Biological Potential of Alternative Kombucha Beverages Fermented on Essential Oil Distillation By-Products
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil Production and Waste Streams Obtaining
2.2. Kombucha Fermentation
2.3. Antioxidant Capacity
2.4. Pharmacological Activities
2.4.1. Anti-Inflammatory Activity
2.4.2. Antihyperglycemic Assay
2.5. Sensory Analysis
- Smell: vinegar mild (1); vinegar strong (2); yeast (3); plant (4); other (5).
- Taste: mild vinegar (1); vinegar strong (2); yeast (3); plant (4); other (5).
- Acidity: imperceptibly acidic (1); slightly sour (2); medium sour (3); noticeably sour (4); too sour (5).
- Sweetness: imperceptibly sweet (1); slightly sweet (2); average sweet (3); noticeably sweet (4); too sweet (5).
- Color tone: pale (transparent) (1); light yellow (2); golden-yellow (3); light brown (4); dark brown (5).
- Overall acceptability: completely unacceptable (1); partially unacceptable (2); indifferent (3); partially acceptable (4); completely acceptable (5).
2.6. Statistical Analysis
3. Results and Discussion
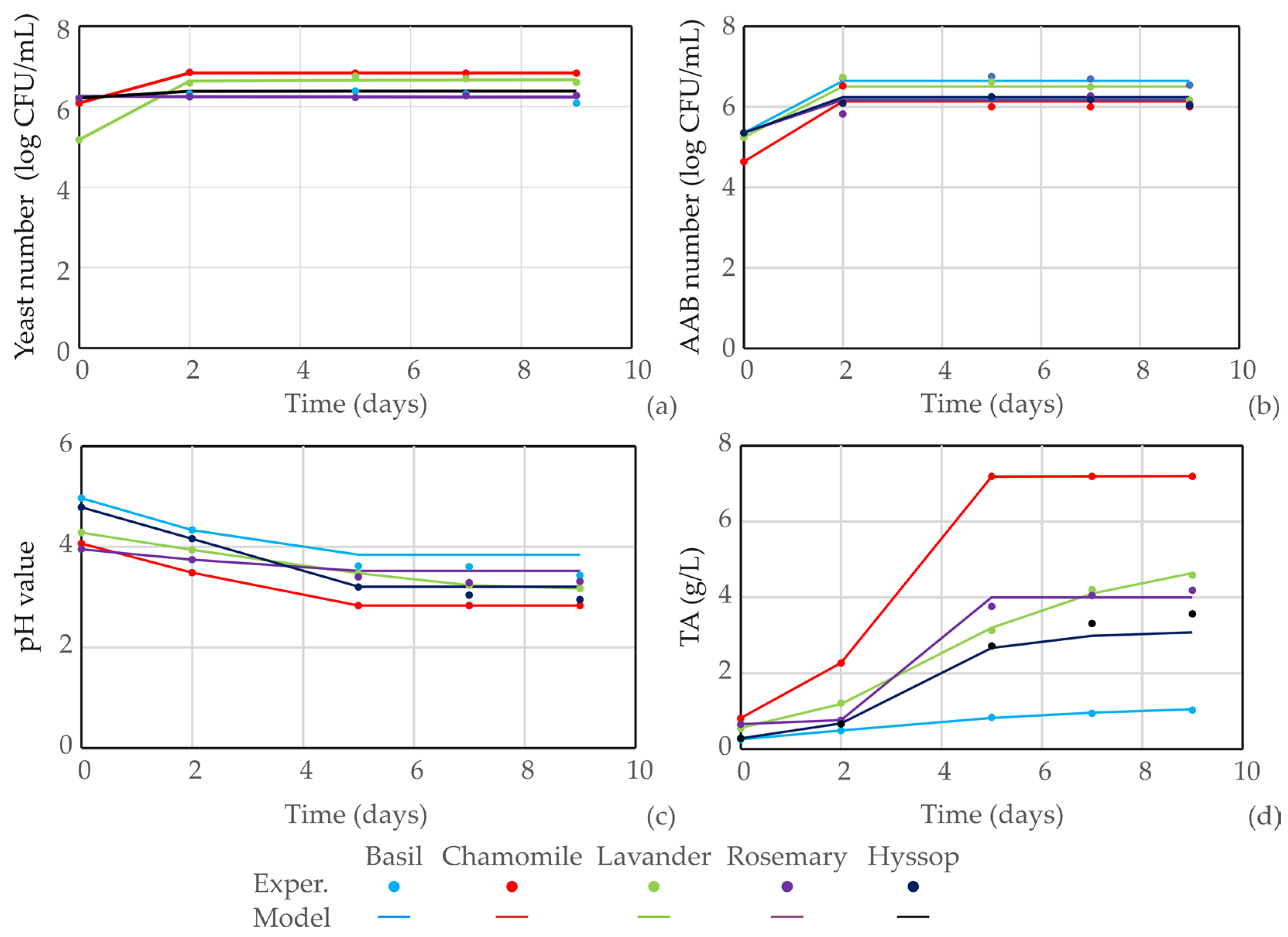
3.1. Kombucha Fermentation
3.2. Antioxidant Capacity
3.3. Pharmacological Activities
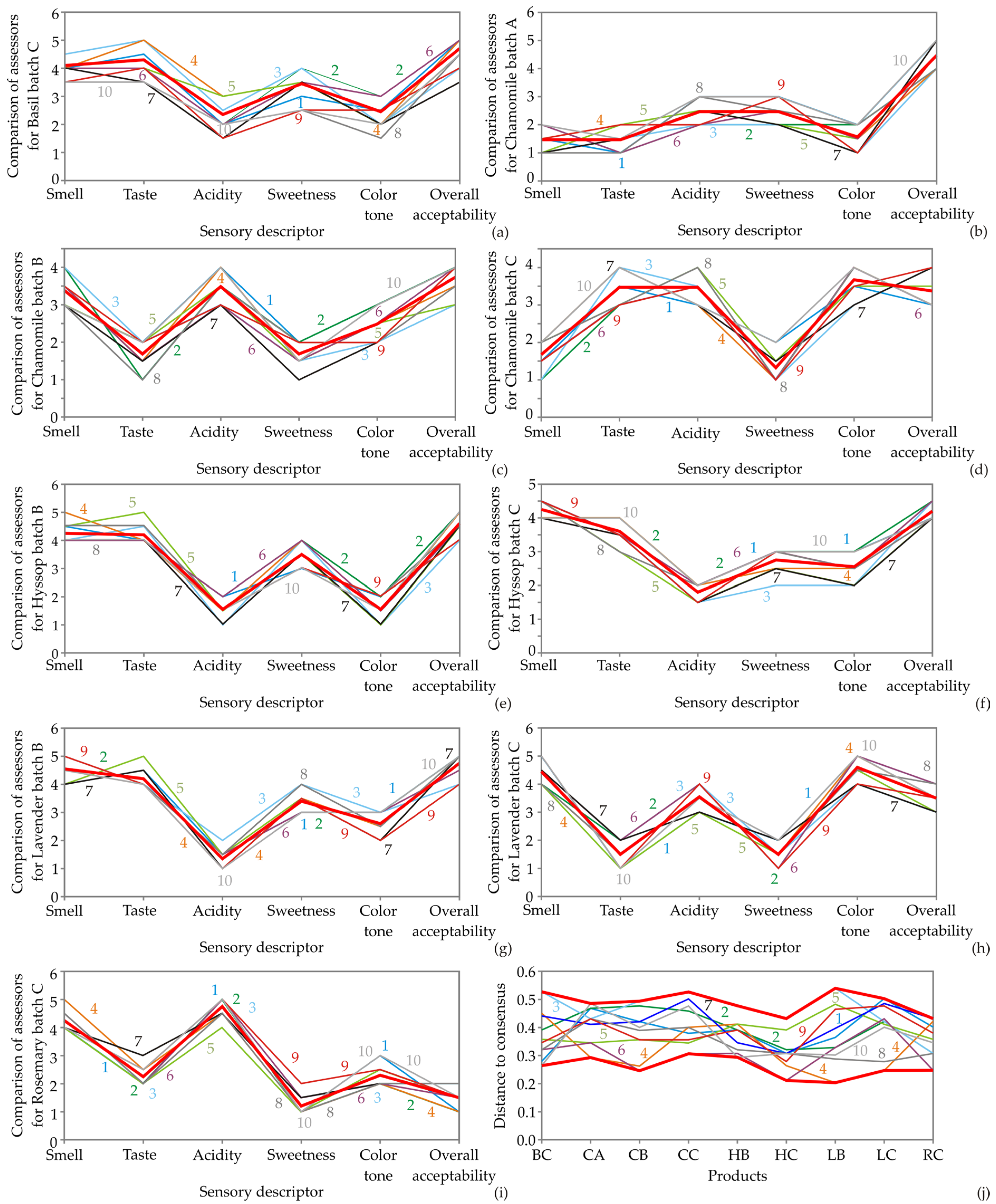
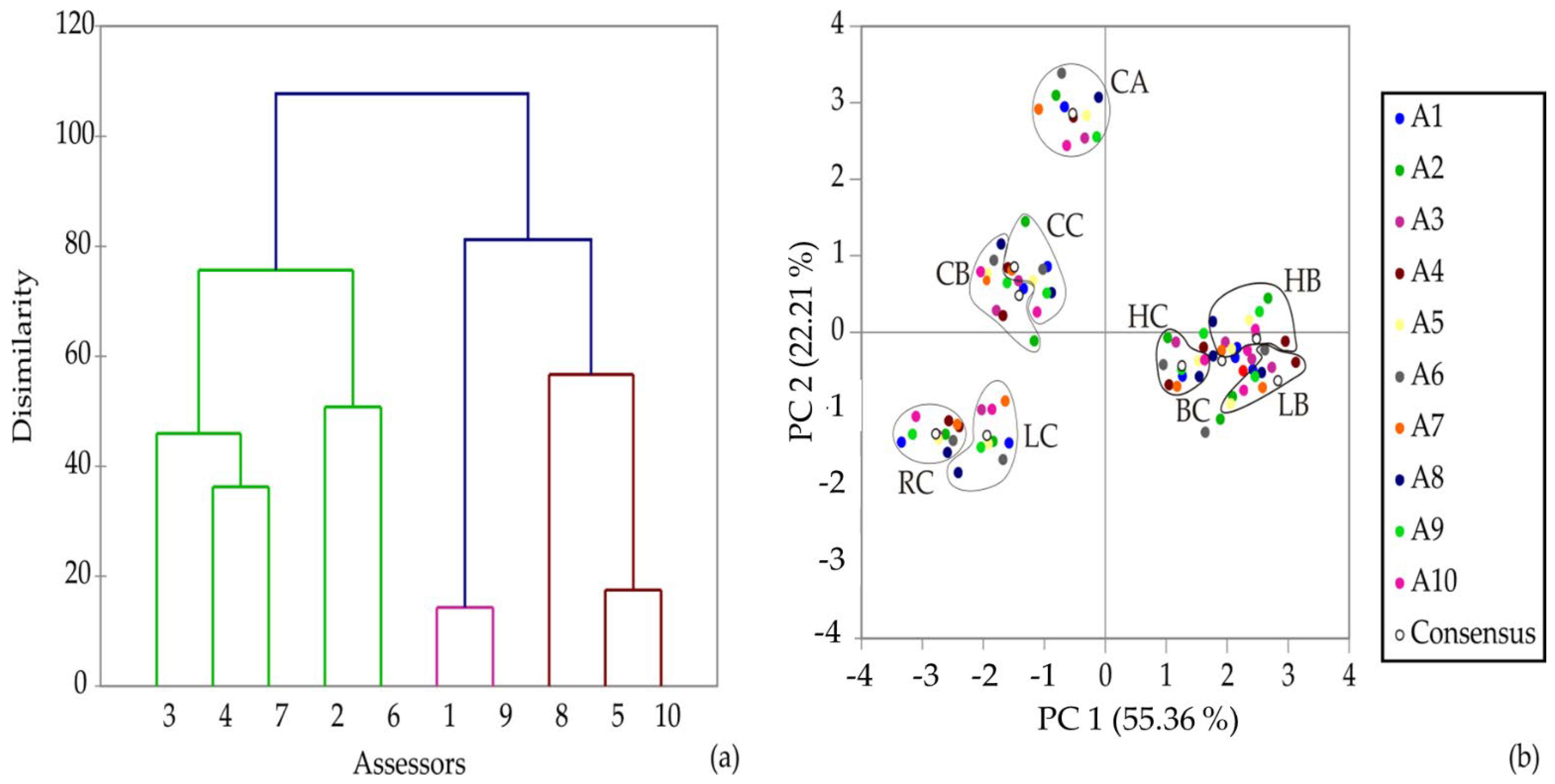
3.4. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elguea-Culebras, G.O.; MeleroBravo, E.; Sánchez-Vioquea, R. Potential sources and methodologies for the recovery of phenolic compounds from distillation residues of Mediterranean aromatic plants. An approach to the valuation of by-products of the essential oil market–A review. Ind. Crops Prod. 2022, 175, 114261. [Google Scholar] [CrossRef]
- Jensch, C.; Strube, J. Proposal of a New Green Process for Waste Valorization and Cascade Utilization of Essential Oil Plants. Sustainability 2022, 14, 3227. [Google Scholar] [CrossRef]
