Phytochemical Analysis, Antibacterial and Antibiofilm Activities of Aloe vera Aqueous Extract against Selected Resistant Gram-Negative Bacteria Involved in Urinary Tract Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material
2.2. Extraction
2.3. Analysis of the Extract by HPLC–MS/MS
2.3.1. Sample Preparation
2.3.2. Analysis Conditions
2.4. Elemental Composition
2.5. Antibacterial Investigations
2.5.1. Preparation Bacterial Cultures and Assessment of Their Susceptibility to Common Antibiotics
2.5.2. Preparation of the Antimicrobial Solution
2.5.3. Antibacterial Assay by the Well Diffusion Method
2.5.4. Determination of Minimum Inhibitory and Bactericidal Concentrations
2.6. Antibiofilm Formation Screening
2.7. Statistical Analysis
3. Results
3.1. Phytochemical and Mineral Composition of the Optimized Extract (O-ECB)
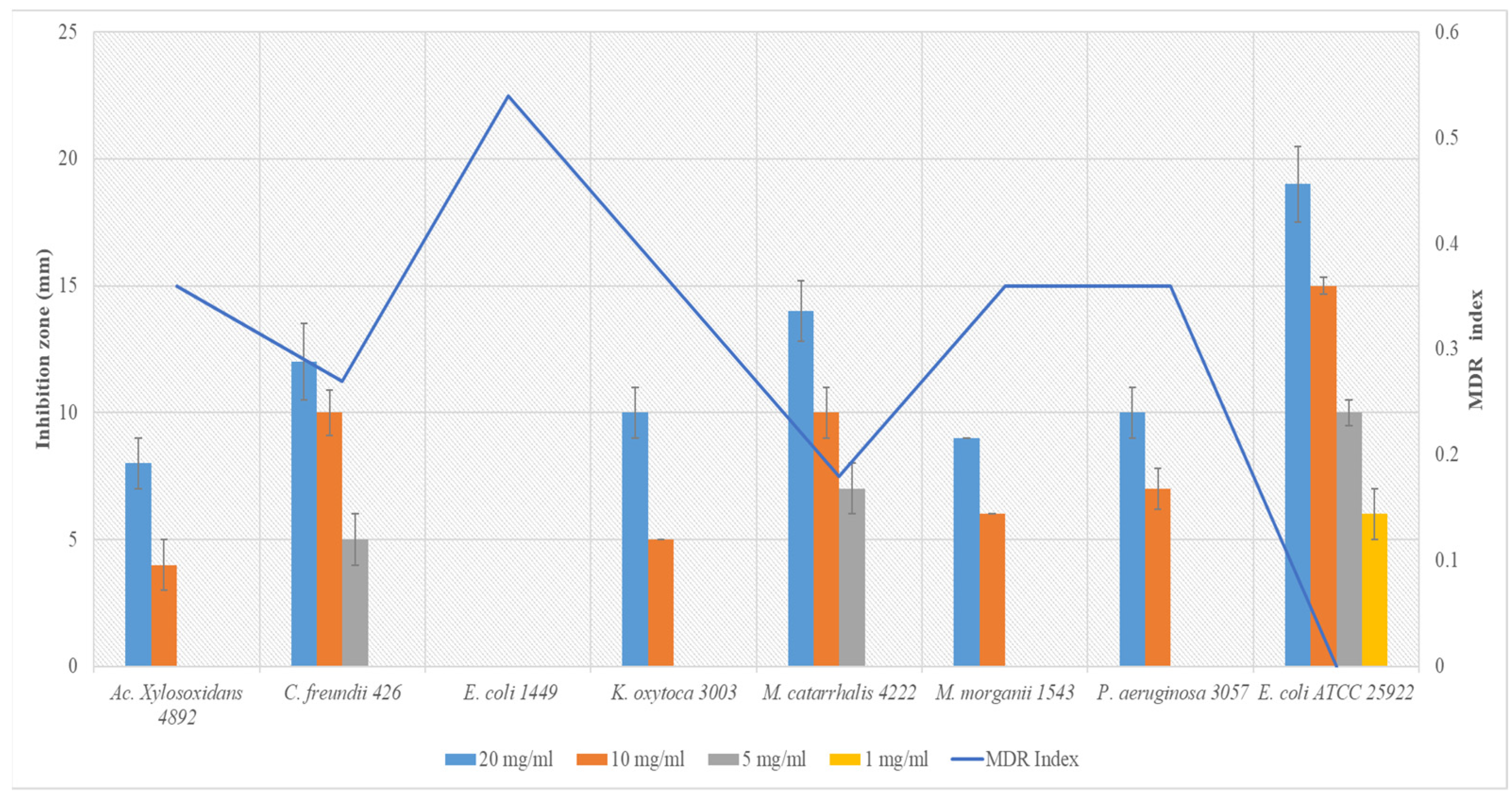
