Abstract
Food waste-based biorefineries are considered an essential concept for the implementation of a sustainable circular economy. In this study, cheese whey powder (CWP), a dairy industry waste, was utilized to produce cyclosporin A (CsA). As it is difficult to valorize CWP because its components vary depending on the origin, a process for sugar conversion via acid hydrolysis was designed to obtain reproducible results using refined whey powder (WP) of a consistent quality. Acid hydrolysis was carried out using 2% (w/w) HCl and biomass loading of 50 g/L at 121 °C for 20 min. CWP hydrolysates were utilized to ferment Tolypocladium inflatum ATCC 34921. CsA production was found to be 51.3 mg/L at 12 days, a 1.4-fold increase compared to the control (commercial glucose, 36.3 mg/L). Our results showed that 100 g CWP can be converted to 81.8 mg of CsA. This finding demonstrated that CWP can be used as a sustainable feedstock for biorefineries.
1. Introduction
The prediction that the global population will reach 9.6 billion by 2050 has led scientists to focus solely on increasing food production without considering food loss and waste [1]. Consequently, food waste (FW) is inevitably generated worldwide, causing serious environmental pollution. In Europe and China, 100 and 600 million tons of FW is generated each year, respectively, and is estimated to increase by 44% in 2050 compared to that in 2005 as per the global increasing trend [2]. According to the Food and Agriculture Organization (FAO) of the United Nations, greenhouse gas (GHG) emissions by FW accounted for more than 20% of global GHG emissions; the carbon footprint of FWs, except land-use changes, is estimated to be 4.4 GT CO2 [3]. Even in developed countries, a considerable proportion of FW still ends up in landfills (75% in the US and 91% in Australia) [4,5]. Governments around the world are working to reduce environmental pollution from FW by introducing the concept of a circular economy (CE), defined as realizing a sustainable economic system by recycling resources [6]. The use of biorefineries is an innovative strategy to realize CE, and the current legislation emphasizes the use of FW as a sustainable feedstock for biorefineries [7]. Furthermore, FW-based biorefineries could meet the criteria of the United States Department of Agriculture (USDA) and the Department of Energy (DOE) to increase the usage rate of biobased chemicals and materials from 5% in 2005 to 25% in 2030 [8].
Cheese whey (CW) is a food processing waste generated from cheese production, resulting in the production of 9 kg CW per kg of cheese [9]. Globally, CW generation is estimated to be approximately 191.7 Mt per year [10]. CW is composed of various ingredients, such as lactose (45–50 g/L), protein (6–8 g/L), lipids (4–5 g/L), and mineral salts (8–10% of dried extracts), and could be utilized as a resource in various fields [11]. CW is rich in nutrients, but most of it is discharged directly into sewage systems, with the exception of some being used as animal feed [12]. During the treatment of CW wastewater, the high BOD (26–60 g/L) and COD (50–70 g/L) of CW, due to lactose, can lead to eutrophication, toxicity, and impermeabilization as well as an increase in treatment costs [13,14]. Moreover, Yang et al. [15] reported that high COD in wastewater significantly affected the emission of GHGs such as CO2 and CH4. To prevent environmental pollution caused by CW and realize CE, studies related to the possibility of the innovative application of CW in biorefineries have been conducted, and various studies in the literature have demonstrated the potential of CW as a sustainable feedstock by producing value-added substances such as hydrogen, polyhydroxyalkanoates, lactic acid, and bacterial cellulose [16,17,18,19]. Therefore, biorefineries utilizing the lactose in CW as a feedstock are expected to be a viable approach for realizing a sustainable CE.
Lactose, a major component of carbohydrates in cheese whey, is a disaccharide in which glucose and galactose are linked by β-1,4-glycosidic bonds [19]. Representative technologies for converting the carbohydrates in biomass into monosaccharides, which microorganisms can metabolize, include acid and enzymatic hydrolysis [20,21]. The advantage of acid hydrolysis over enzymatic hydrolysis is that it does not involve pretreatment, and hydrolysis occurs at a fast hydrolysis rate, thereby reducing the overall sugar conversion cost [22]. In the acid hydrolysis of lactose, H+ ions in the acid solutions break the β-1,4-glycosidic bonds of lactose to produce glucose and galactose in the presence of water at a temperature of 100–150 °C [23]. This converted monosaccharide can be utilized as a carbon source for microbial fermentation.
Cyclosporine (Cs) is a cyclic undecapeptide, first isolated from Tolypocladium inflatum [24]. Currently, Cs is used as an immunosuppressant and has been commercialized with the approval of the U.S. Food and Drug Administration (FDA) for the treatment of dry eye syndrome and transplant rejection [25,26]. The global Cs market is forecasted to grow to USD 6.1 billion in 2027, at a compound annual growth rate (CAGR) of 15.0% from 2020 to 2027 [27]. Cyclosporin A (CsA) exhibits outstanding pharmacological activity among various isolated natural Cs derivatives (A to I and K to Z) [28]. CsA has been reported to have a cytokine storm preventive effect [29], and recently, studies have been conducted to assess the applicability of this effect on the inhibition of the cytokine storm caused by COVID-19 [30]. CsA is primarily produced through microbial fermentation, a more economical method than enzymatic synthesis [31]. Microorganisms such as T. inflatum, Neocosmospora vasinfecta, Aspergillus fumigatus and Fusarium solani are known to produce CsA, but among them, T. inflatum has been reported to have the highest productivity [32]. Since the use of purified carbon sources for microbial fermentation is the main cause of cost increases, it is necessary to search for inexpensive carbon sources to replace them [33]. However, except for wheat bran, few studies in the literature have reported on the utilization of biomass as an alternative carbon source for CsA production [32].
