Analysis of the Influence of Microbial Community Structure on Flavor Composition of Jiang-Flavor Liquor in Different Batches of Pre-Pit Fermented Grains
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Determination of Organic Acids in Base Baijiu
2.3. Determination of the Flavor Components of the Base Baijiu
2.4. DNA Extraction and High-Throughput Sequencing of Microbial Community
2.5. Analysis of Data
3. Results
3.1. Microbial Community Diversity Analysis
3.2. Microbial Community Dynamic of Pre-Pit Fermentation Grains in Different Rounds
3.3. Analysis of Flavor Substances in Different Rounds of Base Baijiu
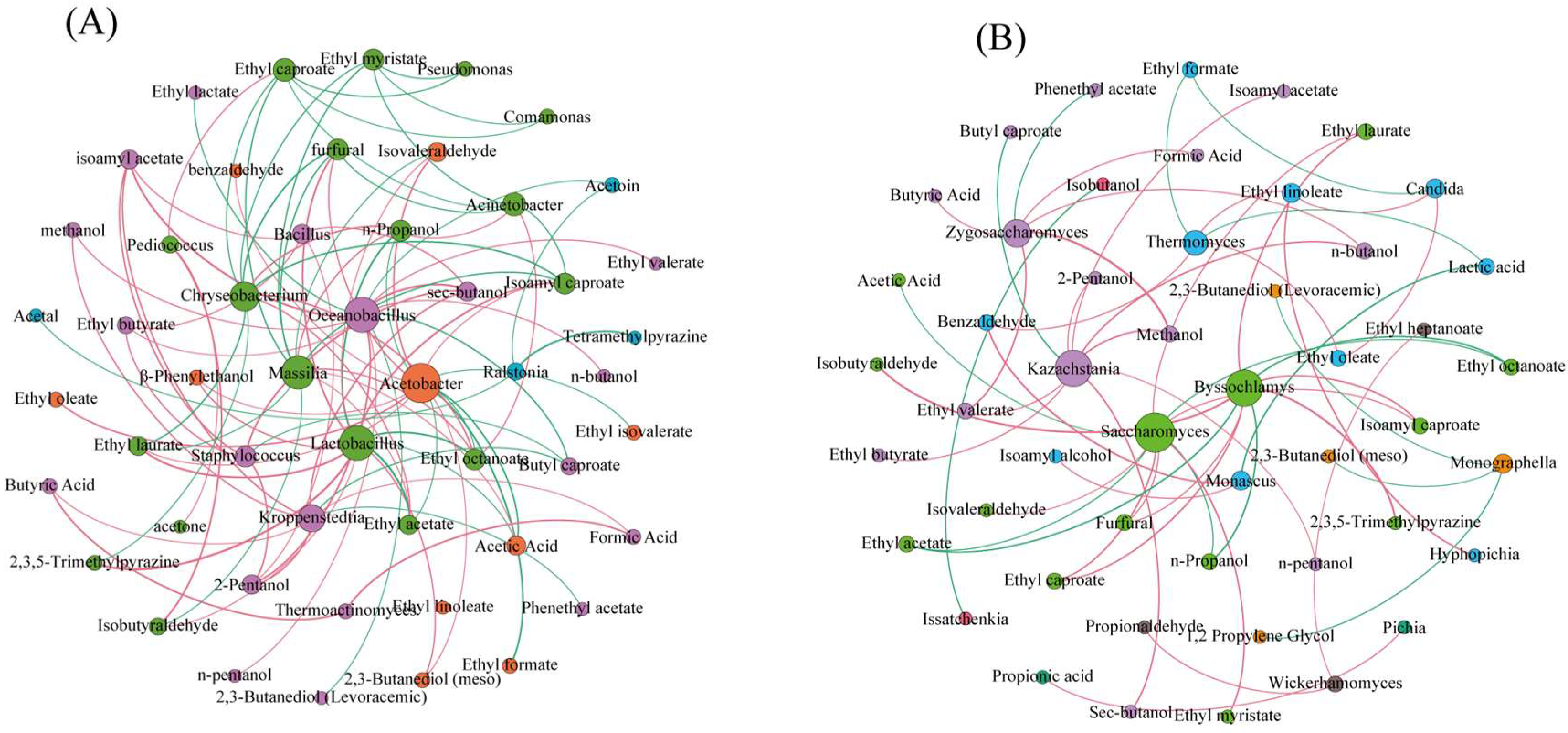
3.4. Correlation between Microbial Communities of Pre-Pit Fermentation Grains and Flavor Substances of Base Baijiu in Different Rounds
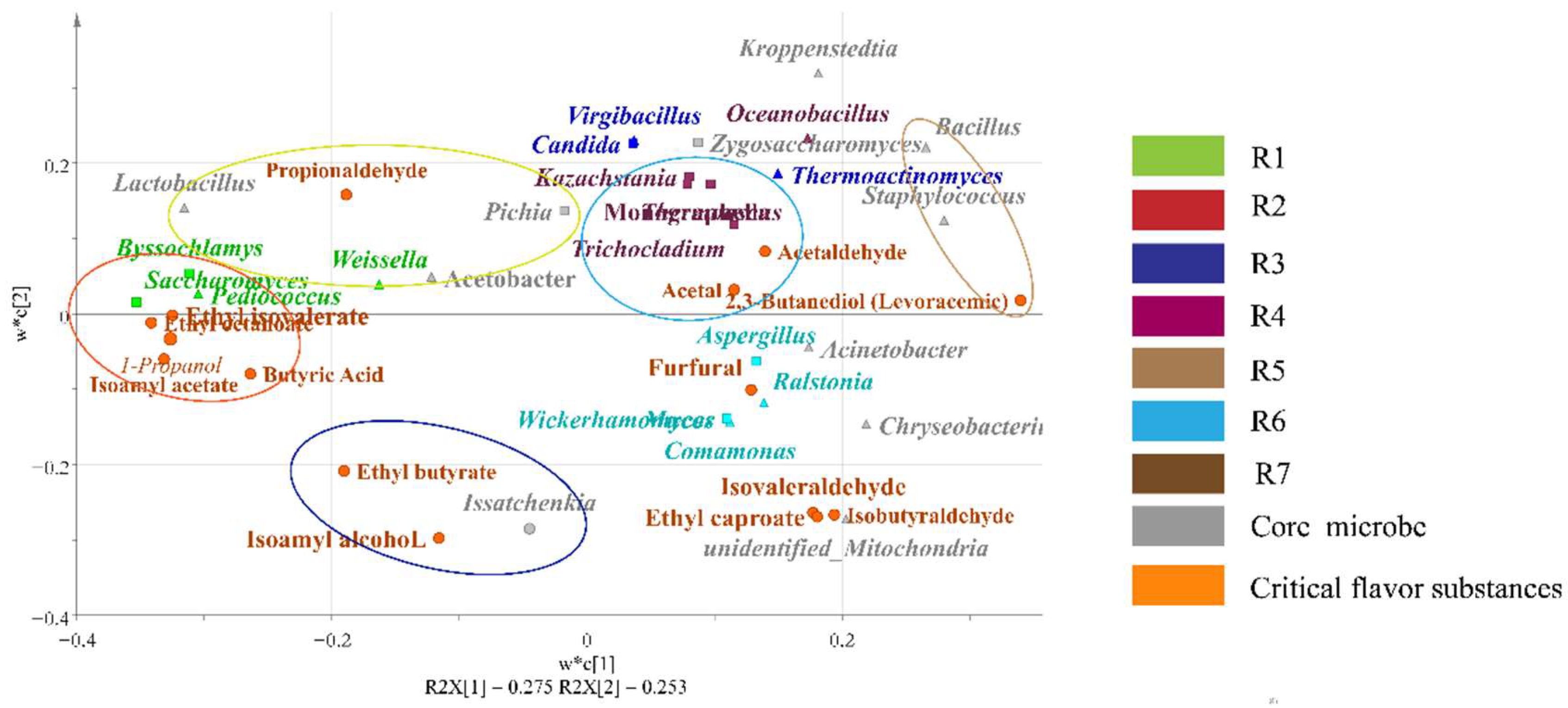
3.5. Regression Analysis of Microbial of Pre-Pit Fermentation Grains and Key Flavor Substances in Different Rounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, X.-W.; Han, B.-Z. Baijiu, Chinese Liquor: History, Classification and Manufacture. J. Ethn. Foods 2016, 3, 19–25. [Google Scholar] [CrossRef]
- Hao, H.; Yan, R.; Miao, Z.; Wang, B.; Sun, J.; Sun, B. Volatile Organic Compounds Mediated Endogenous Microbial Interactions in Chinese Baijiu Fermentation. Int. J. Food Microbiol. 2022, 383, 109955. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lu, X.; Zhang, X.; Zheng, F.; Yu, D.; Li, C.; Zheng, S.; Chen, B.; Liu, X.; Ma, M.; et al. In-Depth Profiling of Carboxyl Compounds in Chinese Baijiu Based on Chemical Derivatization and Ultrahigh-Performance Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Food Chem. X 2022, 15, 100440. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yang, S.; Li, H.; Qin, D.; Shen, Y.; Li, H.; Sun, J.; Zheng, F.; Sun, B. Why the Key Aroma Compound of Soy Sauce Aroma Type Baijiu Has Not Been Revealed Yet? LWT 2022, 154, 112735. [Google Scholar] [CrossRef]
