Bioactivity of Organic Fermented Soymilk as Next-Generation Prebiotic/Probiotics Mixture
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Soymilk Preparation
2.3. Bacteria Used and Standard Inoculum Preparation
2.3.1. Preparation of Standard Fermented Bacterial Inoculum and Inoculation of Soymilk
2.3.2. Preparation of Standard Pathogenic Bacterial Inoculum
2.4. Antibacterial Activity
2.4.1. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.4.2. Evaluation of the Bacteriostatic and Bactericidal Effect
2.5. Quantitative Analysis of Antioxidants by Measuring a Di Phenyl Picrylhydrazyl (DPPH) Free
2.6. Sensory Properties Evaluation of Fermented Soymilk
2.7. Shelf Life of Fermented Soymilk
2.8. Analytical Methods
2.8.1. pH Value
2.8.2. Determination of Total Acidity
2.8.3. Determination of Total Phenolic Content, Free Amino Acids, Saponin, and Isoflavone
- Preparation of solvent extracts
- Determination of total phenolic contents
- The free amino acid content measurement:
- Quantification of total saponin
- HPLC analysis of the soy isoflavone
2.9. Statistical Analysis
3. Results and Discussion
3.1. Antibacterial Effect of Fermented Soymilk Products against Pathogenic Bacterial Strains
3.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Fermented Soymilk Products
3.3. Antibacterial Mode of Action
3.4. The Total Phenolic, Antioxidant, Aglycone Isoflavones, Free Amino Acids, and Saponin Contents in the Soymilk Fermented by Consortia Probiotic Bacterial Cultures L. plantarum + Lc. thermophilus + B. longum Probiotic Bacterial Strains
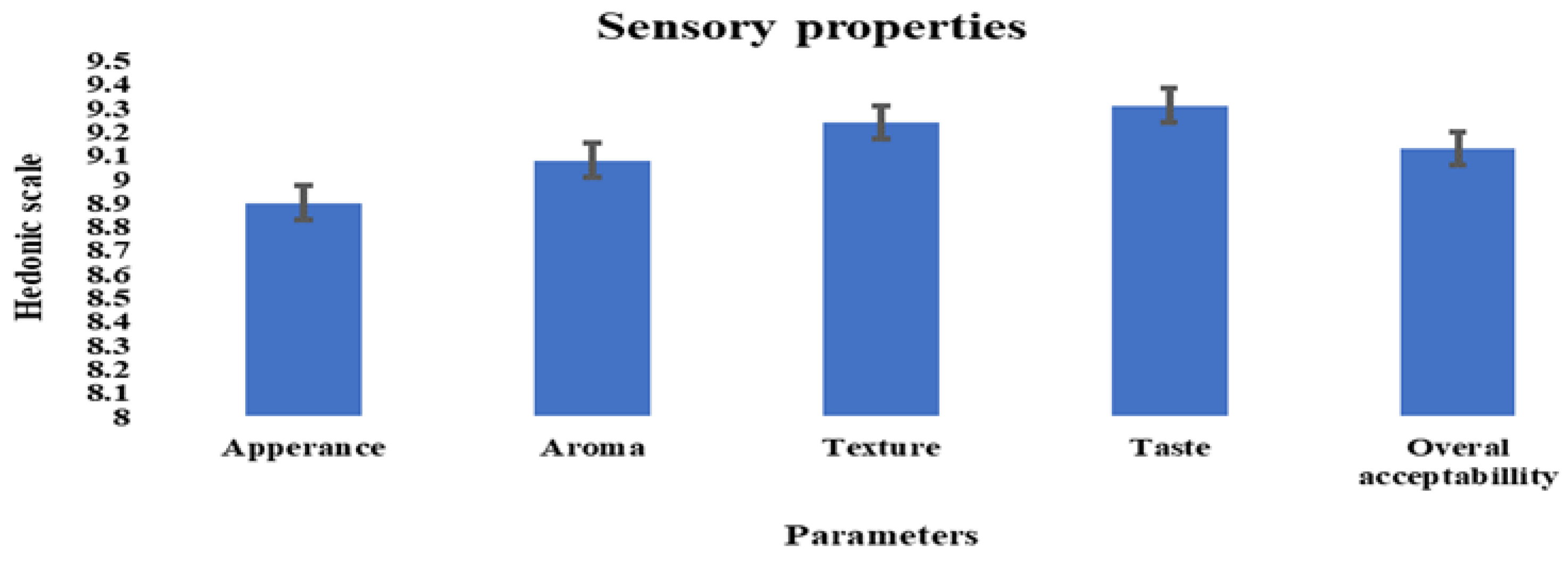
3.5. Sensory Properties Evaluation of Fermented Soymilk
3.6. Shelf Life (Storage) Period of Fermented Soymilk
Viability of Probiotic Bacterial Cultures and Change in pH and Total Acidity during Shelf Life of Fermented Soymilk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galdeano, C.M.; Cazorla, S.I.; Dumit, J.M.L.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of probiotics for human health: A review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Nasr, N. Psychological impact of probiotics and fermented foods on mental health of human in integrated healthy lifestyle. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2815–2822. [Google Scholar] [CrossRef]
- Jeske, S.; Zannini, E.; Arendt, E.K. Past, present and future: The strength of plant-based dairy substitutes based on gluten-free raw materials. Food Res. Int. 2018, 110, 42–51. [Google Scholar] [CrossRef]
- Rizzo, G.; Baroni, L. Soy, soy foods and their role in vegetarian diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Tyagi, S.K.; Anurag, R.K. Plant-based milk alternatives an emerging segment of functional beverages: A review. J. Food Sci. Technol. 2016, 53, 3408–3423. [Google Scholar] [CrossRef]