- Aćimović, M.; Tešević, V.; Smiljanić, K.; Cvetković, M.; Stanković, J.; Kiprovski, B.; Sikora, V. Hydrolates—By-products of essential oil destilation: Chemical composition, biological activity and potential uses. Advant. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Navarrete, A.; Herrero, M.; Martín, A.; Cocero, M.J.; Ibáñez, E. Valorization of solid wastes from essential oil industry. J. Food Eng. 2011, 104, 196–201. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Roscigno, G.; Pane, C.; Celano, G.; Di Matteo, M.; Mainente, M.; Vuotto, A.; Mencherini, T.; Esposito, T.; Vitti, A.; et al. Essential oils and quality composts sourced by recycling vegetable residues from the aromatic plant supply chain. Ind. Crops Prod. 2021, 162, 113255. [Google Scholar] [CrossRef]
- Truzzi, E.; Chaouch, M.A.; Rossi, G.; Tagliazucchi, L.; Bertelli, D.; Benvenuti, S. Characterization and Valorization of the Agricultural Waste Obtained from Lavandula Steam Distillation for Its Reuse in the Food and Pharmaceutical Fields. Molecules 2022, 27, 1613. [Google Scholar] [CrossRef]
- Dini, I. Bio Discarded from Waste to Resource. Foods 2021, 10, 2652. [Google Scholar] [CrossRef]
- Dzamic, A.M.; Mileski, K.S.; Ciric, A.D.; Ristic, M.S.; Sokovic, M.D.; Marin, P.D. Essential Oil Composition, Antioxidant and Antimicrobial Properties of Essential Oil and Deodorized Extracts of Helichrysum italicum (Roth) G. Don. J. Essent. Oil-Bear. Plants 2019, 22, 493–503. [Google Scholar] [CrossRef]
- Aćimović, M.; Tešević, V.; Mara, D.; Cvetković, M.; Stanković, J.; Filipović, V. The analysis of cumin seeds essential oil and total polyphenols from postdestillation waste material. Adv. Technol. 2016, 5, 23–30. [Google Scholar] [CrossRef]
- Slavov, A.M.; Karneva, K.B.; Vasileva, I.N.; Denev, P.N.; Denkova, R.S.; Shikov, V.T.; Manolova, M.N.; Lazarova, Y.L.; Ivanova, V.N. Valorization of lavender waste–obtaining and characteristics of polyphenol rich extracts. Food Sci. Appl. Biotechnol. 2018, 1, 11–18. [Google Scholar]
- Barakat, N.; Beaufort, S.; Rizk, Z.; Bouajila, J.; Taillandier, P.; El Rayess, Y. Kombucha analogues around the world: A review. Crit. Rev. Food Sci. Nutr. 2022, 29, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Emiljanowicz, K.E.; Malinowska-Pańczyk, E. Kombucha from alternative raw materials—The review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3185–3194. [Google Scholar] [CrossRef]