- Kang, J.; Chen, X.; Han, B.-Z.; Xue, Y. Insights into the Bacterial, Fungal, and Phage Communities and Volatile Profiles in Different Types of Daqu. Food Res. Int. 2022, 158, 111488. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Chai, L.-J.; Fang, G.-Y.; Mei, J.-L.; Lu, Z.-M.; Zhang, X.-J.; Xiao, C.; Wang, S.-T.; Shen, C.-H.; Shi, J.-S.; et al. Spatial Heterogeneity of the Microbiome and Metabolome Profiles of High-Temperature Daqu in the Same Workshop. Food Res. Int. 2022, 156, 111298. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Y.; Huang, X.; Han, B. Microbial Diversity and Metabolites Dynamic of Light-Flavor Baijiu with Stacking Process. Fermentation 2022, 8, 67. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, Y.; Lu, Z.-M.; Chai, L.-J.; Zhang, X.-J.; Wang, S.-T.; Shen, C.-H.; Shi, J.-S.; Xu, Z.-H. Daqu Microbiota Exhibits Species-Specific and Periodic Succession Features in Chinese Baijiu Fermentation Process. Food Microbiol. 2021, 98, 103766. [Google Scholar] [CrossRef]
- Wang, H.; Huang, Y.; Huang, Y. Microbiome Diversity and Evolution in Stacking Fermentation during Different Rounds of Jiang-Flavoured Baijiu Brewing. LWT 2021, 143, 111119. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Huang, H.; Pang, Z.; Fu, Z.; Niu, J.; Zhang, C.; Li, W.; Li, X.; Sun, B. Correlation between Microbial Communities and Flavor Compounds during the Fifth and Sixth Rounds of Sauce-Flavor Baijiu Fermentation. Food Res. Int. 2021, 150, 110741. [Google Scholar] [CrossRef]
- Jin, G.; Zhu, Y.; Xu, Y. Mystery behind Chinese Liquor Fermentation. Trends Food Sci. Technol. 2017, 63, 18–28. [Google Scholar] [CrossRef]