3.2. Susceptibility of Uropathogenic Bacteria to Common Antibiotics and Inhibition Zones of Aloe vera Extract
3.3. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
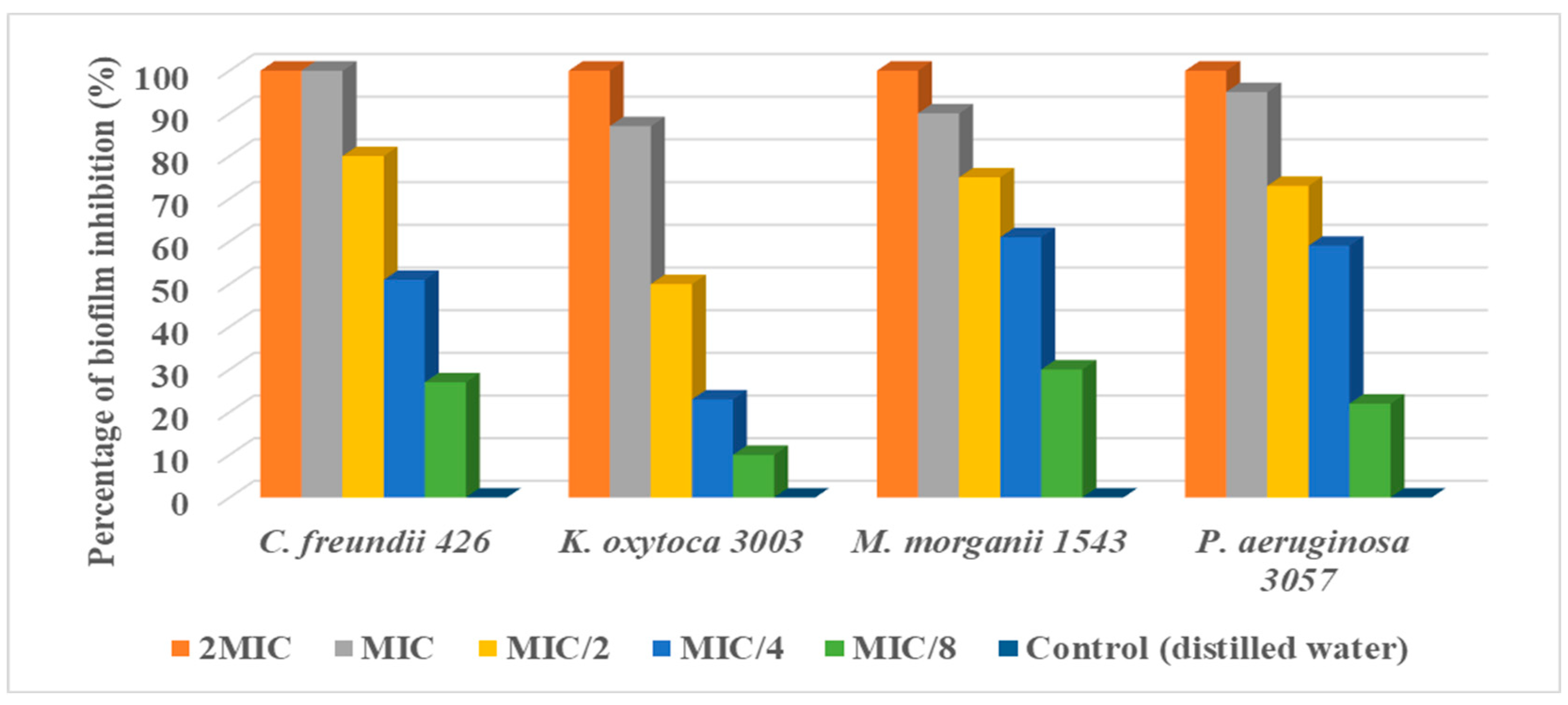
3.4. Antibiofilm Formation Potential of Aloe vera Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palma, E.; Tilocca, B.; Roncada, P. Antimicrobial resistance in veterinary medicine: An overview. Int. J. Mol. Sci. 2020, 21, 1914. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.M.M.; Jorelle, A.B.; Sarra, S.; Viktorovna, P.I.; Davares, A.K.; Ingrid, N.K.; Steve, A.A.F.; Andreevna, S.L.; Vyacheslavovna, Y.N.; Carime, B.Z. Short review on the potential alternatives to antibiotics in the era of antibiotic resistance. J. Appl. Pharm. Sci. 2022, 12, 29–40. [Google Scholar] [CrossRef]
- WHO: World Health Organization. Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance.2020 (accessed on 31 May 2022).