In this study, cheese whey powder (CWP), a food waste generated in large quantities by the dairy industry, was valorized for use as a sustainable feedstock for biorefineries. Although CWP is a useful resource containing a variety of nutrients, it is difficult to control its quality. Therefore, to ensure the reproducibility of scientific experiments, acid hydrolysis conditions (acid type; acid concentration; biomass loading) were derived using refined whey powder (WP), and the optimized hydrolysis conditions were then applied to CWP. CWP hydrolysates were utilized for CsA production as the carbon sources for T. inflatum ATCC 34921 fermentation, and commercial glucose and WP hydrolysates were used as control groups. Finally, the overall process for CsA production from CWP by T. inflatum ATCC 34921 fermentation was evaluated using mass balance based on 100 g of CWP. To the best of the author’s knowledge, this is the first study to produce CsA using CW.
2. Materials and Methods
2.1. Materials
Cheese whey (CW) was collected from a cheese manufacturer (Cheeseflo, Seoul, Korea) and stored in a 4 °C refrigerator. The collected CW was centrifuged at 13,000 rpm for 20 min, dried at 60 °C for 48 h and again dried at 105 °C for 2 h completely remove moisture. Glucose, ethyl acetate, acetonitrile, calcium carbonate (CaCO3), hydrochloric acid (HCl), nitric acid (HNO3), sulfuric acid (H2SO4), ammonium sulfate ((NH4)2SO4), potassium phosphate monobasic (KH2PO4), magnesium sulfate (MgSO4), and calcium chloride (CaCl2) were purchased from Daejung Chemicals (Gyeonggi-do, Korea). Whey powder (WP) from bovine milk, TWEEN® 80, zinc sulfate heptahydrate (ZnSO4·7H2O), manganese(II) chloride tetrahydrate (MnCl2·4H2O), sodium molybdate (NaMoO4), copper(II) sulfate pentahydrate (CuSO4·5H2O), and Iron(II) sulfate heptahydrate (FeSO4·7H2O) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Yeast extract, malt extract, and potato dextrose agar were purchased from BD Difco™ (Sparks, MD, USA). All reagents were of analytical grade and used without further purification.
2.2. Acid Hydrolysis of Whey Powder
The effects of acid type and concentration on glucose recovery from WP were investigated as follows. 15 g WP was mixed with 150 mL of different acid solution (HCl, HNO3, and H2SO4) at various concentrations (1 to 5%, w/w) in a 500 mL Erlenmeyer flask. The concentrations of all the acid solutions were controlled by measuring the specific gravity using a gravity meter. The mixtures were then allowed to react in an autoclave at 121 °C for 20 min. Biomass loading was determined as follows: Different amounts of biomass (10 to 120 g/L) were added to 150 mL of the acid solution obtained above in a 500 mL Erlenmeyer flask and then allowed to react at 121 °C for 20 min in an autoclave. The hydrolysates of WP and CWP, for use as carbon sources for CsA production, were prepared by adding the determined types and concentrations of acid solutions and biomass loading to WP and CWP and allowing them to react at 121 °C for 20 min.
2.3. Cyclosporin A Production Using Cheese Whey Hydrolysates
T. inflatum ATCC 34921, for CsA production, was obtained from American Type Culture Collection, USA. The spore suspension was obtained from a potato dextrose agar (PDA) slant using 0.08% of TWEEN® 80 after 10 days of cultivation and stored in 50% glycerol at –80 °C deep freezer. The seed culture was prepared by inoculating 1 mL of spore suspension in a 250 mL Erlenmeyer flask containing 50 mL of a YM medium (20 g/L yeast extract; 4 g/L malt extract) and incubating at 27 °C for 5 days in a shaking incubator at 200 rpm. The seed suspension (2 mL) was inoculated into 50 mL of the main culture medium kept in a 250 mL Erlenmeyer flask and incubated at 27 °C for 12 days in a shaking incubator at 200 rpm. To investigate the effect of glucose concentration, 1 to 5% (w/v) of glucose was added to the main culture as the carbon source. The main culture medium for CsA production contained 10 to 50 g/L glucose; 10 g/L (NH4)2SO4; 0.75 g/L KH2PO4; 0.5 g/L MgSO4; 0.1 g/L CaCl2; and 1 mL trace element solution. The trace element solution was prepared by adding 4400 mg ZnSO4·7H2O, 180 mg MnCl2·4H2O, 25 mg NaMoO4, 80 mg CuSO4·5H2O, and 5000 mg FeSO4·7H2O to 1 L deionized water; the initial pH of the main culture medium was adjusted to 5.5. The WP and CWP hydrolysates were used as carbon sources by replacing them with the main culture medium. The hydrolysates were concentrated using a rotary evaporator to determine the optimum glucose concentration required for CsA production. After this process, the CsA was extracted using ethyl acetate. The same amount of ethyl acetate as that of the main culture medium (50 mL) was added to a 250 mL Erlenmeyer flask and extracted at 200 rpm for 24 h. Then, the mixture was centrifuged at 13,000 rpm for 10 min to obtain a pure ethyl acetate layer containing CsA. Ethyl acetate (1 mL) containing CsA was mixed with 1 mL of acetonitrile for utilization in high-performance liquid chromatography (HPLC) analysis.
