1. Introduction
The human gastrointestinal tract comprises indigenous microbiota whose role is to protect the host from exogenous pathogenic infection, increase host nutrients, act as a xenobiotic, metabolize drugs, be involved in immunomodulation, and maintain the structural integrity of the gut mucosal barrier. However, gut dysbiosis occurs due to immune-mediated disorders caused by pathogenic bacteria in the gut, changes in dietary intake, stress, and antibiotic use [
1]. When gut dysbiosis occurs, the exogenous intestinal pathogens might secrete harmful toxins that block the epithelial cell function and the host’s metabolic response to cause pathological disorders, including multi-system organ failure, colon cancer, and irritable bowel syndrome [
1]. Previous studies have indicated that the overgrowth of pathogenic bacterial populations and the significant decline of health-promoting bacteria play an important role in innate intestinal inflammation and the pathogenesis of the gastrointestinal disease [
2,
3,
4].
A proven way to remedy gut dysbiosis is through intake of diets containing living microorganisms (probiotics) with the same beneficial attributes as the indigenous microbiota. However, for a probiotic to exert its beneficial effect in the host, it has to be taken in through a delivery medium commonly called functional foods [
5]. A delivery medium can be in the form of a pharmaceutical drug supplement or in the form of feed/food which, when consumed, can confer the required probiotic benefit on the host [
5]. The two major mediums of delivery for probiotics are dairy and non-dairy probiotic carriers. The widely and commonly used carriers for probiotics are dairy products such as milk (fermented, powder) ice cream, yogurt, and cheese, while non-dairy probiotic carriers include fruits, rice, oatmeal, cereal-based products, and fruit juice [
6].
Non-dairy probiotic carriers can serve dual roles as prebiotics and probiotic delivery mediums. In 2008, at the 6th Meeting of the International Scientific Association of Probiotics and Prebiotics (ISAPP), prebiotics were defined as a selectively fermented ingredient that results in specific changes in the composition and/or activity of the gastrointestinal microbiota, thus conferring benefit(s) upon host health [
7]. Prebiotics also serve as food for probiotics. Non-dairy probiotic carriers serve as excellent functional foods as the challenges of lactose intolerance in the host, refrigeration as a means of preservation of the functional food, and allergies from cholesterol intake are eliminated [
8]. Also, non-dairy products, due to their physical structures, establish a conducive environment for probiotics by limiting the adverse environmental conditions of the gastrointestinal tracts [
8,
9]. Plants contain polysaccharides such as inulin which are prebiotics and are capable of fermenting in the intestinal tract, thus enabling them to interact with probiotics, thereby enhancing the functionality of the organisms [
10]. Plants possess oligosaccharides that deliver probiotics directly into the large intestines, thus preventing the digestion of probiotics in the gastrointestinal tract [
11]. Prebiotics are present in significant amounts in several edible fruits, vegetables, and cereals and can alter the colonic microflora to a healthy composition by inducing beneficial luminal or systemic effects within the host [
1].
Baobab (
Adansonia digitata) is a potential source of polysaccharides such as sugar, carbohydrates, pectin (mainly soluble and insoluble fibers), proteins, vitamin C, calcium, and lipids [
12]. These polysaccharides possess a prebiotic potential and thus can enhance the growth of probiotics in the large intestine, thus promoting the health of the gastrointestinal tract. The pectin also prevents the binding of pathogens to the intestinal wall, thereby chelating heavy metals [
13]. Baobab fruit pulp contains a high concentration of potassium, copper, magnesium, and manganese [
12]. Baobab is rich in fiber, low in fat and protein concentration, but contains different amino acids, thus making it ideal for fiber supplements in foods and increasing the nutritional profile of the food. Due to its low-sugar and high-fiber content, it possesses a low glycemic index which allows for easy digestibility and satiation [
14]. The fruit pulp also contains stearic, palmitic, and arachidic acids, all of which contribute to its pharmacological use. Baobab is an excellent source of vitamins, micronutrients, and soluble fibers, having pre- and probiotic effects, thus serving as an intestinal regulator in the case of gastric disorders [
15,
16].
Lactose intolerant individuals interested in consuming foods that confer probiotic benefits can derive such health benefits from non-dairy foods. The development of synbiotic foods using baobab fruit pulp combined with lactic acid bacteria could ameliorate and eliminate the challenge of the inaccessibility of lactose-intolerant persons to functional foods. The prebiotic potential of baobab fruit pulp and its fit as a constituent in the formulation of a functional beverage were evaluated for the first time in this study. This study aimed to produce synbiotic beverages composed mainly of baobab fruit pulp and probiotic lactic acid bacteria (LAB) and to determine its efficacy as a functional food.
4. Discussion
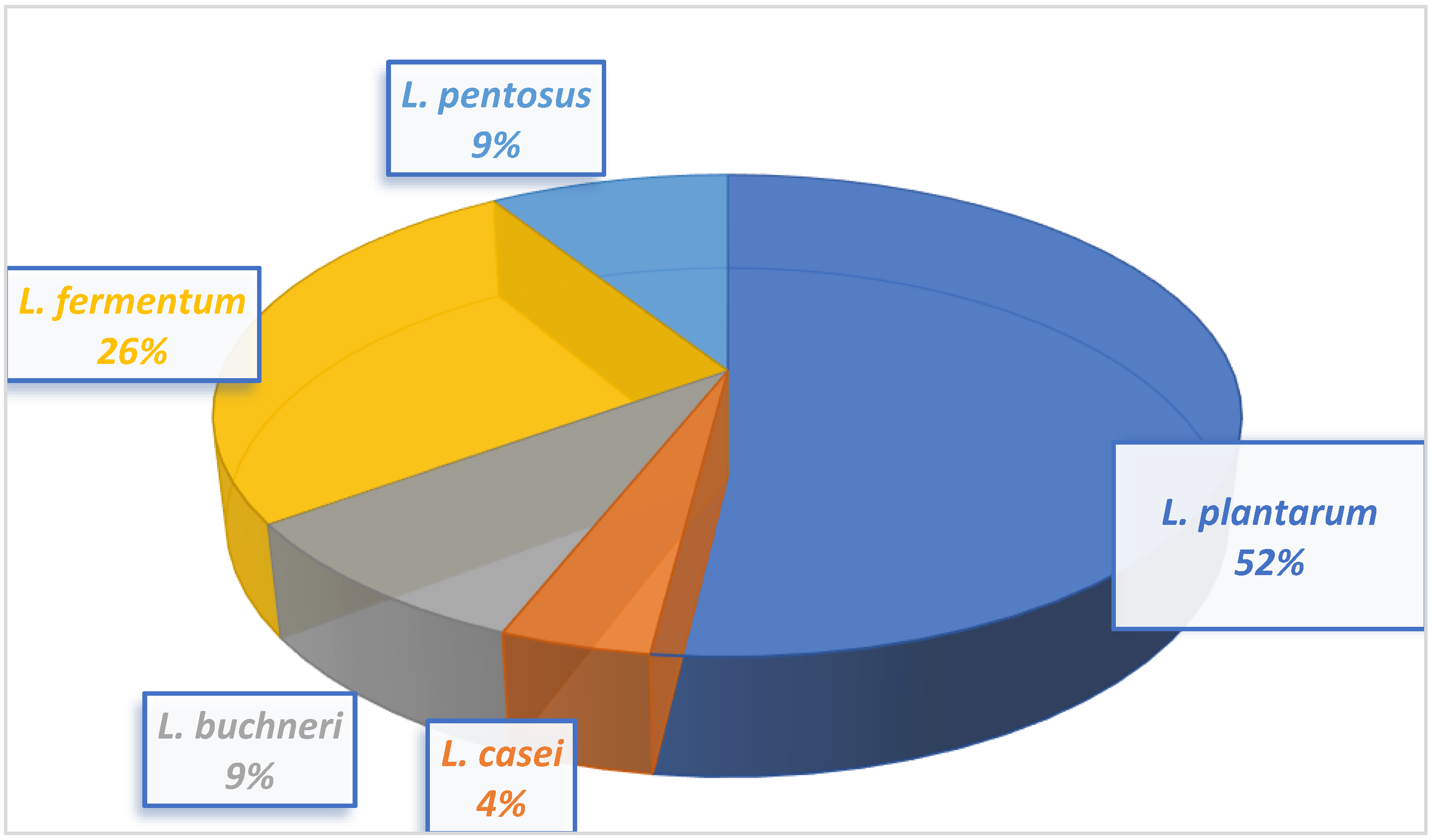
Twenty-three lactic acid bacteria isolated from milk samples were identified as
L. buchneri,
L. casei,
L. fermentum,
L. plantarum, and
L. pentosus. The morphological and biochemical characteristics of the LAB isolates conform to that of Bergey’s Manual of Determinative Bacteriology [
43]. The 16S rRNA gene sequencing revealed the isolates to be closely related to
Lacticaseibacillus casei,
Limosilactobacillus fermentum,
Lactiplantibacillus plantarum,
Lentilactobacillus buchneri, and
Lactiplantibacillus pentosus.Lactobacillus plantarum had the highest occurrence at 48%, and Lactobacillus casei had the lowest occurrence at 4%.