- Jayabalan, R.; Subathradevi, P.; Marimuthu, S.; Sathishkumar, M.; Swaminathan, K. Changes in free-radical scavenging ability of kombucha tea during fermentation. Food Chem. 2008, 109, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Malbaša, R.; Lončar, E.; Djurić, M. Comparison of the products of Kombucha fermentation on sucrose and molasses. Food Chem. 2008, 106, 1039–1045. [Google Scholar] [CrossRef]
- Belloso-Morales, G.; Hernández-Sánchez, H. Manufacture of a beverage from cheese whey using a "tea fungus" fermentation. Rev. Latinoam. Microbiol. 2003, 45, 5–11. [Google Scholar]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on Kombucha Tea-Microbiology, Composition, Fermentation, Beneficial Effects, Toxicity, and Tea Fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Pure, A.E.; Pure, M.E. Antioxidant and Antibacterial Activity of Kombucha Beverages Prepared using Banana Peel, Common Nettles and Black Tea Infusions. Appl. Food Biotechnol. 2016, 3, 125–130. [Google Scholar]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of kombucha consortium to transform soy whey into a novel functional beverage. J. Funct. Foods 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Muzaifa, M.; Rohaya, S.; Nilda, C.; Harahap, K.R. Kombucha Fermentation from Cascara with Addition of Red Dragon Fruit (Hylocereus polyrhizus): Analysis of Alcohol Content and Total Soluble Solid. In Proceedings of the International Conference on Tropical Agrifood, Feed and Fuel (ICTAFF 2021), online, 7 September 2021. [Google Scholar]
- Vukmanović, S.; Vitas, J.; Malbaša, R. Valorization of winery effluent using kombucha culture. J. Food Process. Preserv. 2020, 44, e14627. [Google Scholar] [CrossRef]
- Aćimović, M.; Šovljanski, O.; Pezo, L.; Travičić, V.; Tomić, A.; Zheljazkov, V.D.; Ćetković, G.; Švarc-Gajić, J.; Brezo-Borjan, T.; Sofrenić, I. Variability in Biological Activities of Satureja montana Subsp. montana and Subsp. variegata Based on Different Extraction Methods. Antibiotics 2022, 11, 1235. [Google Scholar]