- Peng, X.; Guo, S. Texture characteristics of soymilk gels formed by lactic fermentation: A comparison of soymilk prepared by blanching soybeans under different temperatures. Food Hydrocoll. 2015, 43, 58–65. [Google Scholar] [CrossRef]
- Battistini, C.; Gullón, B.; Ichimura, E.S.; Gomes, A.M.P.; Ribeiro, E.P.; Kunigk, L.; Moreira, J.U.V.; Jurkiewicz, C. Development and characterization of an innovative synbiotic fermented beverage based on vegetable soybean. Braz. J. Microbiol. 2018, 49, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.-H.; Green-Johnson, J.M.; Buckley, N.D.; Lin, Q.-Y. Bioactivity of soy-based fermented foods: A review. Biotechnol. Adv. 2019, 37, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Olaoye, O.; Ndife, J.; Raymond, V. Use of lactobacillus plantarum as starter culture and its influence on physicochemical, microbiological, and sensory characteristics of Kunnu-Aya produced from sorghum and tigernut. J. Food Qual. 2017, 2017, 6738137. [Google Scholar] [CrossRef]
- Ziaei, S.; Halaby, R. Dietary isoflavones and breast cancer risk. Medicines 2017, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-S.; Yang, C.-Y.; Fang, T.J. Strategic ultrasound-induced stress response of lactic acid bacteria on enhancement of β-glucosidase activity for bioconversion of isoflavones in soymilk. J. Microbiol. Methods 2018, 148, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Patel, N.; Mandal, S. Comparative growth behaviour and biofunctionality of lactic acid bacteria during fermentation of soy milk and bovine milk. Probiotics Antimicrob. Proteins 2018, 10, 277–283. [Google Scholar] [CrossRef]
- Shah, N.P. Functional cultures and health benefits. Int. Dairy J. 2007, 17, 1262–1277. [Google Scholar] [CrossRef]
- TURGAY, Ö.; Erbilir, F. Isolation and characterization of Lactobacillus bulgaricus and Lactobacillus casei from various foods. Turk. J. Biol. 2006, 30, 39–44. [Google Scholar]
- Fijan, S. Antimicrobial effect of probiotics against common pathogens. Probiotics Prebiotics Hum. Nutr. Health 2016, 10, 5772. [Google Scholar] [CrossRef]
- Zhao, G.-R.; Xiang, Z.-J.; Ye, T.-X.; Yuan, Y.-J.; Guo, Z.-X. Antioxidant activities of Salvia miltiorrhiza and Panax notoginseng. Food Chem. 2006, 99, 767–774. [Google Scholar] [CrossRef]
- Ma, L.; Li, B.; Han, F.; Yan, S.; Wang, L.; Sun, J. Evaluation of the chemical quality traits of soybean seeds, as related to sensory attributes of soymilk. Food Chem. 2015, 173, 694–701. [Google Scholar] [CrossRef]
- Olorunnisomo, O.; Ososanya, T.; Adedeji, O. Homogenization of milk and its effect on sensory and physico-chemical properties of yoghurt. Afr. J. Food Sci. 2014, 8, 465–470. [Google Scholar] [CrossRef][Green Version]
- Tang, Y.; Jiang, Z.; Yuan, G.; Huan, L.; Yu, Z.; Yue, G.; Yan, T. Study on Antioxidant Properties of Fermented Soymilk by Lactobacillus casei-16. Food Res. Dev. 2018, 39, 1–9. [Google Scholar]