2.4. Analytical Methods
The carbohydrate composition of WP and CWP was determined using the National Renewable Energy Laboratory (NREL) analysis method [34]. Briefly, 0.3 g of WP and CWP was soaked in 3 mL of 72% (w/w) H2SO4 and allowed to react at 30 °C for 2 h in a water bath. After the reaction, distilled water was added to adjust the acid concentration to 4% (w/w), and the mixture was autoclaved at 121 °C for 1 h. The reaction mixture was then neutralized with CaCO3. The supernatant of the neutralized mixture was filtered through a 0.22 μm syringe filter for HPLC analysis. Glucose concentration was analyzed using HPLC equipped with a refractive index detector (RID-10A, Shimadzu, Japan). The analysis conditions were as follow: Shodex SUGAR SH1011 H+ ion exclusion column (300 mm × 8 mm, Shodex, Japan); mobile phase, 0.005 N H2SO4; flow rate, 0.6 mL/min; temperature of the column, 50 °C; and injection volume, 20 μL. CsA concentration was determined using an HPLC system equipped with a diode array detector at a wavelength of 260 nm (Primaide 1430, Hitachi, Japan). Analytical conditions were as follow: XBridge C18 column (5.0 μm, 4.6 mm × 250 mm, Waters, Milford, MA, USA); mobile phase, 0.01% (v/v) phosphoric acid in 70% (v/v) acetonitrile; flow rate, 0.8 mL/min; temperature of the column, 70 °C; and injection volume, 10 μL.
3. Results and Discussion
3.1. Effect of Acid Type and Concentration on Glucose Recovery from Whey Powder
In the fundamental study, the carbohydrate contents in whey powder (WP, purchased from Sigma-Aldrich) and cheese whey powder (CWP, industrial waste) were found to be 60.5% (27.7% glucan and 32.8% galactan) and 65.4 % (29.0% glucan and 36.4% galactan), respectively. In addition, in the fundamental study stage, it was confirmed that galactose does not significantly affect CsA production by T. inflatum ATCC 34921 (data not shown). These results agreed with Survase et al.’s report [35] that galactose did not significantly affect CsA production, thus galactose from WP and CWP was not considered in this study. By applying a conversion factor of 1.1 to replace glucan with glucose [36], the theoretical maximum glucose recovery based on 100 g of WP and CWP was estimated to be approximately 30.5 g and 31.9 g, respectively. To investigate the effects of different acids and their concentrations on glucose recovery from WP, different concentrations (1–5%, w/w) of three acid solutions, namely HCl, HNO3, and H2SO4, were used for acid hydrolysis of WP. The results are shown in Figure 1. Compared to H2SO4, HCl permeates biomass more easily and is easier to recover after treatment owing to its volatility [37]. The glucose recovery after acid hydrolysis of WP with different concentrations of HCl solutions was as follows: 68.5% with 1% HCl, 71.1% with 2% HCl, 64.3% with 3% HCl, 59.7% with 4% HCl, and 53.9% with 5% HCl. Treatment with HCl concentrations above 2% consistently decreased glucose recovery. Treatment with HNO3 results in a higher hydrolysis yield of biomass and less corrosive to equipment than other acid solutions [38]. In acid hydrolysis of WP by HNO3 solutions, glucose recovery decreased remarkably as the concentration of HNO3 solutions increased; the results for glucose recovery were as follows: 61.9% with 1% HNO3, 56.2% with 2% HNO3, 52.3% with 3% HNO3, 48.2% with 4% HNO3, and 37.1% with 5% HNO3. According to Zhang et al. [39], H2SO4 is less corrosive, less toxic, and cheaper than HCl, which makes it more economical for acid hydrolysis of biomass than other acids. In acid hydrolysis of WP with H2SO4, the glucose recovery rate increased proportionately to the H2SO4 concentration (43.4% with 1% H2SO4, 51.5% with 2% H2SO4, 55.7% with 3% H2SO4, and 57.6% with 4% H2SO4) and then decreased at a 5% H2SO4 concentration to 52.4%. Therefore, a suitable acid solution and concentration for maximum glucose recovery from WP was found to be 2% HCl, and the treatment resulted in 71.1% of the theoretical maximum glucose recovery (21.7 g glucose/100 g WP).
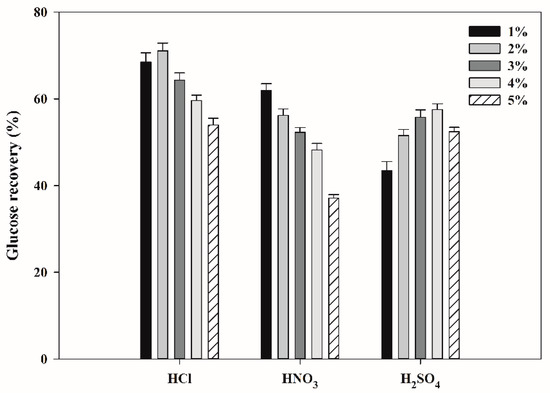
Figure 1.
Effects of acid type and concentration on glucose recovery from whey powder.
3.2. Determination of Biomass Loading for Maximal Glucose Recovery from Whey Powder
Biomass loading is a crucial factor for the economic feasibility of a process at the commercial scale, determining the power demand in downstream processes, including hydrolysis and fermentation [40]. To investigate the effective biomass loading for glucose recovery, WP was hydrolyzed using 2% HCl solution optimized above; the results are shown in Figure 2. Glucose recovery according to different biomass loadings was as follows: 84.8% at 10 g/L, 84.3% at 20 g/L, 83.7% at 30 g/L, 83.2% at 40 g/L, 83.0% at 50 g/L, 74.3% at 60 g/L, 74.8% at 70 g/L, 73.6% at 80 g/L, 71.6% at 90 g/L, 71.1% at 100 g/L, 62.2% at 110 g/L, and 55.2% at 120 g/L. Glucose recovery remained above 80% without significant change until biomass loading was increased to 50 g/L. As biomass loading increased to 60 g/L, the glucose recovery decreased slightly to around 70%, which did not significantly change until biomass loading was 100 g/L. The glucose recovery decreased rapidly when biomass loading exceeded 100 g/L. In the acid hydrolysis of biomass, a decrease in glucose recovery with increasing biomass loading has also been reported in various studies in the literature [41,42]. A high biomass loading increases viscosity, thereby inhibiting efficient mixing and mass transfer, and ultimately reducing glucose recovery [43]. On the other hand, a high biomass loading can reduce overall process cost and ecological footprint [44]. In this study, the appropriate biomass loading was determined to be 50 g/L, which satisfied both the maximum glucose recovery and high biomass loading. The final glucose recovery at this time was found to be 83.0% of the theoretical maximum (25.3 g glucose/100 g WP).