- European Pharmacopoeia, 8th ed.; Supplement 8.0; Consil of Europe: Strasbourg, France, 2014.
- Velićanski, A.S.; Cvetković, D.D.; Markov, S.L.; Šaponjac, V.T.T.; Vulić, J.J. Antioxidant and Antibacterial Activity of the Beverage Obtained by Fermentation of Sweetened Lemon Balm (Melissa officinalis L.) Tea with Symbiotic Consortium of Bacteria and Yeasts. Food Technol. Biotechnol. 2014, 52, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, D.; Markov, S.; Djurić, M.; Savić, D.; Velićanski, A. Specific interfacial area as a key variable in scaling-up Kombucha fermentation. J. Food Eng. 2008, 85, 387–392. [Google Scholar] [CrossRef]
- González-Molina, E.; Moreno, D.A.; García-Viguera, C. A new drink rich in healthy bioactives combining lemon and pomegranate juices. Food Chem. 2009, 115, 1364–1372. [Google Scholar] [CrossRef]
- Markham, K.R. Flavones, flavonols and their glycosides. In Methods in Plant Biochemistry; Harborne, J.B., Dey, P.M., Eds.; Academic Press: London, UK, 1989; pp. 193–237. [Google Scholar]
- Mena, P.; García-Viguera, C.; Navarro-Rico, J.; Moreno, D.A.; Bartual, J.; Saura, D.; Martí, N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011, 91, 1893–1906. [Google Scholar] [CrossRef] [PubMed]
- Aborus, E.N.; Čanadanović-Brunet, J.; Ćetković, G.; Tumbas Šaponjac, V.; Vulić, J.; Ilić, N. Powdered barley sprouts: Composition, functionality and polyphenol digestibility. Int. J. Food Sci. Technol. 2016, 52, 231–238. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction–antioxidant activities of products of browning reaction prepared from glucoamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Tumbas Šaponjac, V.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.A.; Čanadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Krunić, M. Anthocyanin profiles and biological properties of caneberry (Rubuss pp.) press residues. J. Sci. Food Agric. 2014, 94, 2393–2400. [Google Scholar] [CrossRef]
- Mintel. Tea and RTD Teas—US—August 2019. Reasons for Drinking Kombucha. 2019. Available online: www.reports.mintel.com (accessed on 1 September 2022).