- Agarwal, D.K.; Krambeck, A.E.; Sharma, V.; Maldonado, F.J.; Westerman, M.E.; Knoedler, J.J.; Rivera, M.E. Treatment of non-obstructive, non-struvite urolithiasis is effective in treatment of recurrent urinary tract infections. World J. Urol. 2020, 38, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Arsene, M.M.J.; Viktorovna, P.I.; Davares, A.K.L.; Esther, N.; Nikolaevich, S.A. Urinary tract infections: Virulence factors, resistance to antibiotics, and management of uropathogenic bacteria with medicinal plants—A review. J. Appl. Pharm. Sci. 2021, 11, 001–012. [Google Scholar] [CrossRef]
- Gupta, K.; Hooton, T.M.; Naber, K.G.; Wullt, B.; Colgan, R.; Miller, L.G.; Moran, G.J.; Nicolle, L.E.; Raz, R.; Schaeffer, A.J.; et al. Infectious Diseases Society of America, European Society for Microbiology and Infectious Diseases. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin. Infect. Dis. 2011, 52, e103–e120. [Google Scholar] [CrossRef]
- Bader, M.S.; Loeb, M.; Leto, D.; Brooks, A.A. Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgrad. Med. 2020, 132, 234–250. [Google Scholar] [CrossRef]
- Amer, M.W.; Awwad, A.M. Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization, and antibacterial activity. Chem. Int. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Ansaldi, M.; Boulanger, P.; Brives, C.; Debarbieux, L.; Dufour, N.; Froissart, R.; Gandon, S.; Hénaff, C.L.; Petit, M.-A.; Rocha, E.; et al. Les applications antibactériennes des bactériophages. Virologie 2020, 24, 23–36. [Google Scholar] [CrossRef]
- Andersson, D.I.; Hughes, D.; Kubicek-Sutherland, J.Z. Mechanisms, and consequences of bacterial resistance to antimicrobial peptides. Drug Resist. Updat. 2016, 2, 43–57. [Google Scholar] [CrossRef]
- Arsene, J.M.M.; Davares, A.K.; Andreevna, S.L.; Vladimirovich, E.A.; Carime, B.Z.; Marouf, R.; Khelifi, I. The use of probiotics in animal feeding for safe production and as potential alternatives to antibiotics. Vet. World 2021, 14, 319–328. [Google Scholar] [CrossRef]
- Manga, A.M.J.; Podoprigora, I.V.; Davares AK, L.; Razan, M.; Das, M.S.; Senyagin, A.N. Antibacterial activity of grapefruit peel extracts and green-synthesized silver nanoparticles. Vet. World 2021, 14, 1330–1341. [Google Scholar] [CrossRef]
- Wojnicz, D.; Kucharska, A.Z.; Sokół-Łętowska, A.; Kicia, M.; Tichaczek-Goska, D. Medicinal plants extracts affect virulence factors expression and biofilm formation by the uropathogenic Escherichia coli. Urol. Res. 2012, 40, 683–697. [Google Scholar] [CrossRef]
- Kumar, S.; Budhwar, L.; Yadav, A.; Yadav, M.; Parkash Yadav, J. Phytochemical screening and antibacterial activity of Aloe vera collected from different climatic regions of India. Nat. Prod. J. 2016, 6, 73–82. [Google Scholar] [CrossRef]
- Kumar, S.; Kalita, S.; Das, A.; Kumar, P.; Singh, S.; Katiyar, V.; Mukherjee, A. Aloe vera: A contemporary overview on scope and prospects in food preservation and packaging. Prog. Org. Coat. 2022, 166, 106799. [Google Scholar] [CrossRef]
- Sarker, A.; Grift, T.E. Bioactive properties and potential applications of Aloe vera gel edible coating on fresh and minimally processed fruits and vegetables: A review. J. Food Meas. Charact. 2021, 15, 2119–2134. [Google Scholar] [CrossRef]