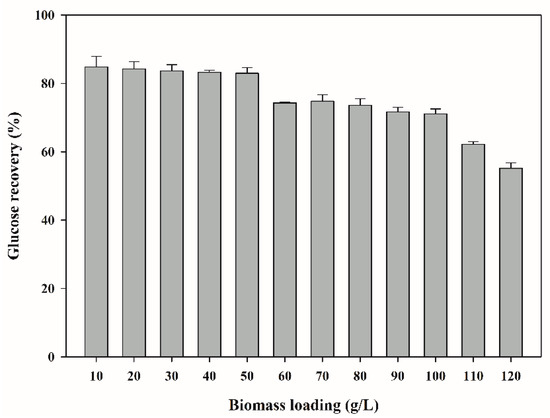
Figure 2.
Effect of biomass loading on glucose recovery from whey powder.
3.3. Cyclosporin A Production by Tolypocladium inflatum ATCC 34921
The initial concentration of the carbon source can affect microbial viability and productivity of fermentation products [45]. Paradoxically, the presence of an excessive carbon source may inhibit its utilization efficiency, cell growth, and cell viability [46]. Therefore, it is necessary to determine the appropriate carbon source concentration for the fermentation of the final product in the designed reaction system. To determine the appropriate concentration in the medium, the effect of glucose concentration on CsA production was investigated in the main medium, using commercial glucose as the carbon source (Figure 3). The results of CsA production by T. inflatum ATCC 34921 fermentation at different glucose concentrations (1–5%, w/v) were as follows: 31.0 mg/L at 1% glucose, 36.3 mg/L at 2% glucose, 34.0 mg/L at 3% glucose, 33.4 mg/L at 4% glucose, and 33.9 mg/L at 5% glucose. Accordingly, the initial glucose concentration in the medium for CsA production was determined to be 2% glucose, in line with highest CsA production (36.3 mg/L).
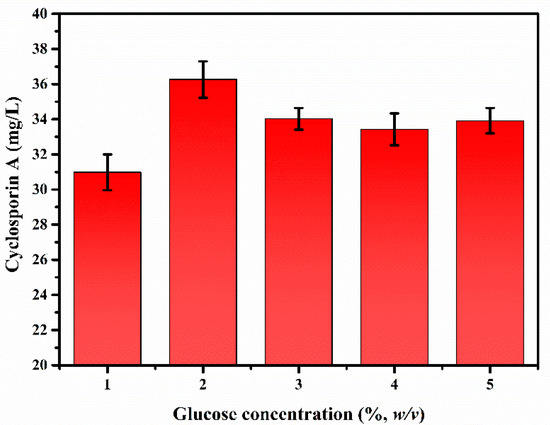
Figure 3.
Effect of glucose concentration on cyclosporin A production by Tolypocladium inflatum ATCC 34921.
Figure 4 shows the fermentation profile of T. inflatum ATCC 34921 during CsA production in the main medium using, commercial glucose as the carbon source at a concentration of 2% (w/v). Glucose in the medium decreased steadily and was almost completely consumed after 12 days. The production of CsA markedly increased after 2 days, steadily enhanced from 30.9 mg/L at 6 days to 36.3 mg/L at 12 days. Dry cell weight (DCW) increased rapidly until 4 days; after 6 days, DCW showed a tendency to stabilize, reaching 11.8 g/L at 12 days, similar to CsA production. CsA is produced when mycelium is formed. The maximum production of CsA was exhibited at maximum mycelium growth, and similar results have been reported in other studies [47,48,49]. Sporulation usually occurs under severe conditions. The development of conidiospores can inhibit CsA production. At 12 days, conidiospores were formed as glucose was completely consumed, and it was confirmed that the CsA production reached the maximum (Figure 5).
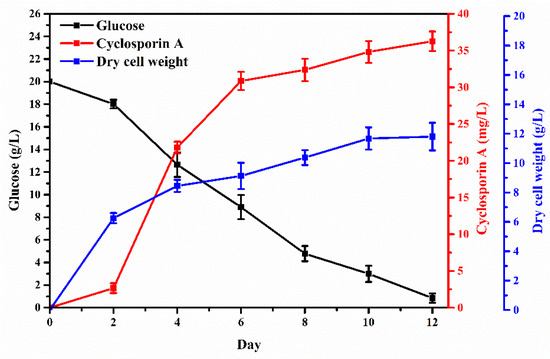
Figure 4.
Fermentation profiling of Tolypocladium inflatum ATCC 34921 for cyclosporin A production in the main medium (carbon source: 2% (w/v) commercial glucose).
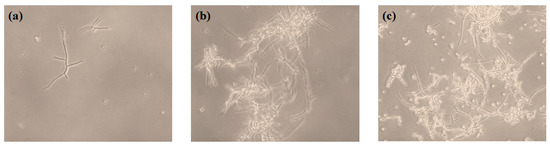
Figure 5.
Morphologies of Tolypoclzdium inflatum ATCC 34921 growth. 2 days (a), 8 days (b), and 12 days (c).