- Chen, Y.-F.; Chiang, M.-L.; Chou, C.-C.; Lo, Y.-C. Enhancing the antitumor cell proliferation and Cu2 + -chelating effects of black soybeans through fermentation with Aspergillus awamori. J. Biosci. Bioeng. 2013, 115, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Rui, X.; Wang, D.; Liu, M.; Chen, X.; Dong, M. Effect of fermentation pH on protein bioaccessibility of soymilk curd with added tea polyphenols as assessed by in vitro gastrointestinal digestion. J. Agric. Food Chem. 2017, 65, 11125–11132. [Google Scholar] [CrossRef]
- Dini, I.; Schettino, O.; Simioli, T.; Dini, A. Studies on the constituents of Chenopodium quinoa seeds: Isolation and characterization of new triterpene saponins. J. Agric. Food Chem. 2001, 49, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Bryman, A.; Cramer, D. Quantitative Data Analysis with IBM SPSS 17, 18 & 19: A Guide for Social Scientists; Routledge: London, UK, 2012. [Google Scholar]
- Singh, B.P.; Vij, S.; Hati, S.; Singh, D.; Kumari, P.; Minj, J. Antimicrobial activity of bioactive peptides derived from fermentation of soy milk by Lactobacillus plantarum C2 against common foodborne pathogens. Int. J. Fermented Foods 2015, 4, 91–99. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Wu, Y.-F.; Wang, Y.-S.; Wang, X.-Z.; Piao, C.-H.; Liu, J.-M.; Liu, Y.-L.; Wang, Y.-H. The protective effects of probiotic-fermented soymilk on high-fat diet-induced hyperlipidemia and liver injury. J. Funct. Foods 2017, 30, 220–227. [Google Scholar] [CrossRef]
- Zhao, D.; Shah, N.P. Influence of tea extract supplementation on bifidobacteria during soymilk fermentation. Int. J. Food Microbiol. 2014, 188, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Buljat, A.M.; Jurina, T.; Jurinjak Tušek, A.; Valinger, D.; Gajdoš Kljusurić, J.; Benković, M. Applicability of foam mat drying process for production of instant cocoa powder enriched with lavender extract. Food Technol. Biotechnol. 2019, 57, 159–170. [Google Scholar] [CrossRef]
- Singh, B.P.; Bhushan, B.; Vij, S. Antioxidative, ACE inhibitory and antibacterial activities of soy milk fermented by indigenous strains of lactobacilli. Legume Sci. 2020, 2, e54. [Google Scholar] [CrossRef]
- Rekha, C.; Vijayalakshmi, G. Isoflavone phytoestrogens in soymilk fermented with β-glucosidase producing probiotic lactic acid bacteria. Int. J. Food Sci. Nutr. 2011, 62, 111–120. [Google Scholar] [CrossRef]
- Donkor, O.; Shah, N.P. Production of β-Glucosidase and Hydrolysis of Isoflavone Phytoestrogens by Lactobacillus acidophilus, Bifidobacterium lactis, and Lactobacillus casei in Soymilk. J. Food Sci. 2008, 73, M15–M20. [Google Scholar] [CrossRef] [PubMed]