- Nizam, N.H.M.; Rawi, N.F.M.; Ramle, S.F.M.; Abd Aziz, A.; Abdullah, C.K.; Rashedi, A.; Kassim, M.H.M. Physical, thermal, mechanical, antimicrobial, and physicochemical properties of starch-based film containing Aloe vera: A review. J. Mater. Res. Technol. 2021, 15, 1572–1589. [Google Scholar] [CrossRef]
- Saleem, A.; Naureen, I.; Naeem, M.; Murad, H.S.; Maqsood, S.; Tasleem, G. Aloe vera Gel Effect on Skin and Pharmacological Properties. Sch. Int. J. Anat. Physiol. 2022, 5, 1–8. [Google Scholar] [CrossRef]
- Mbarga, M.J.A.; Viktorovna, P.I.; Mikhaïlovitch, M.K.; Davares AK, L.; Parfait, K.; Rehailia, M.; Shommiya, D. In vitro antimicrobial activity, antibioresistance reversal properties, and toxicity screen of ethanolic extracts of Heracleum mantegazzianum Sommier and Levier (giant hogweed), Centaurea jacea L.(brown knapweed), and Chenopodium album L.(Pigweed): Three invasive plants. Open Vet. J. 2022, 12, 584. [Google Scholar] [CrossRef]
- Arsene, M.M.J.; Viktorovna, P.I.; Davares, A.K.L.; Parfait, K.; Andreevna, S.L.; Mouafo, H.T.; Rehailia, M.; Vyacheslavovna, Y.N.; Pavlovna, S.I.; Manga, I.A.M.; et al. Antimicrobial and Antibiotic-Resistance Reversal Activity of Some Medicinal Plants from Cameroon against Selected Resistant and Non-Resistant Uropathogenic Bacteria. Front. Biosci.-Elite 2022, 14, 25. [Google Scholar] [CrossRef]
- Arsene, M.M.J.; Viktorovna, P.I.; Alla, M.V.; Mariya, M.A.; Sergei, G.V.; Cesar, E.; Davares, A.K.L.; Parfait, K.; Wilfrid, K.N.; Nikolay, T.S.; et al. Optimization of Ethanolic Extraction of Enantia chloranta Bark, Phytochemical Composition, Green Synthesis of Silver Nanoparticles, and Antimicrobial Activity. Fermentation 2022, 8, 530. [Google Scholar] [CrossRef]
- Khar’kov, Y.K.; Arsene, M.M.; Aliya, M.V.; Viktorovna, P.I.; Elena, V.G.; Azova, M.M.; Amira, A.A. Assessment of Antimicrobial Activity of Ethanolic and Aqueous Extracts of Aesculus hippocastanum L. (Horse Chestnut) Bark against Bacteria Isolated from Urine of Patients Diagnosed Positive to Urinary Tract Infections. Front. Biosci.-Sch. 2022, 14, 11. [Google Scholar] [CrossRef]
- Habibipour, R.; Moradi-Haghgou, L.; Farmany, A. Green synthesis of AgNPs@ PPE and its Pseudomonas aeruginosa biofilm formation activity compared to pomegranate peel extract. Int. J. Nanomed. 2019, 14, 6891–6899. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, A.M.; Ezzat, S.M.; El Naggar, M.M.; El Hawary, S.S. In vivo diabetic wound healing effect and HPLC–DAD–ESI–MS/MS profiling of the methanol extracts of eight Aloe species. Rev. Bras. Farmacogn. 2016, 26, 352–362. [Google Scholar] [CrossRef]
- Solaberrieta, I.; Jiménez, A.; Garrigós, M.C. Valorization of Aloe vera Skin By-Products to Obtain Bioactive Compounds by Microwave-Assisted Extraction: Antioxidant Activity and Chemical Composition. Antioxidants 2022, 11, 1058. [Google Scholar] [CrossRef]
- Quispe, C.; Villalobos, M.; Bórquez, J.; Simirgiotis, M. Chemical Composition and Antioxidant Activity of Aloe vera from the Pica Oasis (Tarapac´a, Chile) by UHPLC-Q/Orbitrap/MS/MS. J. Chem. 2018, 2018, 6123850. [Google Scholar] [CrossRef]