- Romano, A.; Toraldo, G.; Cavella, S.; Masi, P. Description of leavening of bread dough with mathematical modelling. J. Food Eng. 2007, 83, 142–148. [Google Scholar] [CrossRef]
- Kim, J.; Adhikari, K. Current Trends in Kombucha: Marketing Perspectives and the Need for Improved Sensory Research. Beverages 2020, 6, 15. [Google Scholar] [CrossRef]
- Tran, T.; Grandvalet, C.; Verdier, F.; Martin, A.; Alexandre, H.; Tourdot-Maréchal, R. Microbiological and technological parameters impacting the chemical composition and sensory quality of kombucha. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2050–2070. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.-P.; Renard, T.; Rollan, S.; Taillandier, P. Impact of fermentation conditions on the production of bioactive compounds with anticancer, anti-inflammatory and antioxidant properties in kombucha tea extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Massoud, R.; Jafari-Dastjerdeh, R.; Naghavi, N.; Khosravi-Darani, K. All Aspects of Antioxidant Properties of Kombucha Drink. Biointerface Res. Appl. Chem. 2022, 12, 4018–4027. [Google Scholar]
- Jakubczyk, K.; Kałduńska, J.; Kochman, J.; Janda, K. Chemical Profile and Antioxidant Activity of the Kombucha Beverage Derived from White, Green, Black and Red Tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Essawet, N.A.; Cvetković, D.; Velićanski, D.; Čanadanović-Brunet, J.; Vulić, J.; Maksimović, V.; Markov, S. Polyphenols and antioxidant acitivities of kombucha beverage enriched with Coffeeberry Extract. Chem. Ind. Chem. Eng. Q 2015, 21, 399–409. [Google Scholar] [CrossRef]
- Leonarski, E.; Cesca, K.; Zanella, E.; Stambuk, B.U.; de Oliveira, D.; Poletto, P. Production of kombucha-like beverage and bacterial cellulose by acerola byproduct as raw material. LWT 2020, 135, 110075. [Google Scholar] [CrossRef]
- Shrirari, T.; Satyanarayana, U. Changes in Free Radical Scavenging Activity of Kombucha during Fermentation. J. Pharm. Sci. Res. 2012, 4, 1978–1981. [Google Scholar]
- Srihari, T.; Karthikesan, K.; Ashokkumar, N.; Satyanarayana, U. Antihyperglycaemic efficacy of kombucha in streptozotocin-induced rats. J. Funct. Foods 2013, 5, 1794–1802. [Google Scholar] [CrossRef]
- Lobo, R.O.; Dias, F.O.; Shenoy, C.K. Kombucha for healthy living: Evaluation of antioxidant potential and bioactive compound. Int. Food Res. J. 2017, 24, 541–546. [Google Scholar]
- Neffe-Skocińska, K.; Sionek, B.; Ścibisz, I.; Kołożyn-Krajewska, D. Acid contents and the effect of fermentation condition of Kombucha tea beverages on physicochemical, microbiological and sensory properties. CYTA J. Food 2017, 15, 601–607. [Google Scholar] [CrossRef]
- Alderson, H.; Liu, C.; Mehta, A.; Gala, H.S.; Mazive, N.R.; Chen, Y.; Zhang, Y.; Wang, S.; Serventi, L. Sensory Profile of Kombucha Brewed with New Zealand Ingredients by Focus Group and Word Clouds. Fermentation 2021, 7, 100. [Google Scholar] [CrossRef]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A Double Power of Bioactive Compounds from Tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
| Plant | ||||||
|---|---|---|---|---|---|---|
| Basil | Chamomile | Lavender | Rosemary | Hyssop | ||
| Kombucha fermentation parameters | ||||||
| Yeast number (log CFU/mL) | ||||||
| Regression parameters | d | 6.232 | 6.837 | 7.975 | 6.232 | 6.384 |
| a | 6.257 | 6.085 | 5.175 | 6.257 | 6.210 | |
| c | 1.911 | 0.100 | 0.100 | 1.911 | 0.100 | |