The use of commercial glucose as a carbon source in biorefineries is a major stumbling block to achieving economic feasibility because it increases the overall process cost [50]. To design a sustainable CsA production process, the potential of CWP to replace commercial glucose as the carbon source was investigated. To utilize CWP in CsA production, the lactose in CWP should be converted to glucose, a monosaccharide that can be metabolized by T. inflatum. Acid hydrolysis of CWP, an industrial waste, was performed by applying acid hydrolysis conditions derived from WP (purchased from Sigma-Aldrich) hydrolysis, and the acid hydrolysis conditions are as follows: 2% (w/w) HCl solution; 121 °C; 20 min; 50 g/L of biomass loading. Figure 6 shows the results of CsA production by T. inflatum ATCC 34921 fermentation using three carbon sources (commercial glucose, WP hydrolysates, and CWP hydrolysates). CsA production was found to be 36.3 mg/L from commercial glucose, 62.4 mg/L from WP hydrolysates, and 51.3 mg/L from CWP hydrolysates, respectively. The DCW of T. inflatum ATCC 34921 was determined to be 11.8 mg/L for commercial glucose, 15.2 mg/L for WP hydrolysates, and 13.9 mg/L for CWP hydrolysates, respectively. Both WP hydrolysates and CWP hydrolysates have been demonstrated to be more profitable for CsA production than commercial glucose. This is presumably due to the effect of whey proteins. Whey protein is composed of various amino acids such as L-valine, L-leucine, and L-isoleucine, L-lysine. In particular, among all the constituent amino acids, L-leucine is present in the highest amont in whey protein [51,52]. According to Lee and Agathos [53], amino acids such as L-valine and L-leucine significantly affect the biosynthesis of CsA by T. inflatum. It is estimated that whey protein, which is converted to amino acids during acid hydrolysis, has a significant effect on CsA production. Dewit and Klarenbeek reported [54] that whey protein can be converted to amino acids even at high temperatures without serious damage during acid hydrolysis. Moreover, subcritical water–acid combined hydrolysis has been used to effectively recover amino acids from whey protein [55]. Severe acid hydrolysis of biomass can cause the generation of phenolic compounds, such as furfural and 5-Hydroxymethylfurfural, which are known as fermentation inhibitors [56,57]. During CsA production using CWP hydrolysates, the high DCW of T. inflatum ATCC 34921 demonstrated that the CWP hydrolysates had no fermentation inhibitory effect. In conclusion, CWP hydrolysates showed a 1.4-fold increase in CsA production, at 51.3 mg/L, compared to commercial glucose (36.3 mg/L). These results indicate that CWP has potential as a sustainable and inexpensive carbon source for CsA production. The lower CsA production in CWP than in WP led us to speculate that CsA production can be further improved when CWP was refined.
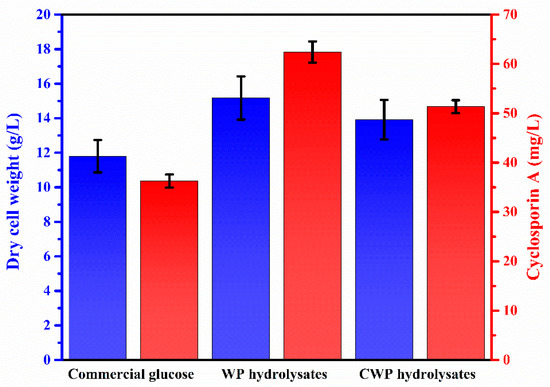
Figure 6.
Cyclosporin A production by Tolypocladium inflatum ATCC 34921 using three carbon sources (commercial glucose, whey powder (WP) hydrolysates, and cheese whey power (CWP) hydrolysates).
3.4. Evaluation of Overall Process for Cyclosporin A Production
The overall process of CsA production from CWP was evaluated using a mass balance based on 100 g of CWP (Figure 7). In the fundamental experiment, CW was found to be composed of 96% moisture and 4% solid fraction, and this mass balance was designed based on the solid fraction. It was found that 100 g of CWP contained 66.0 g of carbohydrate, consisting of 29.0 g of glucan and 36.4 g of galactan. Furthermore, 100 g of CWP was converted to 31.9 g of glucose via acid hydrolysis under the following conditions: 2% (w/w) HCl solution, temperature of 121 °C, time of 20 min, biomass loading of 50 g/L. 40.0 g of galactose recovered under these conditions could not be used in this study because T. inflatum ATCC 34921 cannot metabolize it. New biorefinery strategies are required to convert galactose into value-added substances via microbial fermentation. The recovered 31.9 g of glucose was utilized as a carbon source for CsA production, and T. inflatum ATCC 34921 was fermented in a medium consisting of 20 g/L carbon source, 10 g/L (NH4)2SO4, 0.75 g/L KH2PO4, 0.5 g/L MgSO4, 0.1 g/L CaCl2, and 1 mL trace element solution at 27 °C for 12 days with constant shaking at 200 rpm. Commercial glucose was used as the control to compare CsA production. Approximately 81.8 mg of CsA was produced from glucose converted from CWP, a 1.4-fold increase over 57.9 mg produced from commercial glucose. In conclusion, 31.9 g glucose was recovered from 100 g CWP and converted to 81.8 mg CsA.
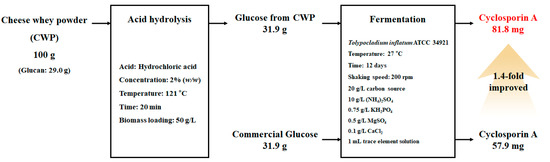
Figure 7.
Mass balance of the overall process for cyclosporin production from cheese whey powder.