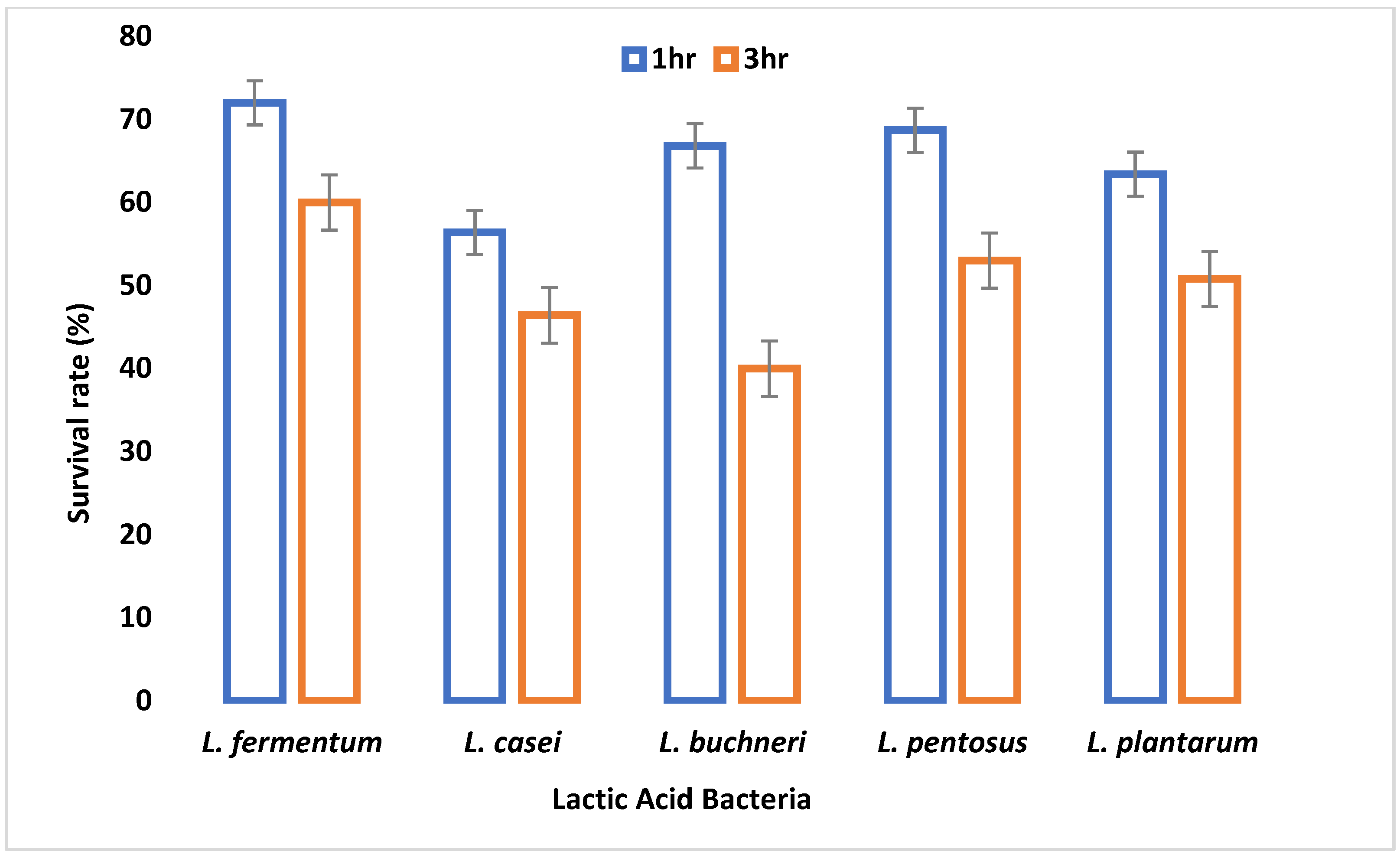
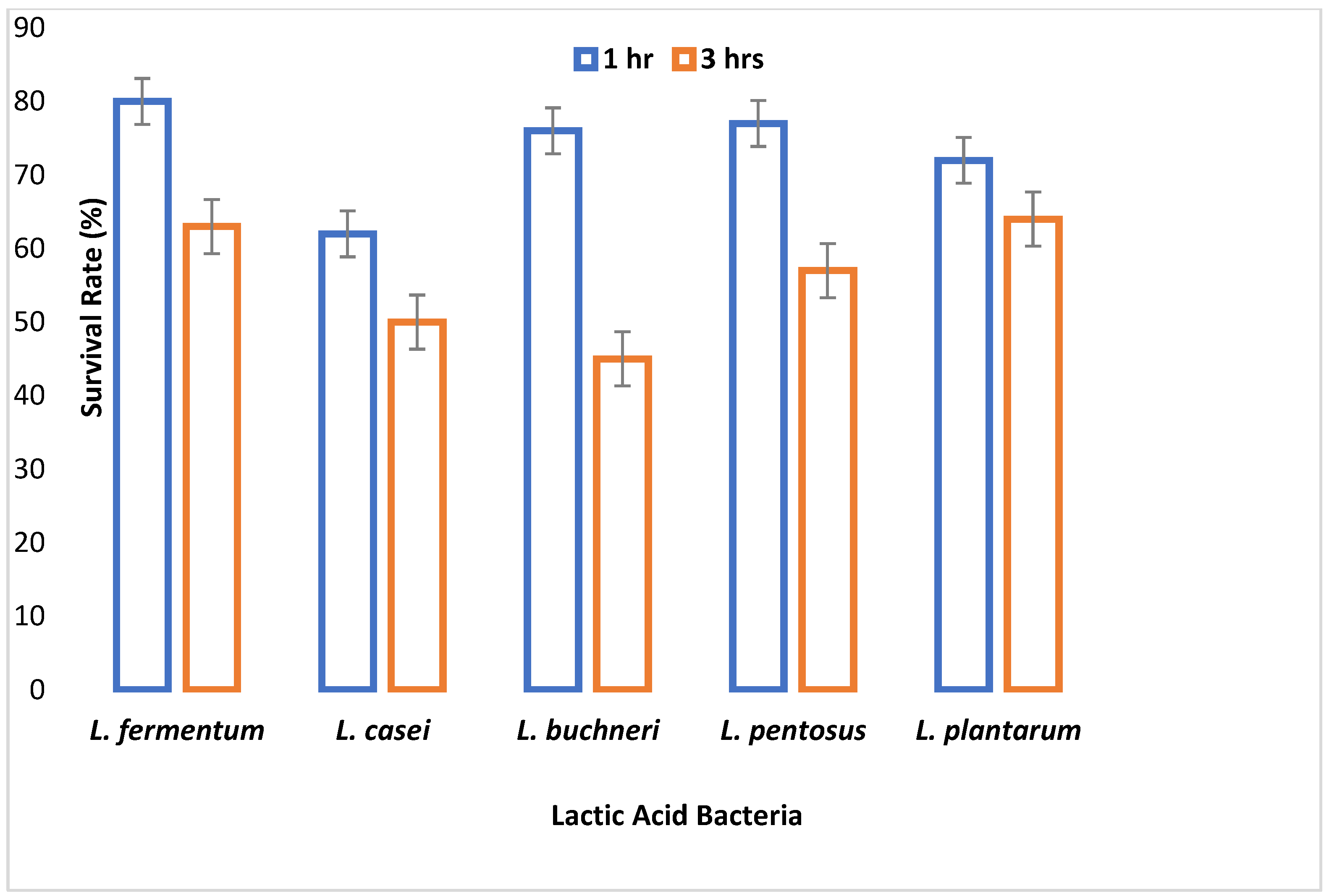
A major criterion used in probiotic selection is the ability to withstand acidity which invariably depicts its inability to inactivate due to the low pH of the gastrointestinal tract. All LAB isolates were able to survive at pH 2.5 and 3.0, although their viability started to decrease after 3 h of incubation. This demonstrates their potential to survive in the extremely acidic pH of the gastrointestinal tract. A similar study by Nami et al. [
29] showed a decrease in the viability of the LAB isolates after 3 h of incubation at pH 3.0. All the organisms had a survival rate above 50% after 1 and 2 h of incubation. After 3 h of incubation, only 67% of the LAB isolates showed a viability of more than 50%. This agrees with the work of Hoque et al. [
44] where all the
Lactobacillus species isolated from yogurt were able to survive an acidic pH of 2.5 to 3.5.