- Li, W.; Fan, G.; Fu, Z.; Wang, W.; Xu, Y.; Teng, C.; Zhang, C.; Yang, R.; Sun, B.; Li, X. Effects of Fortification of Daqu with Various Yeasts on Microbial Community Structure and Flavor Metabolism. Food Res. Int. 2020, 129, 108837. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, L.; Xu, Y. Yeast Community Associated with the Solid State Fermentation of Traditional Chinese Maotai-Flavor Liquor. Int. J. Food Microbiol. 2013, 8, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Rui, J.; Li, J.; Zhang, S.; Yan, X.; Wang, Y.; Li, X. The Core Populations and Co-Occurrence Patterns of Prokaryotic Communities in Household Biogas Digesters. Biotechnol Biofuels 2015, 8, 158. [Google Scholar] [CrossRef]
- Lemanceau, P.; Blouin, M.; Muller, D.; Moënne-Loccoz, Y. Let the Core Microbiota Be Functional. Trends Plant Sci. 2017, 22, 583–595. [Google Scholar] [CrossRef]
- Xu, J.; Yuan, H.; Zhou, H.; Zhao, Y.; Wu, Y.; Zhang, J.; Zhang, S. A Novel Fluorescent Sensor Array to Identify Baijiu Based on the Single Gold Nanocluster Probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 284, 121787. [Google Scholar] [CrossRef]
- He, X.; Yangming, H.; Górska-Horczyczak, E.; Wierzbicka, A.; Jeleń, H.H. Rapid Analysis of Baijiu Volatile Compounds Fingerprint for Their Aroma and Regional Origin Authenticity Assessment. Food Chem. 2021, 337, 128002. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Fu, G.; Chen, Y.; Wan, Y.; Deng, M.; Cai, W.; Li, M. Improvement of the Flavor of Major Ethyl Ester Compounds during Chinese Te-Flavor Baijiu Brewing by Wickerhamomyces Anomalus. Food Biosci. 2022, 50, 102022. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Sun, B. Low Quantity but Critical Contribution to Flavor: Review of The Current Understanding of Volatile Sulfur-Containing Compounds in Baijiu. J. Food Compos. Anal. 2021, 103, 104079. [Google Scholar] [CrossRef]
