ± 0.01 |
| 06 | 06 | 0.25 ± 0.03 g | 2.88 ± 0.02 | 0.09 ± 0.05 |
| 07 | 07 | 4.78 ± 0.12 a | 3.23 ± 0.02 | 1.48 ± 0.14 |
| 08 | 08 | 4.03 ± 0.04 d | 3.87 ± 0.02 | 1.04 ± 0.06 |
| 09 | 09 | 0.10 ± 0.04 g | 0.17 ± 0.08 | 0.60 ± 0.11 |
| 10 | 10 | 0.00 ± 0.01 g | 0.12 ± 0.11 | 0.00 ± 0.13 |
| 11 | 11 | 1.46 ± 0.01 e | 2.24 ± 0.09 | 0.65 ± 0.10 |
| 12 | 12 | 0.58 ± 0.01 f | 1.50 ± 0.20 | 0.39 ± 0.21 |
| 13 | Optimized | 4.43 ± 0.12 b,c | 3.22 ± 0.01 | 1.37 ± 0.13 |
| Experiment | Medium | MC (US¢·L−1) | PP (U·L−1) | PC (US¢·U−1) |
|---|---|---|---|---|
| 01 | Standard | 1.57 | 0.1596 | 9.84 |
| 02 | 02 | 2.22 | 0.1880 | 11.81 |
| 03 | 03 | 1.90 | 0.1716 | 11.07 |
| 04 | 04 | 1.12 | 0.1832 | 6.11 |
| 05 | 05 | 2.87 | 0.0000 | * |
| 06 | 06 | 1.87 | 0.0100 | 187.00 |
| 07 | 07 | 0.79 | 0.1912 | 4.13 |
| 08 | 08 | 1.07 | 0.1612 | 6.64 |
| 09 | 09 | 1.10 | 0.004 | 275.00 |
| 10 | 10 | 1.19 | 0.0000 | * |
| 11 | 11 | 1.21 | 0.0580 | 20.86 |
| 12 | 12 | 1.51 | 0.0232 | 65.09 |
| 13 | Optimized | 0.46 | 0.1772 | 2.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, R.C.; Maciel, V.M.M.; Hissa, D.C.; França, Í.W.; Gonçalves, L.R.B. Production of the Food Enzyme Acetolactate Decarboxylase (ALDC) from Bacillus subtilis ICA 56 Using Agro-Industrial Residues as Feedstock. Fermentation 2022, 8, 675. https://doi.org/10.3390/fermentation8120675
Oliveira RC, Maciel VMM, Hissa DC, França ÍW, Gonçalves LRB. Production of the Food Enzyme Acetolactate Decarboxylase (ALDC) from Bacillus subtilis ICA 56 Using Agro-Industrial Residues as Feedstock. Fermentation. 2022; 8(12):675. https://doi.org/10.3390/fermentation8120675
Chicago/Turabian StyleOliveira, Ravena Casemiro, Vania Maria Melo Maciel, Denise Cavalcante Hissa, Ítalo Waldimiro França, and Luciana Rocha Barros Gonçalves. 2022. "Production of the Food Enzyme Acetolactate Decarboxylase (ALDC) from Bacillus subtilis ICA 56 Using Agro-Industrial Residues as Feedstock" Fermentation 8, no. 12: 675. https://doi.org/10.3390/fermentation8120675
APA StyleOliveira, R. C., Maciel, V. M. M., Hissa, D. C., França, Í. W., & Gonçalves, L. R. B. (2022). Production of the Food Enzyme Acetolactate Decarboxylase (ALDC) from Bacillus subtilis ICA 56 Using Agro-Industrial Residues as Feedstock. Fermentation, 8(12), 675. https://doi.org/10.3390/fermentation8120675