L. fermentum demonstrated the highest capacity to withstand low pH among the isolates tested. The adaptive acid tolerance reaction could be controlled by the production of some genes during adaptation, which comprises two different responses, pre-challenge adaptation and transient adaptation that occurs during low pH challenge.
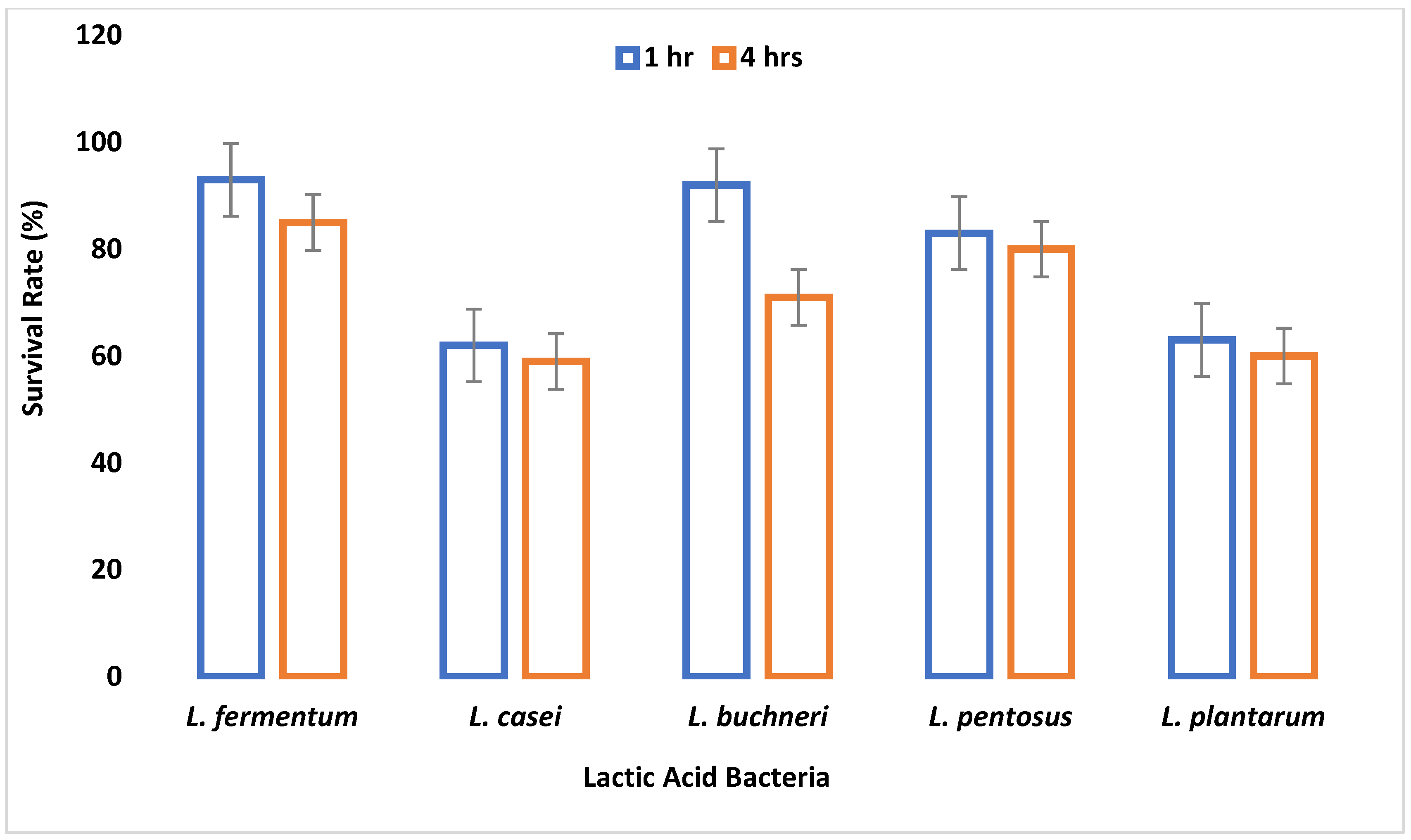
It takes 4 h for food to digest in the intestine [
29]. All the isolates were able to resist bile salt concentrations of 0.3%, 0.5%, and 1% in varying degrees for 4 h. The ability to resist the effect of bile salt is an important criterion for probiotic selection, because the mean intestinal concentration of humans is supposedly 0.3% (
w/
v) [
45]. This demonstrates that the LAB isolates met these probiotic criteria, although
L. fermentum survived best. As the bile salt concentration increased and time progressed, the viability of the LAB species decreased, and this conforms to the work of Owusu-Kwarteng et al. [
46], where all
L. fermentum strains isolated from West African fermented millet dough were able to resist 0.3%–2% bile concentration with decreased viability.