- Zhu, L.; Song, X.; Li, X.; Geng, X.; Zheng, F.; Li, H.; Sun, J.; Huang, M.; Sun, B. Interactions between Kafirin and Pickle-like Odorants in Soy Sauce Flavor Baijiu: Aroma Profile Change and Binding Mechanism. Food Chem. 2023, 400, 133854. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.-T.; Lu, Z.-M.; Zhang, X.-J.; Chai, L.-J.; Shen, C.-H.; Shi, J.-S.; Xu, Z.-H. Metagenomics Unveils Microbial Roles Involved in Metabolic Network of Flavor Development in Medium-Temperature Daqu Starter. Food Res. Int. 2021, 140, 110037. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zeng, Y.; Cui, Y.; Chen, P.; Cai, K.; Guo, T.; Tan, G.; Peng, N.; Liang, Y.; Zhao, S. Unraveling the Composition and Succession of Microbial Community and Its Relationship to Flavor Substances during Xin-Flavor Baijiu Brewing. Int. J. Food Microbiol. 2022, 372, 109679. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Kang, Q.; Yin, Z.; Sun, J.; Wang, B.; Zeng, X.; Zhao, D.; Li, H.; Huang, M. Content Changes of Jiupei Tripeptide Tyr-Gly-Asp during Simulated Distillation Process of Baijiu and the Potential in Vivo Antioxidant Ability Investigation. J. Food Compos. Anal. 2021, 102, 104034. [Google Scholar] [CrossRef]
- Zhu, C.; Cheng, Y.; Zuo, Q.; Huang, Y.; Wang, L. Exploring the Impacts of Traditional Crafts on Microbial Community Succession in Jiang-Flavored Daqu. Food Res. Int. 2022, 158, 111568. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Zhang, S.; Liu, T.; Qin, H.; Shen, C.; Liu, H.; Yang, F.; Yang, C.; Yin, Q.; et al. Spatiotemporal Distribution of Environmental Microbiota in Spontaneous Fermentation Workshop: The Case of Chinese Baijiu. Food Res. Int. 2022, 156, 111126. [Google Scholar] [CrossRef]