| b | 0.759 | 4.509 | 0.029 | 0.759 | 1.280 | |
| AAB number (log CFU/mL) | ||||||
| Regression parameters | d | 6.642 | 6.131 | 6.503 | 6.171 | 6.234 |
| a | 5.350 | 4.635 | 5.240 | 5.350 | 5.350 | |
| c | 0.100 | 0.100 | 0.100 | 0.100 | 0.100 | |
| b | 5.225 | 5.225 | 5.225 | 5.225 | 5.225 | |
| pH value | ||||||
| Regression parameters | d | 3.840 | 2.830 | 3.354 | 3.519 | 3.207 |
| a | 4.965 | 4.063 | 4.280 | 3.950 | 4.783 | |
| c | 1.963 | 2.018 | 2.085 | 2.010 | 2.063 | |
| b | 13.431 | 13.374 | 12.584 | 13.395 | 13.472 | |
| TA (g/L) | ||||||
| Regression parameters | d | 1.405 | 7.193 | 5.696 | 3.999 | 3.141 |
| a | 0.257 | 0.815 | 0.561 | 0.658 | 0.281 | |
| c | 5.121 | 2.319 | 4.882 | 2.408 | 3.252 | |
| b | 1.443 | 8.206 | 2.207 | 18.508 | 3.743 | |
| χ2 | RMSE | MBE | MPE | r2 | Skew | Kurt | Mean | StDev | Var | |
|---|---|---|---|---|---|---|---|---|---|---|
| Yeast number | ||||||||||
| Basil | 0.015 | 0.110 | 0.023 | 1.629 | 0.998 | −0.703 | −0.773 | 0.023 | 0.121 | 0.015 |
| Chamomile | 0.000 | 0.008 | 0.000 | 0.088 | 0.999 | 1.926 | 3.672 | 0.000 | 0.009 | 0.000 |
| Lavender | 0.005 | 0.061 | 0.006 | 0.749 | 0.990 | 0.720 | −0.276 | 0.006 | 0.068 | 0.005 |
| Rosemary | 0.001 | 0.031 | 0.002 | 0.423 | 0.775 | −0.454 | −0.755 | 0.002 | 0.035 | 0.001 |
| Hyssop | 0.042 | 0.182 | 0.069 | 2.135 | 0.885 | 0.876 | −0.880 | 0.069 | 0.189 | 0.036 |
| AAB number | ||||||||||
| Basil | 0.007 | 0.077 | 0.024 | 0.988 | 0.982 | −1.085 | 1.096 | 0.024 | 0.082 | 0.007 |
| Chamomile | 0.050 | 0.200 | 0.000 | 2.480 | 0.899 | 1.925 | 3.667 | 0.000 | 0.224 | 0.050 |
| Lavender | 0.047 | 0.195 | 0.028 | 2.335 | 0.874 | −0.914 | 1.318 | 0.028 | 0.215 | 0.046 |
| Rosemary | 0.036 | 0.170 | −0.067 | 1.949 | 0.789 | −1.352 | 1.788 | −0.067 | 0.175 | 0.031 |
| Hyssop | 0.017 | 0.116 | −0.080 | 1.395 | 0.948 | −0.428 | −2.228 | −0.080 | 0.094 | 0.009 |
| pH value | ||||||||||
| Basil | 0.069 | 0.235 | −0.175 | 4.977 | 0.975 | −0.185 | −1.530 | −0.175 | 0.175 | 0.031 |
| Chamomile | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 | −1.904 | 3.789 | 0.000 | 0.000 | 0.000 |
| Lavender | 0.016 | 0.112 | −0.038 | 2.605 | 0.944 | 0.003 | −0.862 | −0.038 | 0.118 | 0.014 |
| Rosemary | 0.029 | 0.151 | −0.113 | 3.417 | 0.961 | 0.051 | −2.872 | −0.113 | 0.112 | 0.013 |
| Hyssop | 0.024 | 0.138 | −0.088 | 2.934 | 0.987 | −0.913 | −1.549 | −0.088 | 0.120 | 0.014 |
| TA value | ||||||||||
| Basil | 0.000 | 0.014 | −0.004 | 1.427 | 0.998 | 0.682 | −0.078 | −0.004 | 0.015 | 0.000 |
| Chamomile | 0.000 | 0.004 | 0.000 | 0.083 | 1.000 | 1.905 | 3.636 | 0.000 | 0.005 | 0.000 |
| Lavender | 0.005 | 0.064 | 0.000 | 2.140 | 0.998 | 0.910 | 0.192 | 0.000 | 0.072 | 0.005 |
| Rosemary | 0.024 | 0.137 | 0.000 | 2.409 | 0.993 | −0.781 | 2.000 | 0.000 | 0.153 | 0.024 |
| Hyssop | 0.087 | 0.264 | 0.173 | 6.087 | 0.992 | 0.849 | −1.596 | 0.173 | 0.223 | 0.050 |
| Kombucha Samples | Basil Batch C | Chamomile Batch A | Chamomile Batch B | Chamomile Batch C | Rosemary Batch C | Lavender Batch B | Lavender Batch C | Hyssop Batch B | Hyssop Batch C |
|---|---|---|---|---|---|---|---|---|---|
| Phenols | 55.57 ± 0.89 c | 12.65 ± 0.55 a | 12.40 ± 0.1 a | 42.11 ± 0.65 c | 45.57 ± 0.44 c | 24.37± 0.52 b | 51.58 ± 1.73 | 16.48 ± 1.0 a | 56.46 ± 0.44 c |
| Flavonoids | 0.25± 0.11 a | 0.67± 0.06 a | 1.04 ± 0.1 b | 5.07 ± 0.25 c | 1.07 ± 0.14 b | 2.58± 0.1 b | 2.93 ± 0.4 b | 0.84 ± 0.11 a | 0.49 ± 0.09 a |