4. Conclusions
In this study, we converted cheese whey powder (CWP) to cyclosporin A (CsA), which is used as an immunosuppressant and has attracted attention as a potential inhibitor of the cytokine storm caused by COVID-19. Our study demonstrated that CWP hydrolysates produced higher amounts of CsA than commercial glucose via Tolypocladium inflatum ATCC 34921 fermentation. This was attributed to the effects of amino acids in CWP. We plan to investigate amino acid profiling in the CWP and determine its effect on CSA production in a follow-up study. In this study, we focused on replacing refined carbon sources with inexpensive and sustainable carbon sources, regardless of fermentation parameters such as nitrogen source, pH, and solid-state fermentation, which have a significant impact on CsA production. A study on the optimization of fermentation conditions in consideration of various fermentation parameters is expected to have a significant positive effect on improving CsA productivity. In addition, CWP hydrolysates were found to contain unutilized galactose; plans are afoot to utilize galactose in the near future. Our study could provide useful directions towards the development of sustainable biorefineries for converting food waste into value-added products.
Author Contributions
Conceptualization, H.R.K. and K.H.L.; methodology, Y.C. and S.K.L.; software, J.H.L.; validation, S.W.K. and H.Y.Y.; formal analysis, H.R.K. and K.H.L.; investigation, J.H.L.; data curation, Y.C.; writing—original draft preparation, H.R.K. and K.H.L.; writing—review and editing, S.W.K. and H.Y.Y.; visualization, S.K.L.; supervision, S.W.K. and H.Y.Y.; project administration, S.W.K. and H.Y.Y.; funding acquisition, H.Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) NRF-2020R1C1C1005060.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alamar, M.D.C.; Falagán, N.; Aktas, E.; Terry, L.A. Minimising Food Waste: A Call for Multidisciplinary Research. J. Sci. Food Agric. 2018, 98, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, M.; Yue, S.Y.; Zheng, T.L.; Gao, Z.; Ma, X.Y.; Wang, Q.H. Global trends and future prospects of food waste research: A bibliometric analysis. Environ. Sci. Pollut. Res. 2018, 25, 24600–24610. [Google Scholar] [CrossRef]
- O’Connor, J.; Hoang, S.A.; Bradney, L.; Dutta, S.; Xiong, X.; Tsang, D.C.W.; Ramadass, K.; Vinu, A.; Kirkham, M.B.; Bolan, N.S. A review on the valorisation of food waste as a nutrient source and soil amendment. Environ. Pollut. 2021, 272, 115985. [Google Scholar] [CrossRef] [PubMed]
- Dou, Z.; Toth, J.D.; Westendorf, M.L. Food waste for livestock feeding: Feasibility, safety, and sustainability implications. Glob. Food Secur. 2018, 17, 154–161. [Google Scholar] [CrossRef]
- Edwards, J.; Othman, M.; Crossin, E.; Burn, S. Life cycle assessment to compare the environmental impact of seven contemporary food waste management systems. Bioresour. Technol. 2018, 248, 156–173. [Google Scholar] [CrossRef]
- Corona, B.; Shen, L.; Reike, D.; Carreón, J.R.; Worrell, E. Towards sustainable development through the circular economy—A review and critical assessment on current circularity metrics. Resour. Conserv. Recycl. 2019, 151, 104498. [Google Scholar] [CrossRef]
- Ebikade, E.O.; Sadula, S.; Gupta, Y.; Vlachos, D.G. A review of thermal and thermocatalytic valorization of food waste. Green Chem. 2021, 23, 2806–2833. [Google Scholar] [CrossRef]
- Ebikade, E.; Athaley, A.; Fisher, B.; Yang, K.; Wu, C.; Ierapetritou, M.G.; Vlachos, D.G. The Future is Garbage: Repurposing of Food Waste to an Integrated Biorefinery. ACS Sustain. Chem. Eng. 2020, 8, 8124–8136. [Google Scholar] [CrossRef]
- Domingos, J.M.B.; Martinez, G.A.; Scoma, A.; Fraraccio, S.; Kerckhof, F.-M.; Boon, N.; Reis, M.A.M.; Fava, F.; Bertin, L. Effect of Operational Parameters in the Continuous Anaerobic Fermentation of Cheese Whey on Titers, Yields, Productivities, and Microbial Community Structures. ACS Sustain. Chem. Eng. 2016, 5, 1400–1407. [Google Scholar] [CrossRef]
- Reddy, M.B.; Reddy, V.U.N.; Chang, Y. Integration of anaerobic digestion and chain elongation technologies for biogas and carboxylic acids production from cheese whey. J. Clean. Prod. 2022, 364, 132670. [Google Scholar] [CrossRef]
- Dragone, G.; Mussatto, S.I.; Oliveira, J.M.; Teixeira, J.A. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 2009, 112, 929–935. [Google Scholar] [CrossRef]
- Papirio, S.; Matassa, S.; Pirozzi, F.; Esposito, G. Anaerobic co-digestion of cheese whey and industrial hemp residues opens new perspectives for the valorization of agri-food waste. Energies 2020, 13, 2820. [Google Scholar] [CrossRef]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef] [PubMed]
- Mansor, E.S.; Ali, E.A.; Shaban, A. Tight ultrafiltration polyethersulfone membrane for cheese whey wastewater treatment. Chem. Eng. J. 2021, 407, 127175. [Google Scholar] [CrossRef]
- Yang, W.B.; Yuan, C.S.; Huang, B.Q.; Tong, C.; Yang, L. Emission Characteristics of Greenhouse Gases and Their Correlation with Water Quality at an Estuarine Mangrove Ecosystem—The Application of an In-Situ On-site NDIR Monitoring Technique. Wetlands 2018, 38, 723–738. [Google Scholar] [CrossRef]