- Du, J.; Li, Y.; Xu, J.; Huang, M.; Wang, J.; Chao, J.; Wu, J.; Sun, H.; Ding, H.; Ye, H. Characterization of Key Odorants in Langyatai Baijiu with Jian Flavour by Sensory-Directed Analysis. Food Chem. 2021, 352, 129363. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, J.; Liu, X.; Zhang, C.; Zhao, Z.; Li, X.; Sun, B. Flavor Mystery of Chinese Traditional Fermented Baijiu: The Great Contribution of Ester Compounds. Food Chem. 2022, 369, 130920. [Google Scholar] [CrossRef]
- Ding, X.; Wu, C.; Huang, J.; Zhou, R. Characterization of Interphase Volatile Compounds in Chinese Luzhou-Flavor Liquor Fermentation Cellar Analyzed by Head Space-Solid Phase Micro Extraction Coupled with Gas Chromatography Mass Spectrometry (HS-SPME/GC/MS). LWT—Food Sci. Technol. 2016, 66, 124–133. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Zhu, Y.; Ben, A.; Qi, J. A Novel Strategy for Discriminating Different Cultivation and Screening Odor and Taste Flavor Compounds in Xinhui Tangerine Peel Using E-Nose, E-Tongue, and Chemometrics. Food Chem. 2022, 384, 132519. [Google Scholar] [CrossRef]
- Jin, Y.; Li, D.; Ai, M.; Tang, Q.; Huang, J.; Ding, X.; Wu, C.; Zhou, R. Correlation between Volatile Profiles and Microbial Communities: A Metabonomic Approach to Study Jiang-Flavor Liquor Daqu. Food Res. Int. 2019, 121, 422–432. [Google Scholar] [CrossRef]
- Su, Y.; Yang, L.; Hui, L.; Yuan-Yuan, G.; Ming-Juan, Z.; Chun-Hui, X.; Ling, X.; Chi, C. Bacterial Communities during the Process of High-Temperature Daqu Production of Roasted Sesame-like Flavour Liquor: Bacterial Communities during High-Temperature Daqu Production. J. Inst. Brew. 2015, 121, 440–448. [Google Scholar] [CrossRef]