- Trupti, J.U.; Das, S.; Solanki, D.; Kinariwala, D.; Hati, S. Bioactivities and ACE-inhibitory peptides releasing potential of lactic acid bacteria in fermented soy milk. Food Prod. Processing Nutr. 2021, 3, 10. [Google Scholar] [CrossRef]
- Marazza, J.A.; Nazareno, M.A.; de Giori, G.S.; Garro, M.S. Enhancement of the antioxidant capacity of soymilk by fermentation with Lactobacillus rhamnosus. J. Funct. Foods 2012, 4, 594–601. [Google Scholar] [CrossRef]
- Vij, S.; Subrota, H.; Deepika, Y. Biofunctionality of probiotic soy yoghurt. Food Nutr. Sci. 2011, 2, 5819. [Google Scholar] [CrossRef]
- Tachakittirungrod, S.; Okonogi, S.; Chowwanapoonpohn, S. Study on antioxidant activity of certain plants in Thailand: Mechanism of antioxidant action of guava leaf extract. Food Chem. 2007, 103, 381–388. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.; Shaker, R.; Osaili, T.; Al-Taani, M.; Olaimat, A.; Awaisheh, S.; Abushelaibi, A.; Holley, R. Sensory evaluation of flavored soy milk-based yogurt: A comparison between Jordanian and Malaysian consumers. J. Food Sci. Eng. 2014, 4, 27. [Google Scholar]
- Ertem, H.; Çakmakçı, S. Shelf life and quality of probiotic yogurt produced with Lactobacillus acidophilus and Gobdin. Int. J. Food Sci. Technol. 2018, 53, 776–783. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Madrona, G.S.; Garcia, S.; Prudencio, S.H. Probiotic viability, physicochemical characteristics and acceptability during refrigerated storage of clarified apple juice supplemented with Lactobacillus paracasei ssp. paracasei and oligofructose in different package type. LWT-Food Sci. Technol. 2015, 63, 415–422. [Google Scholar] [CrossRef]


| Probiotic Strain | T1 | T4 | T5 | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Pathogenic Bacterial Strains | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Spectrum Activity (%) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Spectrum Activity (%) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Spectrum Activity (%) |
| MIC | 1 | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | - | 100 |
| 0.5 | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | - | 100 | |
| 0.25 | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | - | 100 | |
| 0.125 | + | + | + | - | - | - | + | 43 | - | - | - | - | - | - | + | 85 | - | - | - | - | - | - | + | 85 | |
| 0.0625 | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | 0 | |
| 0.0312 | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | 0 | |
| MIC value | 0.25 | 0.25 | 0.25 | 0.125 | 0.125 | 0.125 | 0.25 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.25 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.125 | 0.25 | ||||
| MBC | 1 | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | - | 100 | - | - | - | - | - | - | - | 100 |
| 0.5 | + | - | + | - | - | - | + | 57 | + | - | - | - | - | - | + | 72 | - | - | - | - | - | - | + | 85 | |