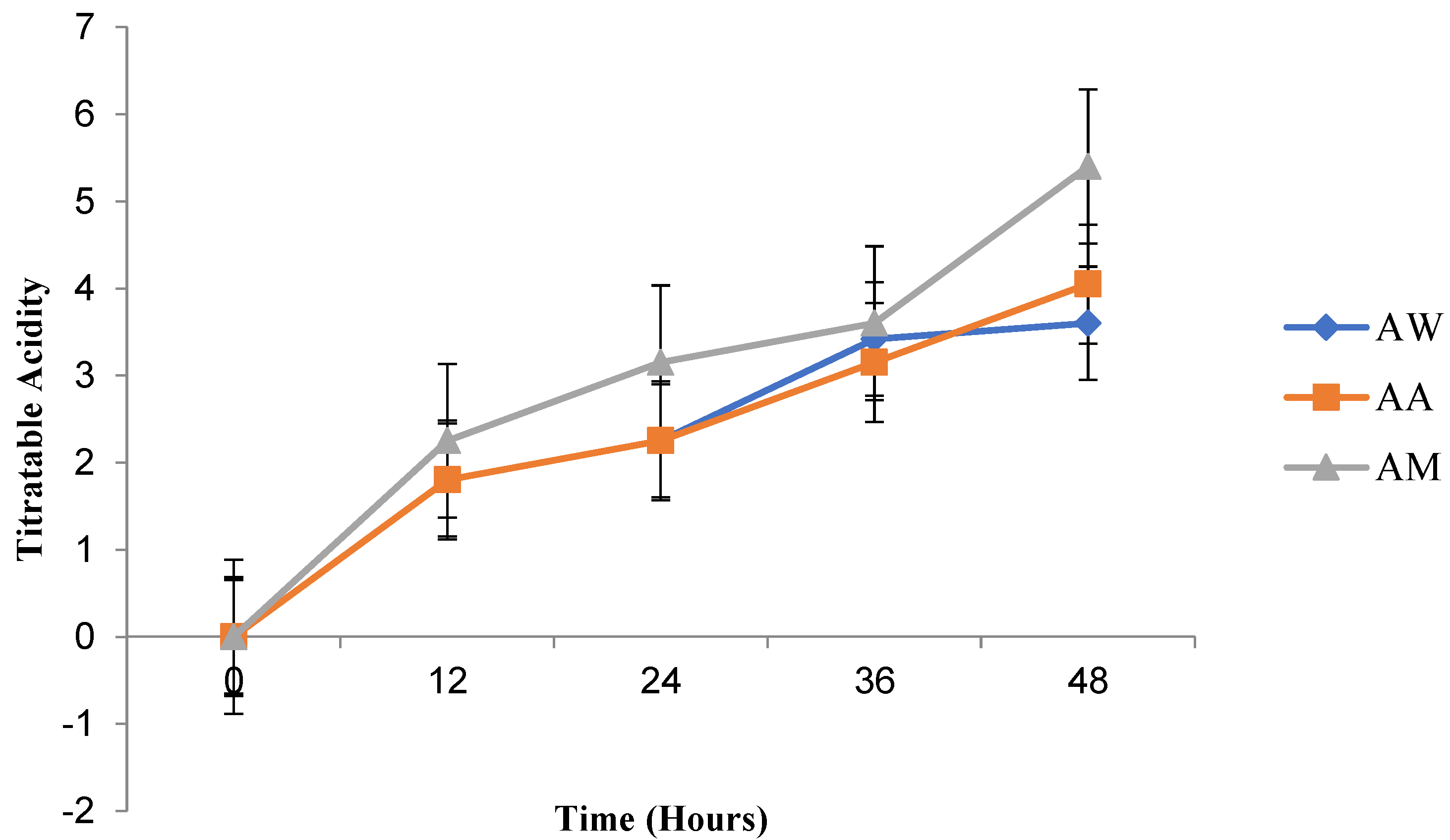
Lactic acid bacteria are capable of lowering pH, producing antibacterial substances such as lactic and organic acids [
47], and producing bacteriocins capable of inhibiting the growth of pathogenic and spoilage bacteria [
48,
49]. In this study, the metabolites produced by LAB present in the culture supernatant demonstrated antagonistic activity against the clinical pathogens and this lends credence to their antimicrobial potential.