- Wu, Q.; Zhu, Y.; Fang, C.; Wijffels, R.H.; Xu, Y. Can We Control Microbiota in Spontaneous Food Fermentation?—Chinese Liquor as a Case Example. Trends Food Sci. Technol. 2021, 110, 321–331. [Google Scholar] [CrossRef]
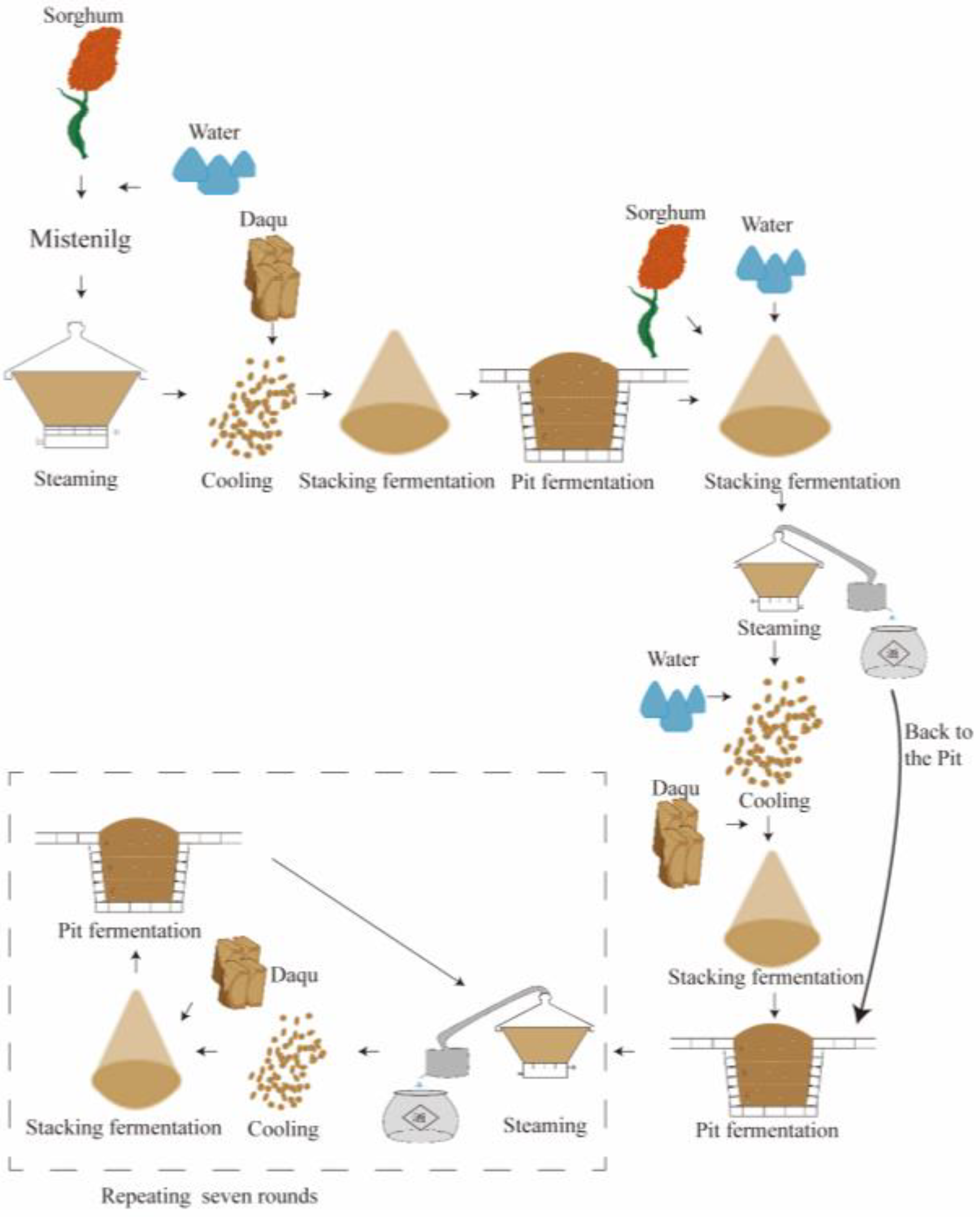
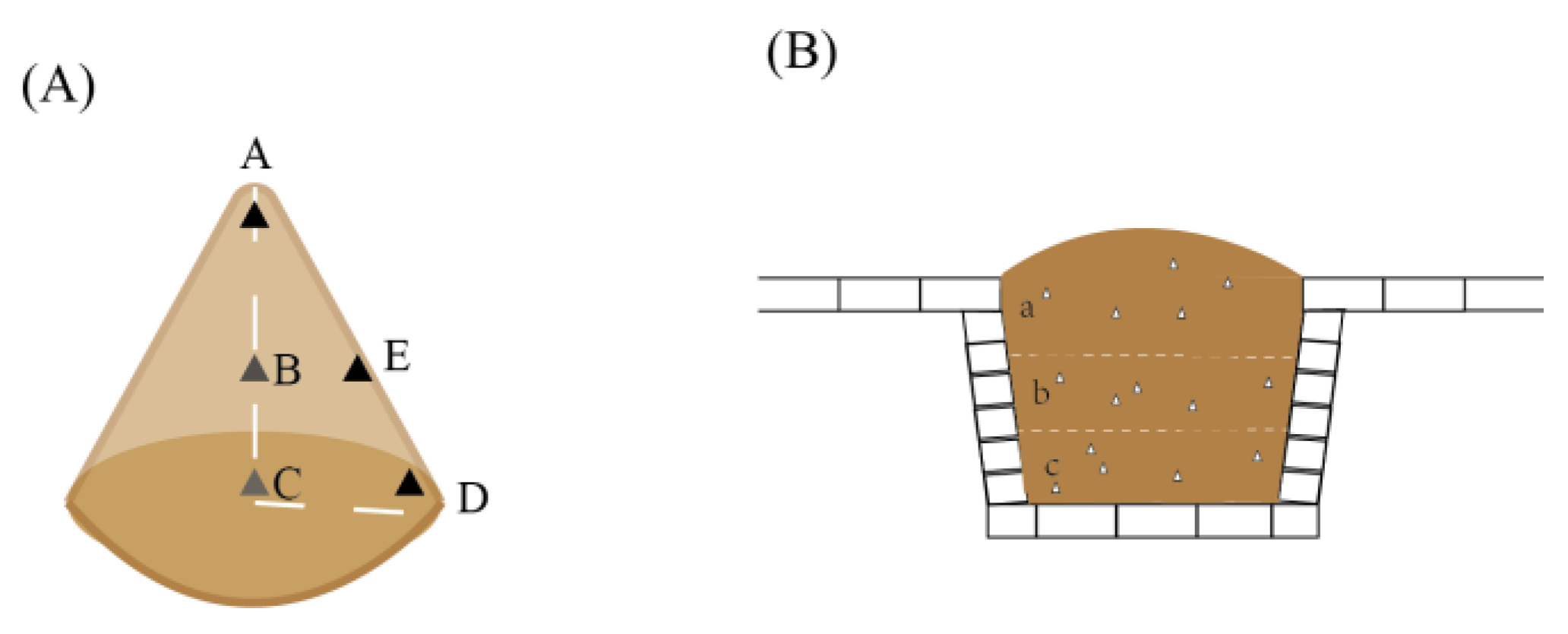
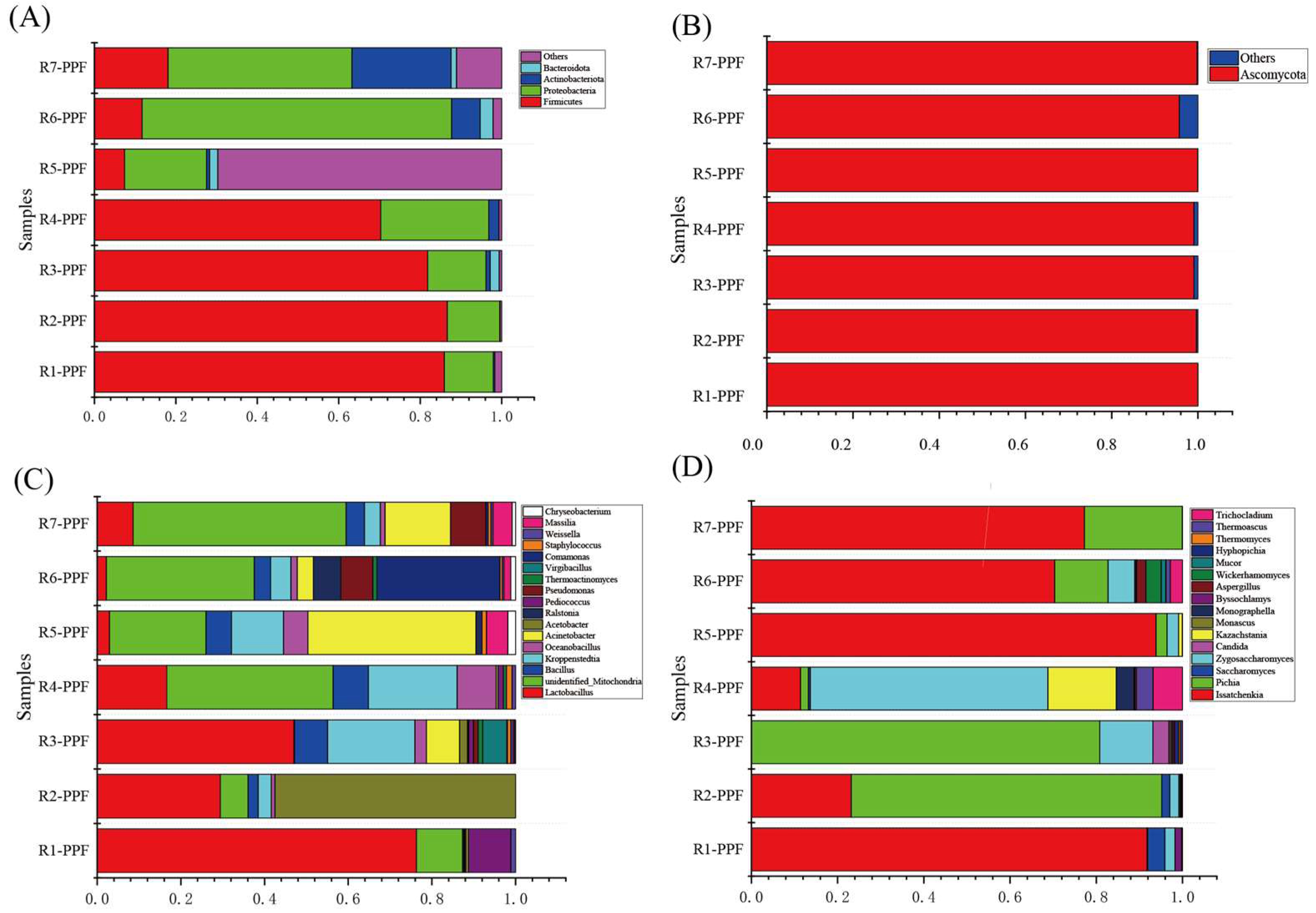
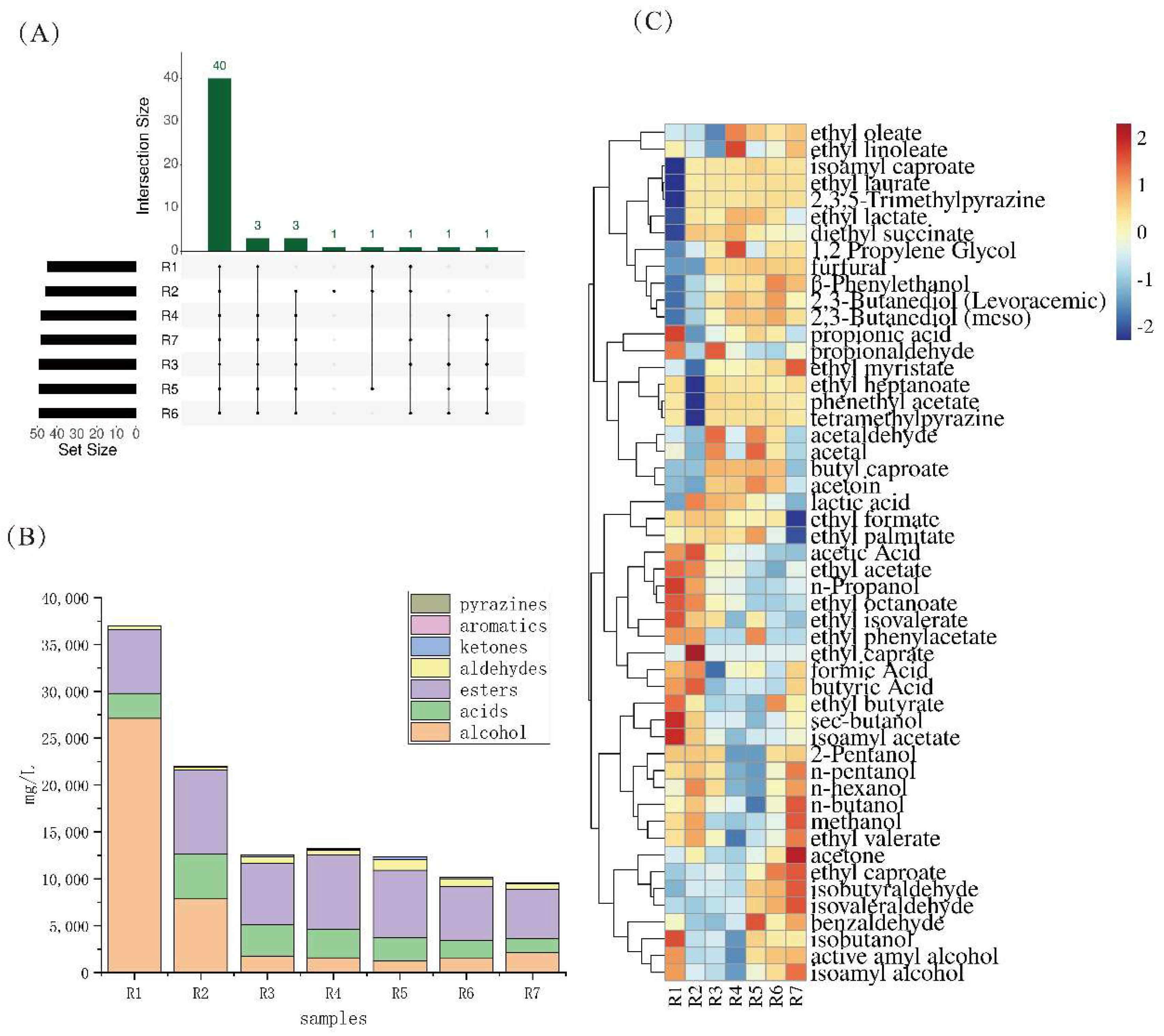
| Samples | Information | Samples | Information |
|---|---|---|---|
| R1-PPF | pre-pit fermentation grains in round 1 | R1 | Base Baijiu in round 1 |
| R2-PPF | pre-pit fermentation grains in round 2 | R2 | Base Baijiu in round 2 |
| R3-PPF | pre-pit fermentation grains in round 3 | R3 | Base Baijiu in round 3 |
| R4-PPF | pre-pit fermentation grains in round 4 | R4 | Base Baijiu in round 4 |
| R5-PPF | pre-pit fermentation grains in round 5 | R5 | Base Baijiu in round 5 |
| R6-PPF | pre-pit fermentation grains in round 6 | R6 | Base Baijiu in round 6 |
| R7-PPF | pre-pit fermentation grains in round 7 | R7 | Base Baijiu in round 7 |
| Samples | Coverage | Shannon | Chao1 | |||
|---|---|---|---|---|---|---|
| Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | |
| R1-PPF | 0.996 | 1.000 | 3.059 | 0.665 | 1026.252 | 62.000 |
| R2-PPF | 0.999 | 1.000 | 1.371 | 1.256 | 205.279 | 36.750 |
| R3-PPF | 1.000 | 1.000 | 4.942 | 3.391 | 993.754 | 192.937 |
| R4-PPF | 1.000 | 1.000 | 4.288 | 2.985 | 340.286 | 156.000 |
| R5-PPF | 0.999 | 1.000 | 2.836 | 0.463 | 704.439 | 15.000 |
| R6-PPF | 1.000 | 1.000 | 4.834 | 1.987 | 246.240 | 118.588 |
| R7-PPF | 0.999 | 1.000 | 4.787 | 0.854 | 341.652 | 46.333 |
| Samples | Core Microbe | Dominant Microbe | Characteristic Microbe | |||
|---|---|---|---|---|---|---|
| Bacteria | Fungi | Bacteria | Fungi | Bacteria | Fungi | |
| R1-PPF | Lactobacillus | Issatchenkia | unidentified_Mitochondria, | Zygosaccharomyce | Pediococcus, Weissella | Byssochlamys, Saccharomyces |