- Añibarro-Ortega, M.; Pinela, J.; Barros, L.; C´iric´, A.; Silva, S.P.; Coelho, E.; Mocan, A.; Calhelha, R.C.; Sokovic´, M.; Coimbra, M.A.; et al. Compositional Features and Bioactive Properties of Aloe vera Leaf (Fillet, Mucilage, and Rind) and Flower. Antioxidants 2019, 8, 444. [Google Scholar] [CrossRef]
- Debora, S.; Giuseppe, P.; Paolo, B. Aloe exudate: Characterization by reversed phase HPLC and headspace GC-MS. J. Agric. Food Chem. 2001, 49, 26–30. [Google Scholar] [CrossRef]
- Aida, P.U.I.A.; Chedea, V.S.; Levai, A.M.; Bocsan, I.C.; Buzoianu, A.D. Pot Aloe vera gel—A natural source of antioxidants. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 2. [Google Scholar] [CrossRef]
- Lee, S.; Do, S.-G.; Kim, S.Y.; Kim, J.; Jin, Y.; Lee, C.H. Mass Spectrometry-Based Metabolite Profiling and Antioxidant Activity of Aloe vera (Aloe barbadensis Miller) in Different Growth Stages. J. Agric. Food Chem. 2012, 60, 11222–11228. [Google Scholar] [CrossRef]
- Aldayel, T.S.; Grace, M.H.; Lila, M.A.; Yahya, M.A.; Omar, U.M.; Alshammary, G. LC-MS Characterization of Bioactive Metabolites from Two Yemeni Aloe Spp. with Antioxidant and Antidiabetic Properties. Arab. J. Chem. 2020, 13, 5040–5049. [Google Scholar] [CrossRef]
- Samira, B.; Ismahene, B.; Aicha, T.; Radia, D.; Abbes, B.; Chawki, B. Analysis of Phytochemical Constituents by using LC-MS, Antifungal and Allelopathic Activities of Leaves Extracts of Aloe Vera. Jordan J. Biol. Sci. 2022, 15, 21–28. [Google Scholar] [CrossRef]
- Mortazavi-Tabatabaei SA, R.; Ghaderkhani, J.; Nazari, A.; Sayehmiri, K.; Sayehmiri, F.; Pakzad, I. Pattern of antibacterial resistance in urinary tract infections: A systematic review and meta-analysis. Int. J. Prev. Med. 2019, 10, 169. [Google Scholar] [CrossRef] [PubMed]
- Hadi, U.; Van den Broek, P.; Kolopaking, E.P.; Zairina, N.; Gardjito, W.; Gyssens, I.C. Cross-sectional study of availability and pharmaceutical quality of antibiotics requested with or without prescription (Over The Counter) in Surabaya, Indonesia. BMC Infect. Dis. 2010, 10, 203. [Google Scholar] [CrossRef] [PubMed]
- De Kraker, M.E.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Arsène MM, J.; Davares AK, L.; Viktorovna, P.I.; Andreevna, S.L.; Sarra, S.; Khelifi, I.; Sergueïevna, D.M. The public health issue of antibiotic residues in food and feed: Causes, consequences, and potential solutions. Vet. World 2022, 15, 662. [Google Scholar] [CrossRef]
- Kirkwood, Z.I.; Millar, B.C.; Downey, D.G.; Moore, J.E. Antimicrobial effect of dimethyl sulfoxide and N, N-Dimethylformamide on Mycobacterium abscessus: Implications for antimicrobial susceptibility testing. Int. J. Mycobacteriol. 2018, 7, 134. [Google Scholar] [CrossRef]
- Corona, F.; Martinez, J.L. Phenotypic resistance to antibiotics. Antibiotics 2013, 2, 237–255. [Google Scholar] [CrossRef]
- Das, P.; Srivastav, A.K. Phytochemical extraction and characterization of the leaves of Aloe vera barbadensis for its anti-bacterial and antioxidant activity. Int. J. Sci. Res. 2015, 4, 658–661. [Google Scholar]