- Policastro, G.; Cesaro, A.; Fabbricino, M. Photo-Fermentative Hydrogen Production from Cheese Whey: Engineering of a Mixed Culture Process in a Semi-Continuous, Tubular Photo-Bioreactor. Int. J. Hydrogen Energy 2022, 47, 10665–10688. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Reddy, M.V.; Imura, K.; Onodera, R.; Kamada, N.; Sano, Y. Two-Stage Polyhydroxyalkanoates (PHA) Production from Cheese Whey Using Acetobacter pasteurianus C1 and Bacillus sp. CYR1. Bioengineering 2021, 8, 157. [Google Scholar] [CrossRef]
- Costa, S.; Summa, D.; Semeraro, B.; Zappaterra, F.; Rugiero, I.; Tamburini, E. Fermentation as a Strategy for Bio-Transforming Waste into Resources: Lactic Acid Production from Agri-Food Residues. Fermentation 2021, 7, 3. [Google Scholar] [CrossRef]
- Lappa, I.K.; Kachrimanidou, V.; Papadaki, A.; Stamatiou, A.; Ladakis, D.; Eriotou, E.; Kopsahelis, N. A Comprehensive Bioprocessing approach to Foster Cheese Whey Valorization: On-Site-Galactosidase Secretion for Lactose Hydrolysis and Sequential Bacterial Cellulose Production. Fermentation 2021, 7, 184. [Google Scholar] [CrossRef]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid hydrolysis of lignocellulosic biomass: Sugars and furfurals formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, S.K.; Lee, J.; Kim, S.; Kim, S.W.; Park, C.; Yoo, H.Y. Energy-efficient glucose recovery from chestnut shell by optimization of NaOH pretreatment at room temperature and application to bioethanol production. Environ. Res. 2022, 208, 112710. [Google Scholar] [CrossRef] [PubMed]
- Lenihan, P.; Orozco, A.; O’Neill, E.; Ahmad, M.N.M.; Rooney, D.W.; Walker, G.M. Dilute acid hydrolysis of lignocellulosic biomass. Chem. Eng. J. 2010, 156, 395–403. [Google Scholar] [CrossRef]
- Gajaendragaadkar, C.N.; Gogate, P.R. Ultrasound assisted acid catalyzed lactose hydrolysis: Understanding into effect of operating parameters and scale up studies. Ultrason. Sonochem. 2017, 37, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Baranova, A.A.; Chistov, A.A.; Shuvalov, M.V.; Tyurin, A.P.; Biryukov, M.V.; Ivanov, I.A.; Sadykova, V.S.; Kurakov, A.V.; Sergeeva, A.I.; Korshun, V.A.; et al. Identification of isocyclosporins by collision-induced dissociation of doulbly protonated species. Talanta 2021, 225, 121930. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Rupenthal, I.D. Modern approaches to the ocular delivery of cyclosporine A. Drug Discov. Today 2016, 21, 977–988. [Google Scholar] [CrossRef] [PubMed]
- Archer, T.M.; Boothe, D.M.; Langston, V.C.; Fellman, C.L.; Lunsford, K.V.; Mackin, A.J. Oral Cyclosporine Treatment in Dogs: A Review of the Literature. J. Vet. Intern. Med. 2013, 28, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Tripathi, P.; Gupta, A.; Yadav, J.S. A comprehensive review on possibilities of treating psoriasis using dermal cyclosporine. Drug Deliv. Transl. Res. 2022, 12, 1541–1555. [Google Scholar] [CrossRef]
- Ganjoo, A.; Sharma, N.; Shafeeq, H.; Bhat, N.A.; Dubey, K.K.; Babu, V. Progress and challenges in the biofoundry of immunosuppressants: From process to practice. Biotechnol. Bioeng. 2022, 119, 3339–3369. [Google Scholar] [CrossRef]
- Choudhary, S.; Sharma, K.; Silakari, O. The interplay between inflammatory pathways and COVID-19: A critical review on pathogenesis and therapeutic options. Microb. Pathog. 2021, 150, 104673. [Google Scholar] [CrossRef] [PubMed]
- Fenizia, C.; Galbiati, S.; Vanetti, C.; Vago, R.; Clerici, M.; Tacchetti, C.; Tiziana, D.T. Cyclosporine A Inhibits Viral Infection and Release as Well as Cytokine Production in Lung Cells by Three SARS-CoV-2 Variants. Microbiol. Spectr. 2022, 10, e0150421. [Google Scholar] [CrossRef]
- Falah, F.; Vasiee, A.; Ramezani, M.; Tabatabaee-Yazdi, F.; Mortazavi, S.A.; Danesh, A. Effect of immobilization, mutation, and microbial stresses on increasing production efficiency of “Cyclosporin A”. Biomass Convers. Biorefinery 2022, 1–16. [Google Scholar] [CrossRef]
- Ramana Murthy, M.V.; Mohan, E.V.S.; Sadhukhan, A.K. Cyclosporin-A production by Tolypocladium inflatum using solid state fermentation. Process Biochem. 1999, 34, 269–280. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.W.; Lee, J.; Kim, S.; Park, C.; Yoo, H.Y. Statistical Optimization of Alkali Pretreatment to Improve Sugars Recovery from Spent Coffee Grounds and Utilization in Lactic Acid Fermentation. Processes 2021, 9, 494. [Google Scholar] [CrossRef]
- Jang, Y.W.; Lee, K.H.; Yoo, H.Y. Improved Sugar Recovery from Orange Peel by Statistical Optimization of Thermo-Alkaline Pretreatment. Processes 2021, 9, 409. [Google Scholar] [CrossRef]
- Survase, S.A.; Annapure, U.S.; Singhal, R.S. The Effect of Medium Supplementation with Second Carbon Source and Amino Acids for Enhanced Production of Cyclosporin A. Curr. Trends Biotechnol. Pharm. 2010, 4, 764–773. [Google Scholar]