| 0.25 | + | + | + | - | - | - | + | 43 | + | + | - | - | - | - | + | 57 | + | - | - | - | - | - | + | 72 | |
| 0.125 | + | + | + | + | + | + | + | 0 | + | + | + | - | - | - | + | 43 | + | - | + | - | - | - | + | 43 | |
| 0.0625 | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | 0 | |
| 0.0312 | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | 0 | |
| MBC value | 1 | 0.5 | 1 | 0.25 | 0.25 | 0.25 | 1 | 1 | 0.5 | 0.25 | 0.125 | 0.125 | 0.125 | 1 | 0.50 | 0.25 | 0.25 | 0.125 | 0.125 | 0.125 | 1 | ||||
| MBC/MIC Ratio | 4 | 2 | 4 | 2 | 2 | 2 | 4 | 8 | 4 | 2 | 1 | 1 | 1 | 4 | 4 | 2 | 2 | 1 | 1 | 1 | 4 | ||||
| * Effect | - | + | - | + | + | + | - | - | - | + | + | + | + | - | - | + | + | + | + | + | - | ||||
| Parameters | Treatment | ||
|---|---|---|---|
| Control | Fermented Soymilk | ||
| Total phenol content (mg/mL) | Ethanol extract | 39.70 ± 0.02 aB | 43.75 ± 0.12 aA |
| Water extract | 25.13 ± 0.43 bB | 31.87 ± 0.30 bA | |
| Inhibition of DPPH (%) | Ethanol extract | 1.00 ± 0.05 aB | 80.0 ± 0.10 aA |
| Water extract | 1.00 ± 0.07 aB | 79.93 ± 0.1 bA | |
| Saponin contents (mg/mL) | Ethanol extract | 3.1 ± 0.01 aA | 2.5 ± 0.03 aB |
| Water extract | 0.9 ± 0.01 bA | 0.5 ± 0.05 bB | |
| Isoflavones concentration (mg/mL) | Diadzin | 1.5 ± 0.01 bA | 0.50 ± 0.07 cB |
| Genistin | 2.50 ± 0.02 aA | 0.39 ± 0.01 dB | |
| Daidzein | 0.02 ± 0.07 dB | 0.75 ± 0.08 bA | |
| Genistein | 0.4 ± 0.03 cB | 1.5 ± 0.02 aA | |
| Free amino acids (mg/mL) | 0.27 ± 0.01 B | 0.5 ± 0.09 A | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelghani, D.Y.; Gad, A.I.; Orabi, M.M.; Abou-Taleb, K.A.; Mahmoud, E.A.; Al Amoudi, S.A.; Zari, A.; Althubaiti, E.H.; Edris, S.; Amin, S.A. Bioactivity of Organic Fermented Soymilk as Next-Generation Prebiotic/Probiotics Mixture. Fermentation 2022, 8, 513. https://doi.org/10.3390/fermentation8100513
Abdelghani DY, Gad AI, Orabi MM, Abou-Taleb KA, Mahmoud EA, Al Amoudi SA, Zari A, Althubaiti EH, Edris S, Amin SA. Bioactivity of Organic Fermented Soymilk as Next-Generation Prebiotic/Probiotics Mixture. Fermentation. 2022; 8(10):513. https://doi.org/10.3390/fermentation8100513
Chicago/Turabian StyleAbdelghani, Dina Y., Abdallah I. Gad, Mona M. Orabi, Khadiga A. Abou-Taleb, Emam A. Mahmoud, Soha A. Al Amoudi, Ali Zari, Eman Hillal Althubaiti, Sherif Edris, and Shimaa A. Amin. 2022. "Bioactivity of Organic Fermented Soymilk as Next-Generation Prebiotic/Probiotics Mixture" Fermentation 8, no. 10: 513. https://doi.org/10.3390/fermentation8100513
APA StyleAbdelghani, D. Y., Gad, A. I., Orabi, M. M., Abou-Taleb, K. A., Mahmoud, E. A., Al Amoudi, S. A., Zari, A., Althubaiti, E. H., Edris, S., & Amin, S. A. (2022). Bioactivity of Organic Fermented Soymilk as Next-Generation Prebiotic/Probiotics Mixture. Fermentation, 8(10), 513. https://doi.org/10.3390/fermentation8100513