- Añibarro-Ortega, M.; Pinela, J.; Ćirić, A.; Lopes, E.; Molina, A.K.; Calhelha, R.C.; Soković, M.; Ferreira, O.; Ferreira, I.C.F.R.; Barros, L. Extraction of aloesin from Aloe vera rind using alternative green solvents: Process optimization and biological activity assessment. Biology 2021, 10, 951. [Google Scholar] [CrossRef]
- Hiruy, M.; Bisrat, D.; Mazumder, A.; Asres, K. Two chromones with antimicrobial activity from the leaf latex of Aloe monticola Reynolds. Nat. Prod. Res. 2021, 35, 1052–1056. [Google Scholar] [CrossRef]
- Li, T.; Lu, Y.; Zhang, H.; Wang, L.; Beier, R.C.; Jin, Y.; Wang, W.; Li, H.; Hou, X. Antibacterial Activity and Membrane-Targeting Mechanism of Aloe-Emodin Against Staphylococcus epidermidis. Front. Microbiol. 2021, 12, 621866. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Zeng, Y.; Liu, Y.; You, L.; Yin, X.; Fu, J.; Ni, J. Aloe-emodin: A review of its pharmacology, toxicity, and pharmacokinetics. Phytother. Res. 2021, 34, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V. Potential of Cameroonian plants and derived products against microbial infections: A review. Planta Med. 2010, 76, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Kuete, V.; Efferth, T. Cameroonian medicinal plants: Pharmacology and derived natural products. Front. Pharmacol. 2010, 1, 123. [Google Scholar] [CrossRef]
- Ouidir, T.; Gabriel, B.; Chabane, Y.N. Overview of multi-species biofilms in different ecosystems: Wastewater treatment, soil and oral cavity. J. Biotechnol. 2022, 350, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gutierrez, F.; Boegli, L.; Agostinho, A.; Sánchez, E.M.; Bach, H.; Ruiz, F.; James, G. Anti-biofilm activity of silver nanoparticles against different microorganisms. Biofouling 2013, 29, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Goller, C.C.; Romeo, T. Environmental influences on biofilm development. Bact. Biofilms 2008, 322, 37–66. [Google Scholar] [CrossRef]
- Mah TF, C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Mann, R.; Holmes, A.; McNeilly, O.; Cavaliere, R.; Sotiriou, G.A.; Rice, S.A.; Gunawan, C. Evolution of biofilm-forming pathogenic bacteria in the presence of nanoparticles and antibiotic: Adaptation phenomena and cross-resistance. J. Nanobiotechnol. 2021, 19, 291. [Google Scholar] [CrossRef]
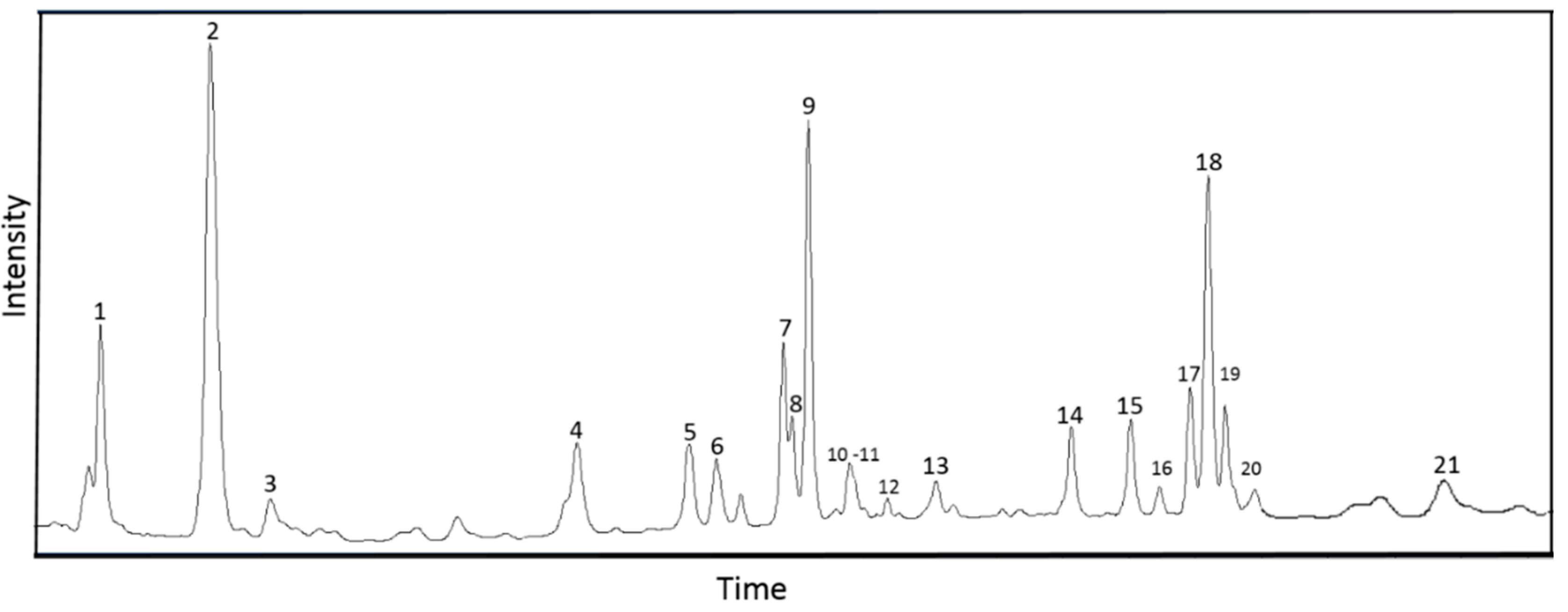
| No. | Compound | Retention Time, min | Molecular Formula | Structural Formula | Molecular Mass, Da | m/z, Registration of Positive Ions | m/z, Registration of Negative Ions | Content, % | References |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Trans-5-O-Caffeoylquinic acid | 3.52 | C16H18O9 |  | 354 | 355 [M + H]+, 377 [M + Na]+, 731 [2M + Na]+ | 191 [C7H11O6]−, 353 [M–H]−, 707 [2M–H]− | 6.68 ± 0.45 | [25,26,27] |
| 2 | Aloesin | 5.34 | C19H22O9 |  | 394 | 395 [M + H]+, 811 [2M + Na]+ | 393 [M–H]− | 30.22 ± 0.43 | [25,26,28,29] |
| 3 | Chlorogenic acid (3-O-Caffeoylquinic acid) | 6.63 | C16H18O9 | 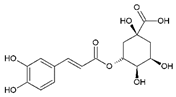 | 354 | 355 [M + H]+, 377 [M + Na]+, 731 [2M + Na]+ | 191 [C7H11O6]−, 353 [M–H]−, 707 [2M–H]− | 1.71 ± 0.05 | [25,26] |
| 4 | Trans-5-p-Coumaroylquinic acid | 10.75 | C16H18O8 |  | 338 | 147 [C9H7O2]+, 339 [M + H]+ | 337 [M–H]−, 675 [2M–H]− | 6.86 ± 0.39 | [25,27] |