- Lee, K.H.; Lee, S.K.; Lee, J.; Kim, S.; Park, C.; Kim, S.W.; Yoo, H.Y. Improvement of Enzymatic Glucose Conversion from Chestnut Shells through Optimization of KOH Pretreatment. Int. J. Environ. Res. Public Health 2021, 18, 3772. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, Y.; Suzuki, T.; Sawanishi, H.; Hasegawa, I.; Mae, K. Effective production of levulinic acid from biomass through pretreatment using phosphoric acid, hydrochloric acid, or ionic liquid. Ind. Eng. Chem. Res. 2014, 53, 11611–11621. [Google Scholar] [CrossRef]
- Dziekonska-Kubczak, U.; Berłowska, J.; Dziugan, P.; Patelski, P.; Pielech-Przybylska, K.; Balcerek, M. Nitric acid pretreatment of Jerusalem artichoke stalks for enzymatic saccharification and biorthanol production. Energies 2018, 11, 2153. [Google Scholar] [CrossRef]
- Zhang, T.; Kumara, R.; Wyman, C.E. Enhanced yields of furfural and other products by simultaneous solvent extraction during thermochemical treatment of cellulosic biomass. RSC Adv. 2013, 3, 9809–9819. [Google Scholar] [CrossRef]
- Kadhum, H.J.; Rajendran, K.; Murthy, G.S. Effect of solids loading on ethanol production: Experimental, economic and environmental analysis. Bioresour. Technol. 2017, 244, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, R.; Shende, R.; Nan, W.; Shende, A. Photocatalytic reforming of pinewood (Pinus ponderosa) acid hydrolysate for hydrogen generation. Int. J. Hydrogen Energy 2017, 42, 2839–2848. [Google Scholar] [CrossRef]
- Sanchis-Sebastiá, M.; Ruuth, E.; Stigsson, L.; Galbe, M.; Wallberg, O. Novel sustainable alternatives for the fashion industry: A method of chemically recycling waste textiles via acid hydrolysis. J. Waste Manag. 2021, 121, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Vani, S.; Sukumaran, R.K.; Savithri, S. Prediction of sugar yields during hydrolysis of lignocellulosic biomass using artificial neural network modeling. Bioresour. Technol. 2015, 188, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Kadhum, H.J.; Mahapatra, D.M.; Murthy, G.S. A novel method for real-time estimation of insoluble solids and glucose concentrations during enzymatic hydrolysis of biomass. Bioresour. Technol. 2019, 275, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Lee, W.G.; Kwon, S.; Lee, S.Y.; Chang, H.N. Succinic acid production by Anaerobiospirillum succiniciproducens: Effects of the H2/CO2 supply and glucose concentration. Enzyme Microb. Technol. 1999, 24, 549–554. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, Y.; Hu, W.; Zheng, X.; Chen, Y. Valorization of food waste fermentation liquid into single cell protein by photosynthetic bacteria via stimulating carbon metabolic pathway and environmental behavior. Bioresour. Technol. 2022, 361, 127704. [Google Scholar] [CrossRef] [PubMed]
- El Enshasy, H.; Fattah, Y.A.; Attu, A.; Anwar, M.; Omar, H.; Magd, S.A.E.; Zahra, R.A. Kinetics of Cell Growth and Cyclosporin A Production by Tolypocladium inflatum when Scailing Up from Shake Flask to Bioreactor. J. Microbiol. Biotechnol. 2008, 18, 128–134. [Google Scholar] [PubMed]
- Ismaiel, A.A. Production of the immunosuppressant cyclosporine A by a new soil isolate, Aspergillus fumigatus, in submerged culture. Appl. Microbiol. Biotechnol. 2017, 101, 3305–3317. [Google Scholar] [CrossRef]
- Survase, S.A.; Kagliwal, L.D.; Annapure, U.S.; Singhal, R.S. Cyclosporin A—A review on fermentative production, downstream processing and pharmacological applications. Biotechnol. Adv. 2011, 29, 418–435. [Google Scholar] [CrossRef]
- Son, J.; Lee, K.H.; Park, C. Enhanced Production of Bacterial Cellulose from Miscanthus as Sustainable Feedstock through Statistical Optimization of Culture Conditions. Int. J. Environ. Res. Public Health 2022, 19, 866. [Google Scholar] [CrossRef]
- Almeida, C.C.; Alvares, T.S.; Costa, M.P.; Conte-Junior, C.A. Protein and amino acid profiles of different whey protein supplements. J. Diet. Suppl. 2016, 13, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Yasmin, A.; Butt, M.S.; Sameen, A.; Shahid, M. Physicochemical and Amino Acid Profiling of Cheese Whey. Pak. J. Nutr. 2013, 12, 455–459. [Google Scholar] [CrossRef][Green Version]
- Lee, J.; Agathos, S.N. Effect of Amino Acids on the Production of Cyclosporin A by Tolypocladium inflatum. Biotechnol. Lett. 1989, 11, 77–82. [Google Scholar] [CrossRef]
- Dewit, J.N.; Klarenbeek, G. Effects of various heat-treatments on structure and solubility of whey proteins. J. Dairy Sci. 1984, 67, 2701–2710. [Google Scholar] [CrossRef]
- Espinoza, A.D.; Morawicki, R.O. Effective Additives on Subcritical Water Hydrolysis of Whey Protein Isolate. J. Agric. Food Chem. 2012, 60, 5250–5256. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, M.J.; Walker, T.W.; Dumesic, J.A.; Rankin, S.A.; Huber, G.W. Production of monosaccharides and whey protein from acid whey waste streams in the dairy industry. Green Chem. 2018, 20, 1824–1834. [Google Scholar] [CrossRef]
- Lee, J.W.; Jeffries, T.W. Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresour. Technol. 2011, 102, 5884–5890. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).