| Controls | Basil batch C | Chamomile batch A | Chamomile batch B | Chamomile batch C | Rosemary batch C | Lavender batch B | Lavender batch C | Hyssop batch B | Hyssop batch C |
| Phenols | 20.36 ± 0.29 b | 2.26 ± 0.24 a | 4.60 ± 0.04 a | 31.32 ± 0.55 c | 41.52 ± 0.48 c | 4.37 ± 0.19 a | 23.02 ± 0.0 b | 1.85 ± 0.06 a | 11.37 ± 0.49 b |
| Flavonoids | 6.99 ± 0.17 b | 0.66 ± 0.15 a | 2.41 ± 0.36 b | 0.06 ± 0.02 a | 9.31 ± 0.13 | 2.84 ± 0.25 b | 3.41 ± 0.53 b | 0.56 ± 0.1 a | 0.96 ± 0.4 a |
| Kombucha Samples | Basil Batch C | Chamomile Batch A | Chamomile Batch B | Chamomile Batch C | Rosemary Batch C | Lavender Batch B | Lavender Batch C | Hyssop Batch B | Hyssop Batch C |
|---|---|---|---|---|---|---|---|---|---|
| DPPH | 67.46 ± 1.91 | 41.30 ± 1.86 | 30.28 ± 1.02 | 64.25 ± 1.21 | 73.70 ± 4.84 | 60.41 ± 2.96 | 65.10 ± 0.61 | 34.51 ± 0.22 | 59.72 ± 2.90 |
| ABTS | 513.20 ± 14.49 | 264.11 ± 13.12 | 131.17 ± 1.37 | 615.62 ± 16.05 | 683.29 ± 16.80 | 395.46 ± 16.36 | 631.30 ± 18.11 | 192.25 ± 7.93 | 533.08 ± 38.48 |
| RP | 51.68 ± 0.79 | 19.45 ± 0.98 | 19.37 ± 0.28 | 65.27 ± 0.99 | 63.34 ± 2.68 | 31.20 ± 0.39 | 72.92 ± 1.12 | 27.21 ± 0.92 | 82.76 ± 0.25 |
| Controls | Basil batch C | Chamomile batch A | Chamomile batch B | Chamomile batch C | Rosemary batch C | Lavender batch B | Lavender batch C | Hyssop batch B | Hyssop batch C |
| DPPH | 54.98 ± 0.2 d | 6.66 ± 0.58 a | 13.74 ± 0.48 b | 56.09 ± 0.68 d | 70.87 ± 1.93 d | 14.14 ± 6.19 b | 50.96 ± 2.86 d | 7.71 ± 1.31 a | 33.04 ± 0.34 c |
| ABTS | 430.84 ± 17.8 c | 140.53 ± 2.62 b | 62.80 ± 8.37 b | 561.57 ± 75.89 c | 548.10 ± 5.5 c | 94.74 ± 12.05 b | 477.52 ± 7.99 c | 36.57 ± 4.5 a | 192.03 ± 28.61 c |
| RP | 50.45 ± 1.53 c | 14.88 ± 0.82 a | 8.72 ± 0.68 a | 50.52 ± 5.31 c | 66.13 ± 1.46 c | 12.89 ± 0.07 a | 69.90 ± 0.07 c | 3.59 ± 0.28 a | 34.07 ± 0.91 b |
| Kombucha Samples | Basil Batch C | Chamomile Batch A | Chamomile Batch B | Chamomile Batch C | Rosemary Batch C | Lavander Batch B | Lavander Batch C | Hyssop Batch B | Hyssop Batch C |
|---|---|---|---|---|---|---|---|---|---|
| AIA | 58.71 ± 0.93 b | 36.98 ± 1.12 a | 31.4 ± 1.08 a | 51.29 ± 0.96 b | 47.25 ± 2.56 b | 30.35 ± 1.04 a | 55.64 ± 3.07 b | 33.25 ± 0.28 a | 61.53 ± 0.59 b |
| AHgA | 25.13 ± 0.08 b | 14.52 ± 0.05 a | 11.64 ± 0.04 a | 26.89 ± 0.4 b | 25.74 ± 0.36 b | 13.98 ± 0.74 a | 29.74 ± 1.02 b | 11.53 ± 0.08 a | 30.69 ± 0.45 b |
| Controls | Basil batch C | Chamomile batch A | Chamomile batch B | Chamomile batch C | Rosemary batch C | Lavander batch B | Lavander batch C | Hyssop batch B | Hyssop batch C |
| AIA | 39.55 ± 2.64 c | 15.43 ± 0.06 a | 17.11 ± 0.12 a | 43.12 ± 3.11 c | 45.17 ± 3.88 c | 12.37 ± 0.09 a | 27.17 ± 0.05 b | 14.83 ± 0.03 a | 29.44 ± 1.65 b |
| AHgA | 17.11 ± 0.03 b | nd | nd | 21.36 ± 0.02 c | 23.12 ± 0.98 c | nd | 10.08 ± 0.09 c | nd | 10.95 ± 0.03 c |
| Products | Smell | Taste | Acidity | Sweetness | Color Tone | Overall Acceptability |
|---|---|---|---|---|---|---|
| Basil batch C | 4.10 ± 0.31 c | 4.30 ± 0.47 d | 2.35 ± 0.49 b | 3.45 ± 0.51 d | 2.45 ± 0.51 b | 4.70 ± 0.47 d |
| Chamomile batch A | 1.45 ± 0.51 a | 1.45 ± 0.51 a | 2.45 ± 0.51 b | 2.45 ± 0.51 b | 1.55 ± 0.51 a | 4.45 ± 0.51 cd |
| Chamomile batch B | 3.35 ± 0.49 b | 1.65 ± 0.49 a | 3.45 ± 0.51 c | 1.65 ± 0.49 a | 2.45 ± 0.51 b | 3.70 ± 0.47 b |
| Chamomile batch C | 1.65 ± 0.49 a | 3.45 ± 0.51 c | 3.45 ± 0.51 c | 1.30 ± 0.47 a | 3.65 ± 0.49 c | 3.35 ± 0.49 b |