| 5 | Luteolin 6,8-di-C-glucoside | 12.47 | C27H30O16 | 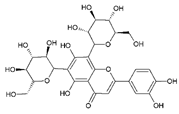 | 610 | 287 [C15H11O6]+, 449 [M–glu]+, 611 [M + H]+, 633 [M + Na]+ | 285 [C15H9O6]−, 447 [M–glu]−, 609 [M–H]− | 3.34 ± 0.01 | [24,25,26,27] |
| 6 | Cis-5-p-Coumaroylquinic acid | 12.92 | C16H18O8 | 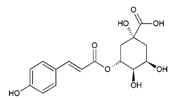 | 338 | 147 [C9H7O2]+, 339 [M + H]+ | 337 [M–H]−, 675 [2M–H]− | 2.83 ± 0.01 | [25,27] |
| 7 | 8-O-Methyl-7-hydroxyaloin B | 13.88 | C22H24O10 |  | 448 | 449 [M + H]+, 471 [M + Na]+ | 447 [M–H]− | 1.03 ± 0.01 | [25,28,30,31] |
| 8 | 8-O-Methyl-7-hydroxyaloin A | 13.91 | C22H24O10 | 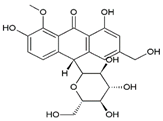 | 448 | 449 [M + H]+, 471 [M + Na]+ | 447 [M–H]− | 7.40 ± 0.08 | [25,28,30,31] |
| 9 | Aloe-emodine-diglucoside | 14.29 | C27H30O15 |  | 594 | 595 [M + H]+, 617 [M + Na]+ | 593 [M–H]− | 12.58 ± 0.34 | [24,32] |
| 10 | Aloinoside B | 14.72 | C27H32O13 | 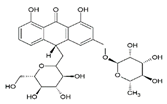 | 564 | 565 [M + H]+ | 563 [M–H]− | 1.81 ± 0.01 | [24,28] |
| 11 | Unidentified component, MM = 716 Da | 14.9 | - | - | 716 | 717 [M + H]+ | 715 [M–H]− | 1.92 ± 0.08 | |
| 12 | Aloinoside A | 15.2 | C27H32O13 |  | 564 | 565 [M + H]+ | 563 [M–H]− | 0.51 ± 0.01 | [24,28,29] |
| 13 | Unidentified component, MM = 716 Da | 16.18 | - | - | 716 | 717 [M + H]+ | 715 [M–H]− | 1.25 ± 0.01 | |
| 14 | 2′-O-Feruloyaloesin | 18.23 | C29H30O12 | 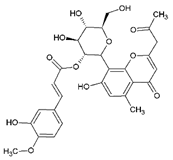 | 570 | 571 [M + H]+ | 569 [M–H]− | 3.65 ± 0.03 | [24] |
| 15 | Aloin B | 19.12 | C21H22O9 |  | 418 | 419 [M + H]+, 441 [M + Na]+ | 297 [C17H13O5]−, 418 [M–H]− | 1.16 ± 0.02 | [24,25,26,28,29,32] |
| 16 | Aloe-emodin-glucoside | 19.55 | C27H30O15 |  | 432 | 271 [C15H11O5]+, 433 [M + H]+ | 269 [C15H9O5]−, 431 [M–H]− | 0.80 ± 0.01 | [24,26,29] |
| 17 | Aloin A | 20.02 | C21H22O9 | 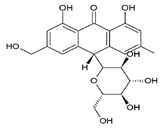 | 418 | 419 [M + H]+, 441 [M + Na]+ | 297 [C17H13O5]−, 418 [M–H]− | 1.29 ± 0.07 | [24,25,26,28,29,32] |
| 18 | 2′-p-Methoxycoumaroylaloeresin B | 20.28 | C29H30O11 |  | 554 | 555 [M + H]+ | 553 [M–H]− | 9.64 ± 0.13 | [26,29] |
| 19 | 6′-Malonylnataloin | 20.53 | C24H24O12 |  | 504 | 505 [M + H]+, 527 [M + Na]+ | 459 [C23H23O10]−, 503 [M–H]− | 0.94 ± 0.04 | [24,26] |
| 20 | Aloe-emodin-glucoside | 20.66 | C21H20O10 |  | 432 | 271 [C15H11O5]+, 433 [M + H]+ | 269 [C15H9O5]−, 431 [M–H]− | 3.89 ± 0.15 | [26,29] |
| 21 | Aloe-emodin | 24.57 | C15H10O5 |  | 270 | 271 [C15H11O5]+ | 269 [C15H9O5]− | 0.82 ± 0.01 | [25,28,29] |
| Chemical Elements | Average Fluorescence Intensity, imp/µA | Standard Deviation |
|---|---|---|
| Si | 0.0617 | 0.0031 |
| S | 0.1335 | 0.0019 |
| Cl | 0.0274 | 0.0026 |
| K | 0.2459 | 0.0033 |
| Br | 0.0492 | 0.0023 |
| NIT | TE | CTR | AMC | FO | CAZ | IPM | CAC | CIP | AMP | TR | MDR Index | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ac. Xylosoxidans 4892 | 6 ± 0 (I) | 11 ± 0 (R) | 23 ± 2 (S) | 36 ± 4 (S) | 6 ± 0 (R) | 16 ± 0 (I) | 32 ± 3 (S) | 16 ± 1 (I) | 20 ± 2 (I) | 20 ± 1 (S) | 6 ± 0 (R) | 0.36 |
| C. freundii 426 | 21 ± 1 (S) | 30 ± 3 (S) | 27 ± 2 (S) | 35 ± 1 (S) | 40 ± 2 (S) | 12 ± 0 (R) | 37 ± 1 (S) | 10 ± 0 (R) | 30 ± 2 (S) | 6 ± 0 (R) | 22 ± 2 (S) | 0.27 |