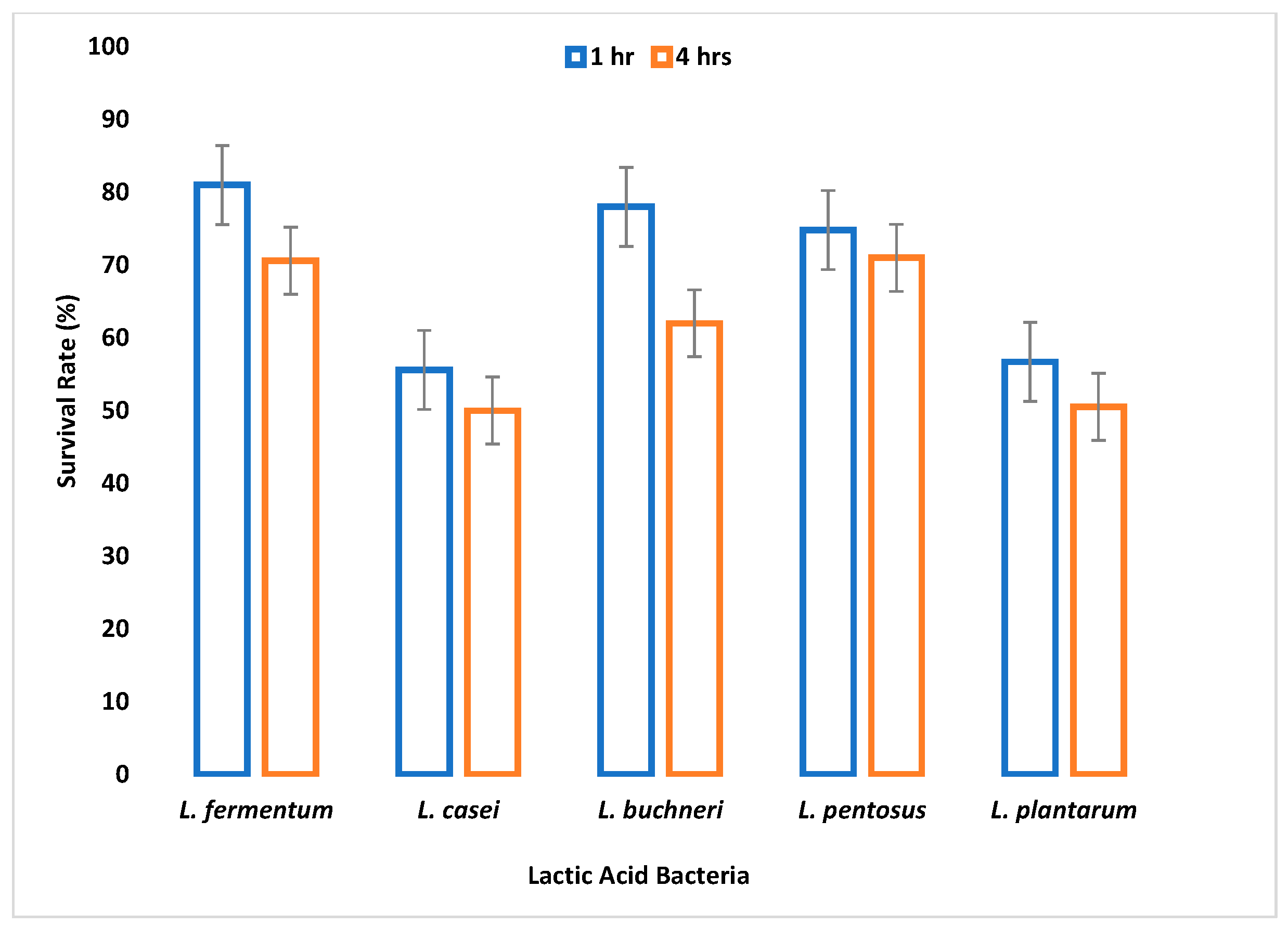
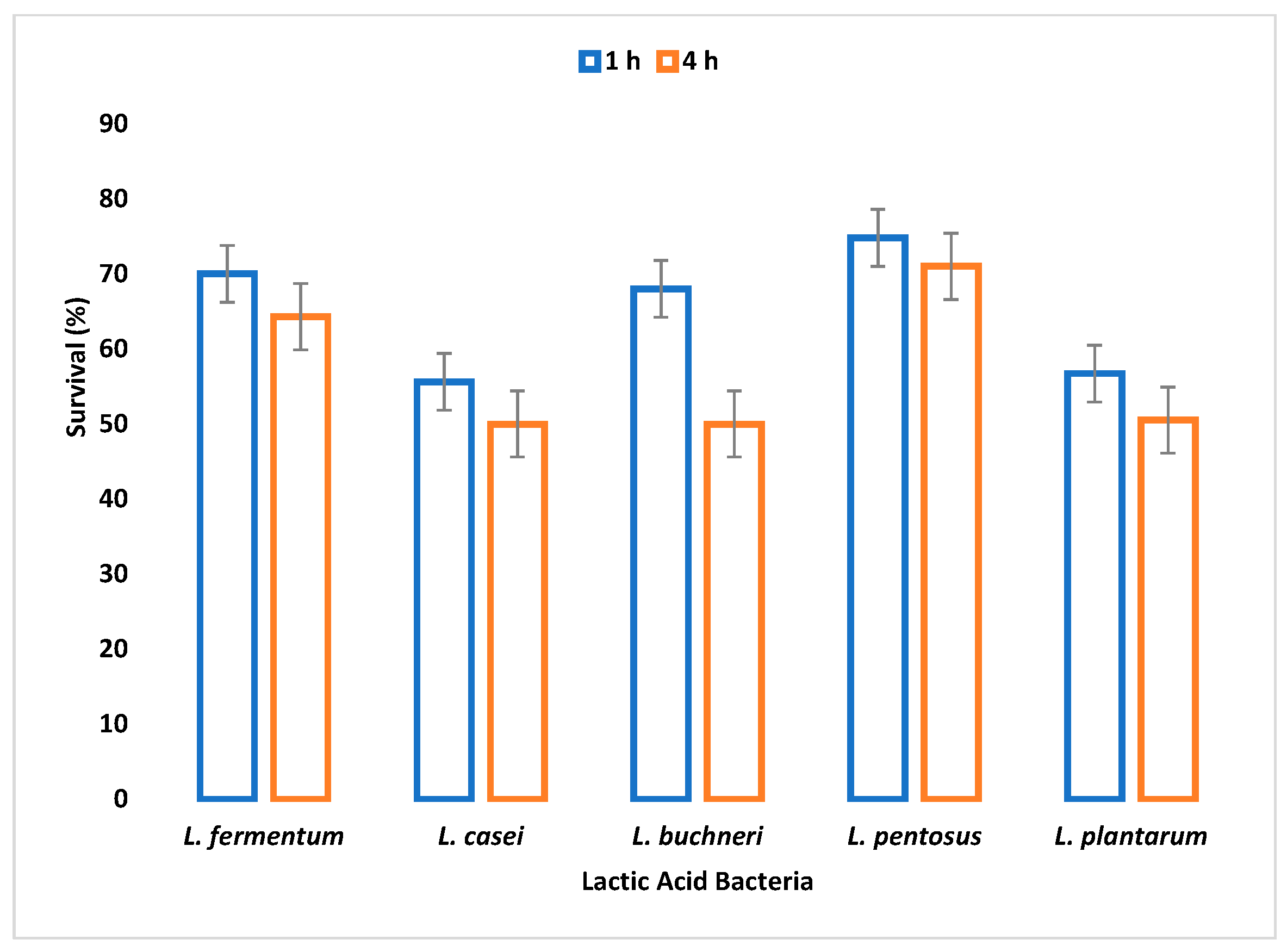
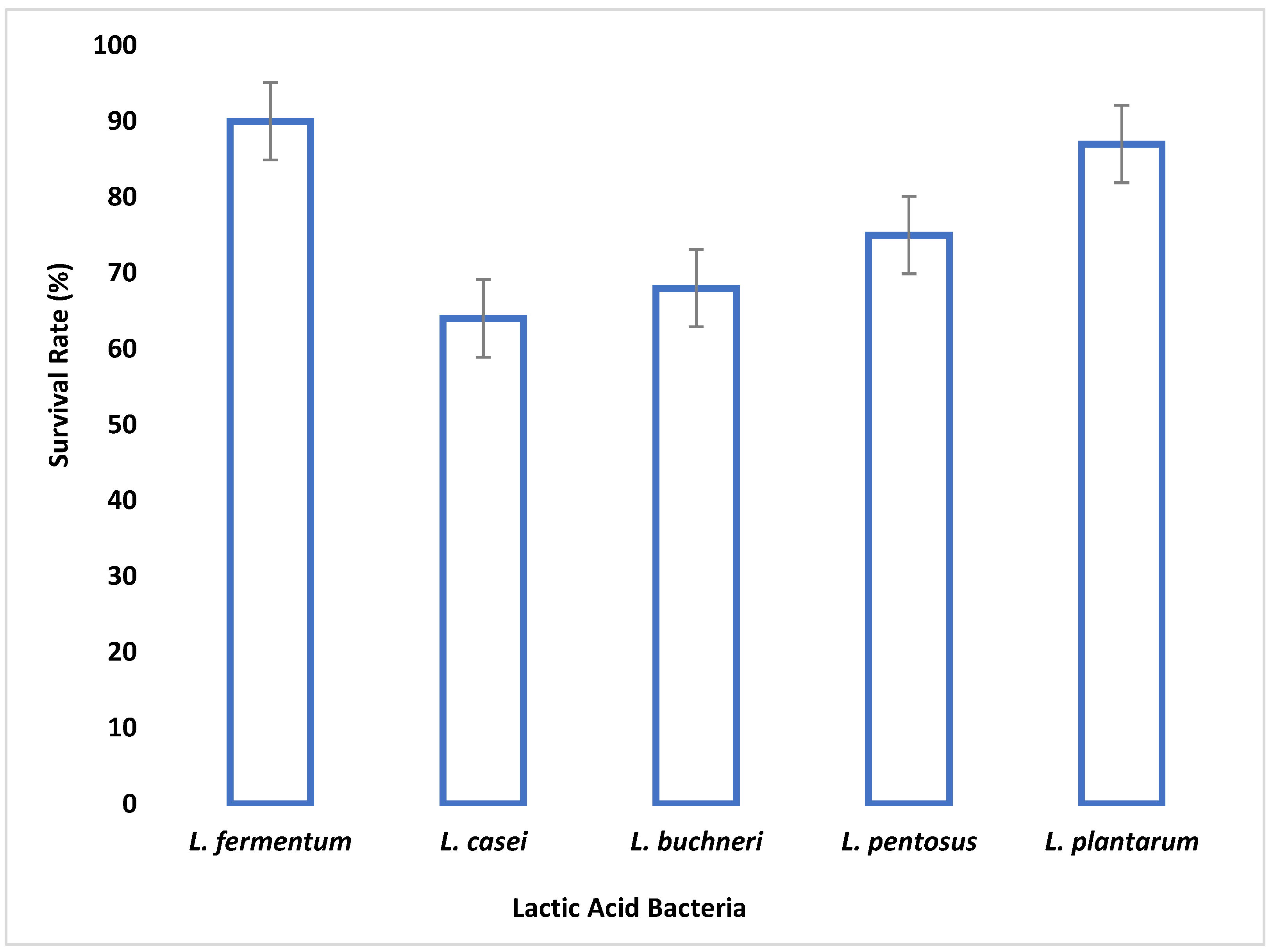
The small and large intestine is colonized by probiotic microorganisms and must be able to survive the harsh conditions therein to be functional. Gastric juice is secreted in the stomach where high acid persists. Therefore, the survival of an organism in gastric juice is a major criterion for probiotic selection. In this study, all tested LAB species were able to survive simulated gastric juice at pH 2.5 with a percentage survival rate ranging between 64% and 90% after 4 h.
L. fermentum had the highest survival rate, while
L. casei had the least survival rate. Reference [
50] reported that the survival rate of
Lactobacillus species in gastric juice differs with different strains.
Safety assessment of probiotic organisms is an important factor that must be considered before they are used in producing any functional food [
51]. Thus, in the guidelines set by [
52], safety assessment is a recommended criterion for choosing probiotics. In this study, the LAB isolates did not liquefy gelatin or produce DNase enzyme and were thereby incapable of hydrolyzing host DNA and unable to lyse red blood cells. The absence of DNase, gelatinase, and hemolytic activity is an indication that the organisms are GRAS organisms and non-virulent, which is a criterion for probiotic selection.
Baobab pulp contains considerable quantities of phytochemicals which makes it suitable as a dietary supplement and valuable for pharmaceuticals. Phytochemical compounds are secondary metabolites of plants that contribute to their use as pharmaceutical, nutritional, and dietary supplements [
53]. These components are also referred to as nutraceuticals owing to their health-promoting properties as well as preventive and therapeutic use in the treatment of chronic diseases [
54].
In this study, the fruit pulp of baobab contained a substantial amount of oleic and linoleic acid with a moderate amount of linolenic acid; therefore, baobab pulp can serve as a source of omega-3, -6, and -9 supplements and can help in improving health. Polyunsaturated fatty acids (PUFA), which are mainly linoleic and α-linolenic acids, are both synthesized by plants and are essential for the prevention of diseases and good health [
55]. Fatty acids are valuable in the pharmaceutical and food industries. Linolenic acids (omega-3 supplements, omega-6 supplements) have been indicated for the prevention and treatment of cardiovascular diseases, improved concentration, memory, motivation, and motor abilities; they are also indicated during pregnancy for the reduction of postpartum, depression, and mood swings as well as improved health after birth [