| R2-PPF | Lactobacillus, Acetobacter | Issatchenkia, Pichia | unidentified_Mitochondria, Bacillus, Kroppenstedtia | Saccharomyces, Zygosaccharomyce | ||
| R3-PPF | Lactobacillus, Kroppenstedtia | Pichia | Acinetobacter, Acetobacter, Pediococcus, Pseudomonas | Zygosaccharomyce, Candida | Thermoactinomyces, Virgibacillus | Candida |
| R4-PPF | unidentified_Mitochondria, Kroppenstedtia | Zygosaccharomyces | Lactobacillus, Bacillus, Pediococcus, Staphylococcus | Issatchenkia, Pichia | Oceanobacillus | Kazachstania, Monographella, Thermoascus, Trichocladium |
| R5-PPF | unidentified_Mitochondria, Acinetobacter | Issatchenkia | Ralstonia, Staphylococcus, Massilia, Chryseobacterium | Pichia, Zygosaccharomyce | ||
| R6-PPF | unidentified_Mitochondria, Comamonas | Issatchenkia | Acinetobacter, Pseudomonas, Massilia, Chryseobacterium | Pichia, Zygosaccharomyce, Thermoascus, Trichocladium | Ralstonia, Comamonas | Aspergillus, Wickerhamomyces, Mucor |
| R7-PPF | unidentified_Mitochondria | Issatchenkia, Pichia | Acinetobacter, Massilia | |||
| Number | Compound | Characteristics | OAV |
|---|---|---|---|
| 1 | Acetaldehyde | Spicy, pungent odour | 339.40–696.28 |
| 2 | Propionaldehyde | Green Aroma | 168.33–658.97 |
| 3 | Isobutyraldehyde | Floral, fruity aroma | 1005.28–2926.81 |
| 4 | Isovaleraldehyde | Floral, fruity aroma | 1367.65–5416.53 |
| 5 | Acetal | fruity aroma | 6807.28–14,685.58 |
| 6 | Furfural | Almond aroma, sweet aroma, burnt, bitter | 0–4543.78 |
| 7 | isoamyl acetate | Banana, sweet, apple and fruit sugar aroma | 19.95–177.93 |
| 8 | Ethyl butyrate | Fruity, floral | 319.27–715.52 |
| 9 | Ethyl isovalerate | Apple, pineapple, banana and fruit aroma | 1058.13–7547.29 |
| 10 | Ethyl caproate | Sweet, fruity, pit and cucumber aroma | 1057.82–1897.14 |