| E. coli 1449 | 24 ± 3 (S) | 11 ± 0 (R) | 8 ± 0 (R) | 27 ± 1 (S) | 30 ± 1 (S) | 7 ± 0 (R) | 22 ± 2 (S) | 12 ± 0 (R) | 26 ± 1 (S) | 6 ± 0 (R) | 6 ± 0 (R) | 0.54 |
| K. oxytoca 3003 | 20 ± 1 (S) | 8 ± 0 (R) | 22 ± 0 (S) | 24 ± 1 (S) | 25 ± 3 (S) | 15 ± 1 (I) | 27 ± 3 (S) | 6 ± 0 (R) | 30 ± 2 (S) | 6 ± 0 (R) | 6 ± 0 (R) | 0.36 |
| M. catarrhalis 4222 | 12 ± 1 (R) | 22 ± 2 (S) | 24 ± 1 (S) | 36 ± 3 (S) | 27 ± 2 (S) | 16 ± 0 (I) | 27 ± 1 (S) | 21 ± 0 (S) | 32 ± 4 (S) | 10 ± 0 (R) | 15 ± 1 (I) | 0.18 |
| M. morganii 1543 | 15 ± 0 (I) | 6 ± 0 (R) | 33 ± 2 (S) | 17 ± 1 (I) | 13 ± 0 (R) | 23 ± 1 (S) | 22 ± 0 (S) | 23 ± 1 (S) | 22 ± 2 (S) | 10 ± 0 (R) | 6 ± 0 (R) | 0.36 |
| P. aeruginosa 3057 | 6 ± 0 (R) | 13 ± 1 (R) | 21 ± 1 (S) | 16 ± 0 (I) | 27 ± 1 (S) | 15 ± 0 (I) | 34 ± 4 (S) | 22 ± 2 (S) | 30 ± 1 (S) | 6 ± 0 (R) | 6 ± 0 (R) | 0.36 |
| E. coli ATCC 25922 | 21 ± 3 (S) | 30 ± 1 (S) | 24 ± 3 (S) | 20 ± 0 (S) | 40 ± 3 (S) | 18 ± 1 (S) | 32 ± 2 (S) | 16 ± 0 (S) | 24 ± 3 (S) | 26 ± 1 (S) | 24 ± 3 (S) | 0.00 |
| MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | |
|---|---|---|---|
| Ac. Xylosoxidans 4892 | 5 | 10 | 2 |
| C. freundii 426 | 2.5 | 10 | 4 |
| E. coli 1449 | >20 | >20 | - |
| K. oxytoca 3003 | 1.25 | 10 | 8 |
| M. catarrhalis 4222 | 1.25 | 5 | 4 |
| M. morganii 1543 | 5 | 10 | 2 |
| P. aeruginosa 3057 | 2.5 | 10 | 4 |
| E. coli ATCC 25922 | 0.625 | 1.25 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsene, M.M.J.; Viktorovna, P.I.; Sergei, G.V.; Hajjar, F.; Vyacheslavovna, Y.N.; Vladimirovna, Z.A.; Aleksandrovna, V.E.; Nikolayevich, S.A.; Sachivkina, N. Phytochemical Analysis, Antibacterial and Antibiofilm Activities of Aloe vera Aqueous Extract against Selected Resistant Gram-Negative Bacteria Involved in Urinary Tract Infections. Fermentation 2022, 8, 626. https://doi.org/10.3390/fermentation8110626
Arsene MMJ, Viktorovna PI, Sergei GV, Hajjar F, Vyacheslavovna YN, Vladimirovna ZA, Aleksandrovna VE, Nikolayevich SA, Sachivkina N. Phytochemical Analysis, Antibacterial and Antibiofilm Activities of Aloe vera Aqueous Extract against Selected Resistant Gram-Negative Bacteria Involved in Urinary Tract Infections. Fermentation. 2022; 8(11):626. https://doi.org/10.3390/fermentation8110626
Chicago/Turabian StyleArsene, Mbarga M. J., Podoprigora I. Viktorovna, Goriainov V. Sergei, Fadi Hajjar, Yashina N. Vyacheslavovna, Zhigunova A. Vladimirovna, Vasilyeva E. Aleksandrovna, Senyagin A. Nikolayevich, and Nadezhda Sachivkina. 2022. "Phytochemical Analysis, Antibacterial and Antibiofilm Activities of Aloe vera Aqueous Extract against Selected Resistant Gram-Negative Bacteria Involved in Urinary Tract Infections" Fermentation 8, no. 11: 626. https://doi.org/10.3390/fermentation8110626
APA StyleArsene, M. M. J., Viktorovna, P. I., Sergei, G. V., Hajjar, F., Vyacheslavovna, Y. N., Vladimirovna, Z. A., Aleksandrovna, V. E., Nikolayevich, S. A., & Sachivkina, N. (2022). Phytochemical Analysis, Antibacterial and Antibiofilm Activities of Aloe vera Aqueous Extract against Selected Resistant Gram-Negative Bacteria Involved in Urinary Tract Infections. Fermentation, 8(11), 626. https://doi.org/10.3390/fermentation8110626