56]. They also serve both preventive and therapeutic purposes in conditions relating to premenstrual syndrome, diabetes, skin problems, and inflammation, to mention but a few [
57]. Omega-9 supplements (oleic acid) are useful in increasing the healthy lifestyle of consumers and also help in combating total and bad cholesterol (LDL) and increasing the good ones (HDL), preventing coronary diseases and aging [
58].
The amount of vitamin C in baobab pulp was very high (224.6 mg/100 g), and this shows that it is a good source of antioxidants. The vitamin C content in this research is higher and agrees with that of [
59] who reported a range of 169–231 mg/100 g. Vitamins play an essential role in human nutrition and health but cannot be synthesized by humans and are therefore taken up in the diet. According to Aluko et al. [
60], the daily intake of ascorbic acid in children and adults is about 17 mg/day and 30 mg/day, respectively; thus, baobab fruit can serve as a good source of ascorbic acid.
Minerals are inorganic nutrients required in minute quantity which are needed for the maintenance of physiochemical processes essential to life. Macronutrients (calcium and sodium) and micronutrients (iron and magnesium) are required for normal growth and development [
61]. Deficiency of any of these minerals may result in impairment in cognitive performance, predisposition to infections due to lowered immunity, anemia, and many other conditions [
62]. In this study, baobab was found to contain a higher amount of calcium and other essential nutrients such as iron, magnesium, and sodium. Consumption of this fruit can serve as a good source of micro and macronutrients.
Adequate nutrition is an important factor of a public health concern as it provides the basis for dietary assessment and formulation of healthier diets before consumption. High amounts of carbohydrates in food serve as a source of energy [
63]. The result of this study showed that baobab is rich in carbohydrates (75.92%) which is comparable with the findings of Elmadfa et al. [
64] that reported a carbohydrate content of 70.03% in baobab fruit pulp obtained from Adamawa State, Nigeria. Storage conditions, processing procedure, the difference in ripening age, well as the possibility of genetic variation may have been responsible for the result variation.