| 11 | Ethyl octanoate | Pear, lychee, fruit, sweet, lily of the valley | 133.31–6904.38 |
| 12 | 1-Propanol | Fruity, floral and grassy aroma | 5.20–462.96 |
| 13 | 2,3-Butanediol (Levoracemic) | Sweetness | 129.17–381.94 |
| 14 | isoamyl alcohol | Fruity, floral aroma | 166.39–412.74 |
| 15 | Butyric Acid | Stench of sweat, sourness and pit mud | 77.33–691.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, S.; Liu, J.; Luo, R.; Zhang, J.; Zhao, D.; Xue, X.; Zheng, J.; Qiao, Z.; Zhang, Q.; Feng, Z.; et al. Analysis of the Influence of Microbial Community Structure on Flavor Composition of Jiang-Flavor Liquor in Different Batches of Pre-Pit Fermented Grains. Fermentation 2022, 8, 671. https://doi.org/10.3390/fermentation8120671
Shen S, Liu J, Luo R, Zhang J, Zhao D, Xue X, Zheng J, Qiao Z, Zhang Q, Feng Z, et al. Analysis of the Influence of Microbial Community Structure on Flavor Composition of Jiang-Flavor Liquor in Different Batches of Pre-Pit Fermented Grains. Fermentation. 2022; 8(12):671. https://doi.org/10.3390/fermentation8120671
Chicago/Turabian StyleShen, Shiming, Jinlong Liu, Ruiqi Luo, Jiaojiao Zhang, Dong Zhao, Xinxin Xue, Jia Zheng, Zongwei Qiao, Qiang Zhang, Zheng Feng, and et al. 2022. "Analysis of the Influence of Microbial Community Structure on Flavor Composition of Jiang-Flavor Liquor in Different Batches of Pre-Pit Fermented Grains" Fermentation 8, no. 12: 671. https://doi.org/10.3390/fermentation8120671
APA StyleShen, S., Liu, J., Luo, R., Zhang, J., Zhao, D., Xue, X., Zheng, J., Qiao, Z., Zhang, Q., Feng, Z., & Han, X. (2022). Analysis of the Influence of Microbial Community Structure on Flavor Composition of Jiang-Flavor Liquor in Different Batches of Pre-Pit Fermented Grains. Fermentation, 8(12), 671. https://doi.org/10.3390/fermentation8120671