| Lavender batch B | 4.55 ± 0.51 c | 4.20 ± 0.41 d | 1.35 ± 0.49 a | 3.40 ± 0.50 d | 2.60 ± 0.50 b | 4.75 ± 0.44 d |
| Lavender batch C | 4.45 ± 0.51 c | 1.50 ± 0.51 a | 3.55 ± 0.51 c | 1.50 ± 0.51 a | 4.60 ± 0.50 d | 3.50 ± 0.51 b |
| Hyssop batch B | 4.25 ± 0.44 c | 4.20 ± 0.41 d | 1.55 ± 0.51 a | 3.50 ± 0.51 d | 1.55 ± 0.51 a | 4.60 ± 0.50 cd |
| Hyssop batch C | 4.25 ± 0.44 c | 3.60 ± 0.50 c | 1.80 ± 0.41 a | 2.75 ± 0.44 b | 2.55 ± 0.51 b | 4.20 ± 0.41 c |
| Rosemary batch C | 4.25 ± 0.44 c | 2.25 ± 0.44 b | 4.75 ± 0.44 d | 1.20 ± 0.41 a | 2.30 ± 0.47 b | 1.50 ± 0.51 a |
| F test | 134.368 | 134.587 | 106.584 | 78.751 | 73.746 | 91.580 |
| p-value | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.001 | p ≤ 0.001 |
| Source | Type | df | Smell | Taste | Acidity | Sweetness | Color Tone | Overall Acceptance |
|---|---|---|---|---|---|---|---|---|
| Products | Fixed | 8 | 232.578 ** | 243.044 ** | 203.444 ** | 148.844 ** | 148.700 ** | 169.878 ** |
| Assessors | Random | 9 | 1.800 | 4.756 | 3.689 | 0.356 | 15.800 ** | 2.583 |
| Sessions | Random | 1 | 0.356 | 0.200 | 0.200 | 0.022 | 0.022 | 0.006 |
| Products × Assessors | Random | 72 | 21.200 ** | 22.844 ** | 21.111 ** | 26.044 ** | 15.300 * | 24.567 ** |
| Error | 89 | 13.644 | 10.800 | 15.800 | 13.978 | 11.978 | 12.494 |
| Assessors | Smell | Taste | Acidity | Sweetness | Color Tone | Overall Acceptability |
|---|---|---|---|---|---|---|
| 1 | 0.0003 | <0.0001 | <0.0001 | / | 0.0003 | / |
| 2 | 0.0001 | 0.0001 | 0.0002 | <0.0001 | / | 0.0001 |
| 3 | 0.0003 | 0.0001 | 0.0016 | 0.0049 | 0.0014 | 0.0001 |
| 4 | 0.0001 | / | 0.0062 | 0.0214 | 0.0028 | 0.0044 |
| 5 | 0.0012 | 0.0001 | 0.0001 | 0.0019 | 0.0002 | 0.0049 |
| 6 | <0.0001 | 0.0014 | 0.0001 | 0.0028 | <0.0001 | <0.0001 |
| 7 | 0.0007 | 0.0000 | 0.0070 | 0.0003 | 0.0005 | 0.0005 |
| 8 | 0.0006 | 0.0005 | 0.0002 | <0.0001 | 0.0007 | 0.0053 |
| 9 | 0.0000 | 0.0000 | 0.0003 | 0.0001 | 0.0001 | <0.0001 |
| 10 | / | / | <0.0001 | / | / | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranitović, A.; Šovljanski, O.; Aćimović, M.; Pezo, L.; Tomić, A.; Travičić, V.; Saveljić, A.; Cvetković, D.; Ćetković, G.; Vulić, J.; et al. Biological Potential of Alternative Kombucha Beverages Fermented on Essential Oil Distillation By-Products. Fermentation 2022, 8, 625. https://doi.org/10.3390/fermentation8110625
Ranitović A, Šovljanski O, Aćimović M, Pezo L, Tomić A, Travičić V, Saveljić A, Cvetković D, Ćetković G, Vulić J, et al. Biological Potential of Alternative Kombucha Beverages Fermented on Essential Oil Distillation By-Products. Fermentation. 2022; 8(11):625. https://doi.org/10.3390/fermentation8110625
Chicago/Turabian StyleRanitović, Aleksandra, Olja Šovljanski, Milica Aćimović, Lato Pezo, Ana Tomić, Vanja Travičić, Anja Saveljić, Dragoljub Cvetković, Gordana Ćetković, Jelena Vulić, and et al. 2022. "Biological Potential of Alternative Kombucha Beverages Fermented on Essential Oil Distillation By-Products" Fermentation 8, no. 11: 625. https://doi.org/10.3390/fermentation8110625
APA StyleRanitović, A., Šovljanski, O., Aćimović, M., Pezo, L., Tomić, A., Travičić, V., Saveljić, A., Cvetković, D., Ćetković, G., Vulić, J., & Markov, S. (2022). Biological Potential of Alternative Kombucha Beverages Fermented on Essential Oil Distillation By-Products. Fermentation, 8(11), 625. https://doi.org/10.3390/fermentation8110625