The human colon harbors about 10
10–10
12 microbial cells which are mostly anaerobes known as the gut microbiota. Their composition can be modulated by diet variation. Prebiotics are a nutritious, non-digestible group of carbohydrates that can be incorporated into diets so that when fermented by gut microbiota in the gastrointestinal tract, they can provide nutrients to stimulate the growth and activities of the gut microbiota. Carbohydrates in plants such as fructooligosaccharides (FOS) and inulin are the best-known sources of prebiotics. Baobab fruit pulp and the beverages produced in this study contained significant amounts of fructooligosaccharides and inulin. According to the study by Lockett et al. [
65], the prebiotic potential of mango, banana, and apple peel powder at 0%, 2%, and 4% concentrations encouraged the proliferation of several test probiotics evaluated. Their study revealed that even at 0% concentration, the growth of the test probiotics increased significantly. This demonstrates that the concentration of prebiotics presents in baobab fruit pulp and beverages as determined in this study will support the growth of probiotics. Thus, they can be used to stimulate the activities of the gut microbiota and also to enhance host health by improving immune function, improving digestion, colonic integrity, and also down-regulate allergenic response [
66]. Prebiotics can also improve the uptake of zinc, iron, and calcium and decrease colon cancer and cholesterol.
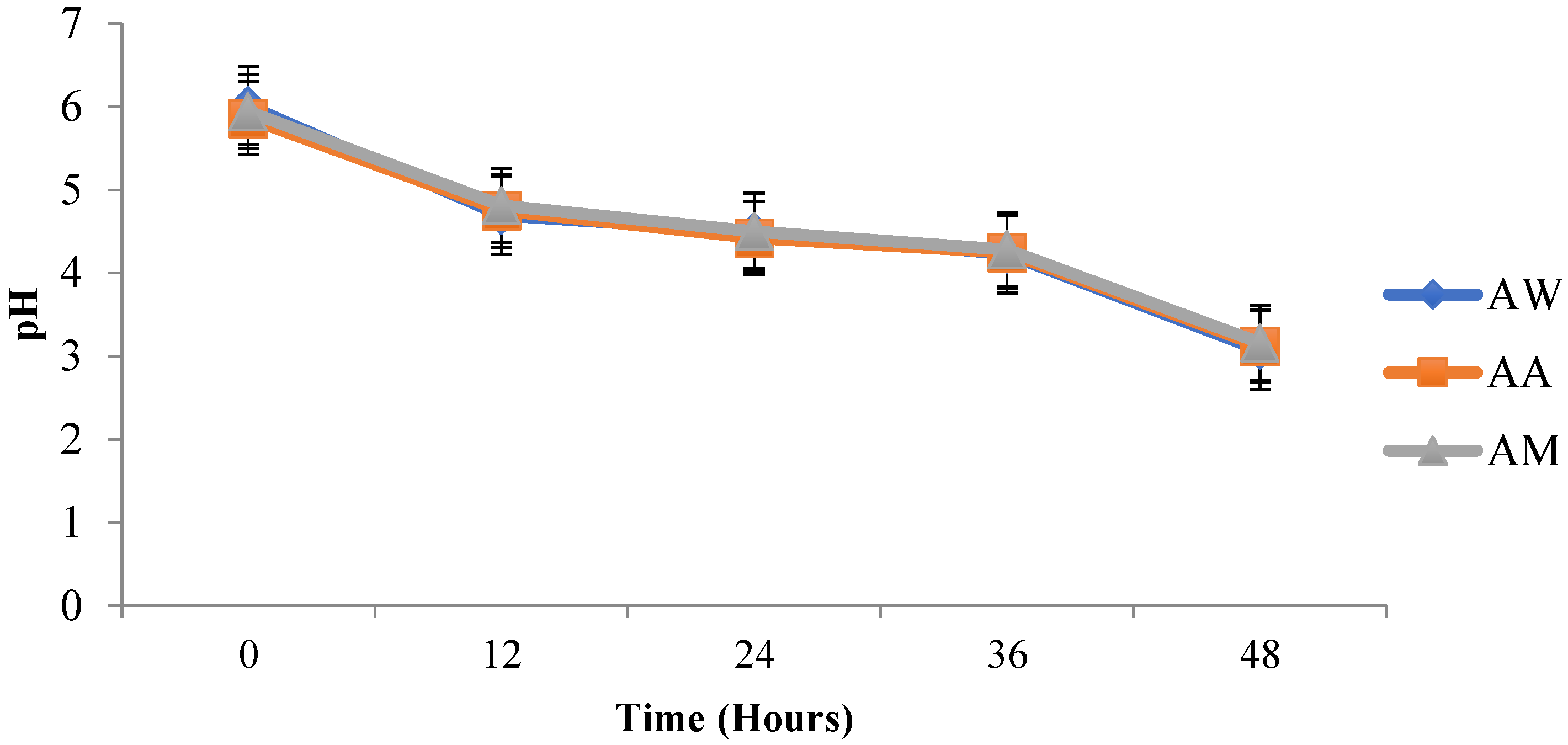
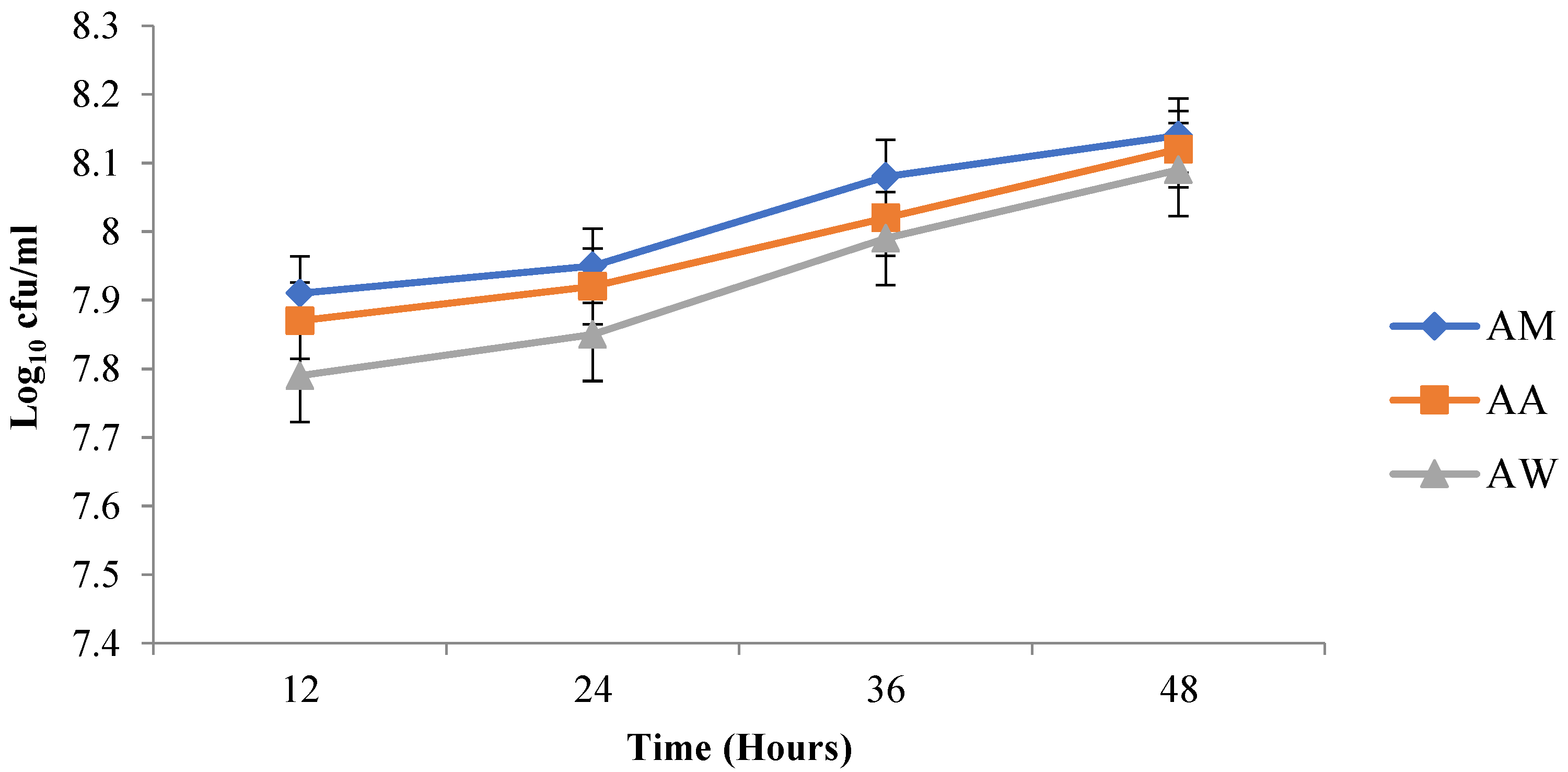
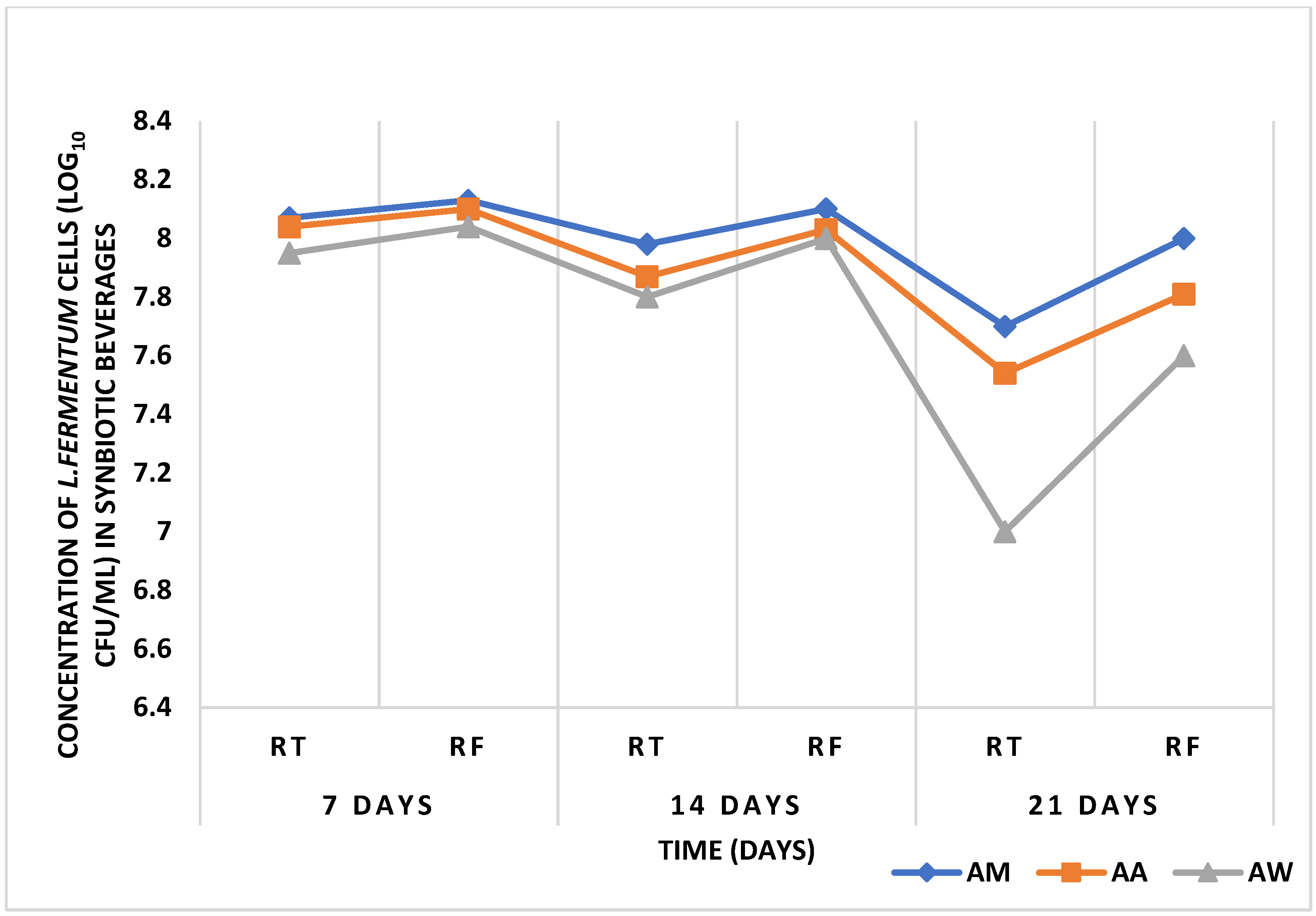
After inoculation of
L. fermentum into the different beverages, pH reduced and acidity increased over time. This increased acidity may be attributed to the production of lactic acid by
L. fermentum and conforms to the study by Sharma et al. [
67], who also noticed a decrease in the pH of probiotic
mutandabota (a traditional food in South Africa produced from baobab fruit) over some time after inoculation with
Lactobacillus rhamnosus.L. fermentum inoculated into the various baobab fruit beverages were able to survive after 48 h. The viability of the probiotic organism increased as time progressed, and this could be due to the presence of additional nutrients in the beverages which were able to support the growth of the organism. Over three weeks, the pH of the synbiotic fruit beverages stored at room and refrigeration temperatures decreased with an increase in acidity and subsequent decrease in viability of
L. fermentum, and this may be associated with the increased production of lactic acid by
L. fermentum, since fermentation commenced over the storage period. According to Othman et al. [
68], lactic acid production is the metabolic end product of LAB fermentation which accumulates over time and inhibits LAB growth due to pH alteration into acidic conditions.
L. fermentum in the synbiotic beverages survived best at refrigeration temperature than at room temperature.
Prebiotics are fermentable carbohydrates used by probiotics, and this is significant as it influences the composition and metabolic activities of the gut microbiota [
69]. Although these carbohydrates are indigestible in the gastrointestinal tract, they can be broken down by the gut microbiota to produce short-chain fatty acids (SCFA) and lactate [
70]. In this study, the test LAB isolates degraded the fructooligosaccharides and inulin content of the fruit beverages after three weeks with the production of lactate, and this may enhance the gut microbiota.