Lacticaseibacillus paracasei KC39 Immobilized on Prebiotic Wheat Bran to Manufacture Functional Soft White Cheese
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Microorganisms
2.2. Ready-to-Use Freeze-Dried Synbiotic Biocatalyst and Cell Survival Assessment
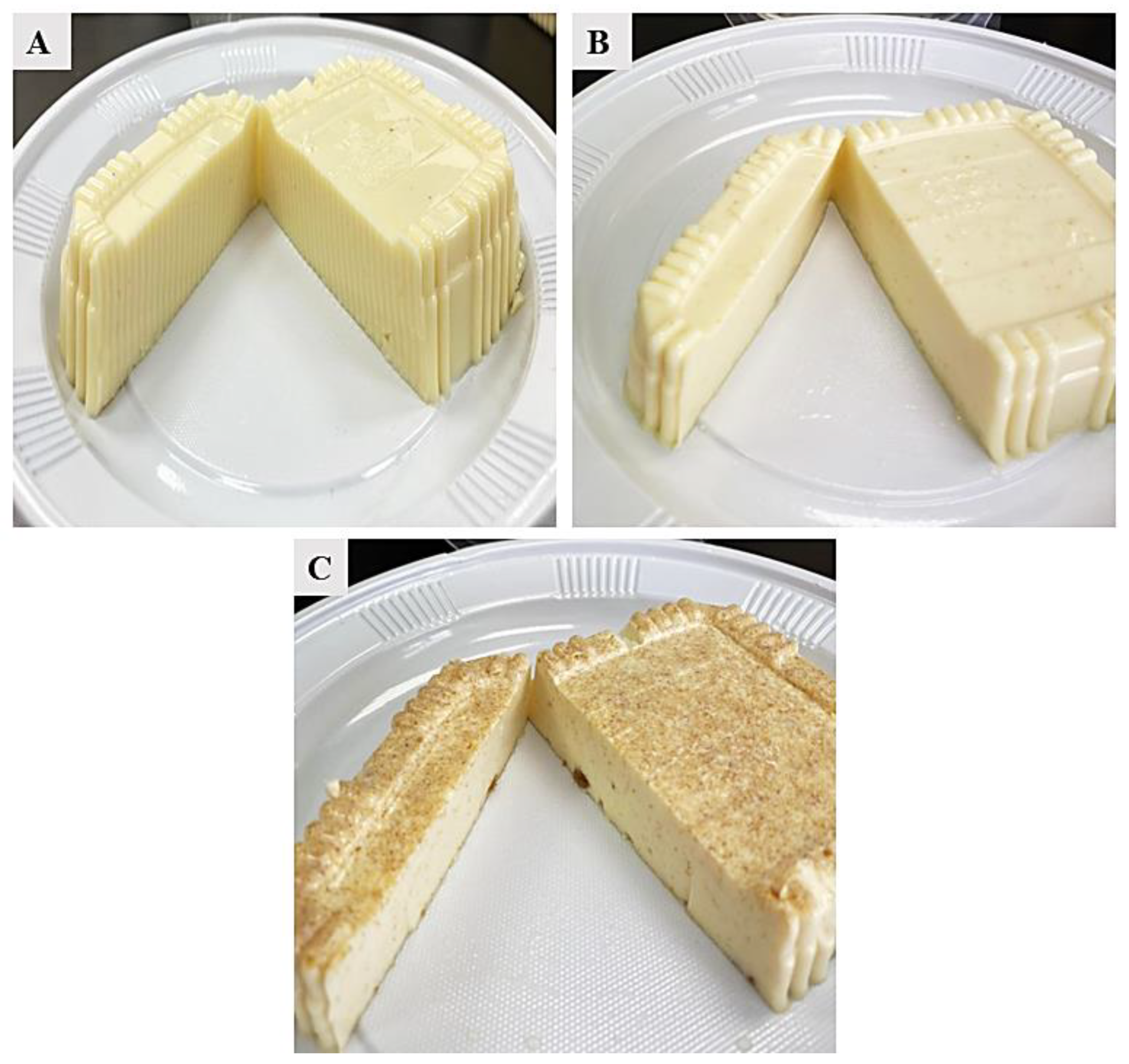
2.3. Functional Soft White Cheese Production
2.4. Physicochemical Analysis
2.5. Texture Profile Analyses
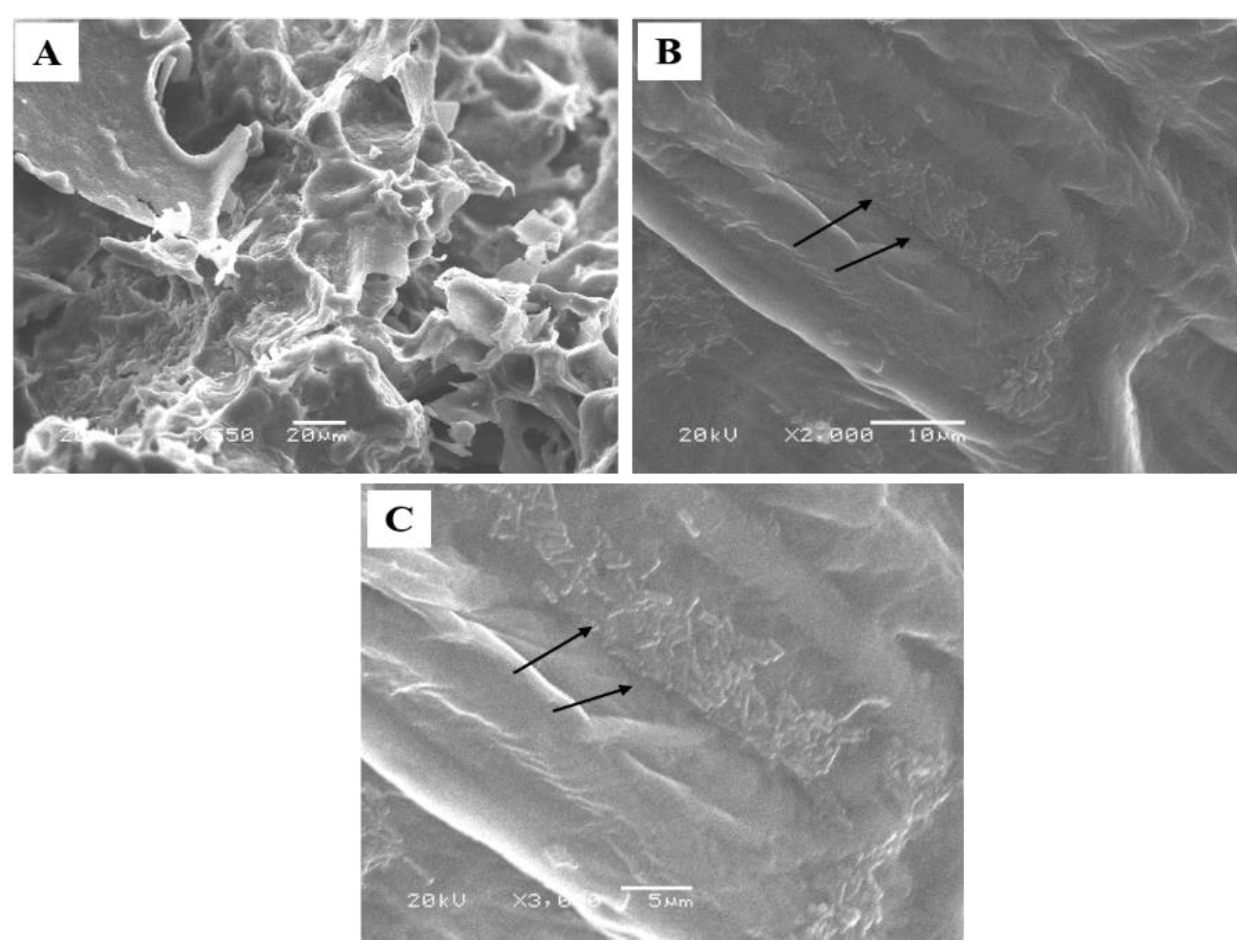
2.6. Scanning Electron Microscopy
2.7. Simulated Gastrointestinal Digestion
2.8. Microbiological Profile Analysis of Cheese
2.9. Solid-Phase Microextraction GC-MS Analysis
2.10. Sensory Evaluation
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Freeze-Dried Immobilized Lc. paracasei
3.1.1. Stability of Immobilized Lc. paracasei during Storage
3.1.2. Morphological Analyses
3.2. Physicochemical Characteristics of Functional White Cheese
3.3. Texture Profile Analyses
3.4. Microstructure of Cheese Samples
3.5. Cell Survival in Simulated Gastric Intestinal Juice
3.6. Lacticaseibacillus Paracasei Growth Capacity
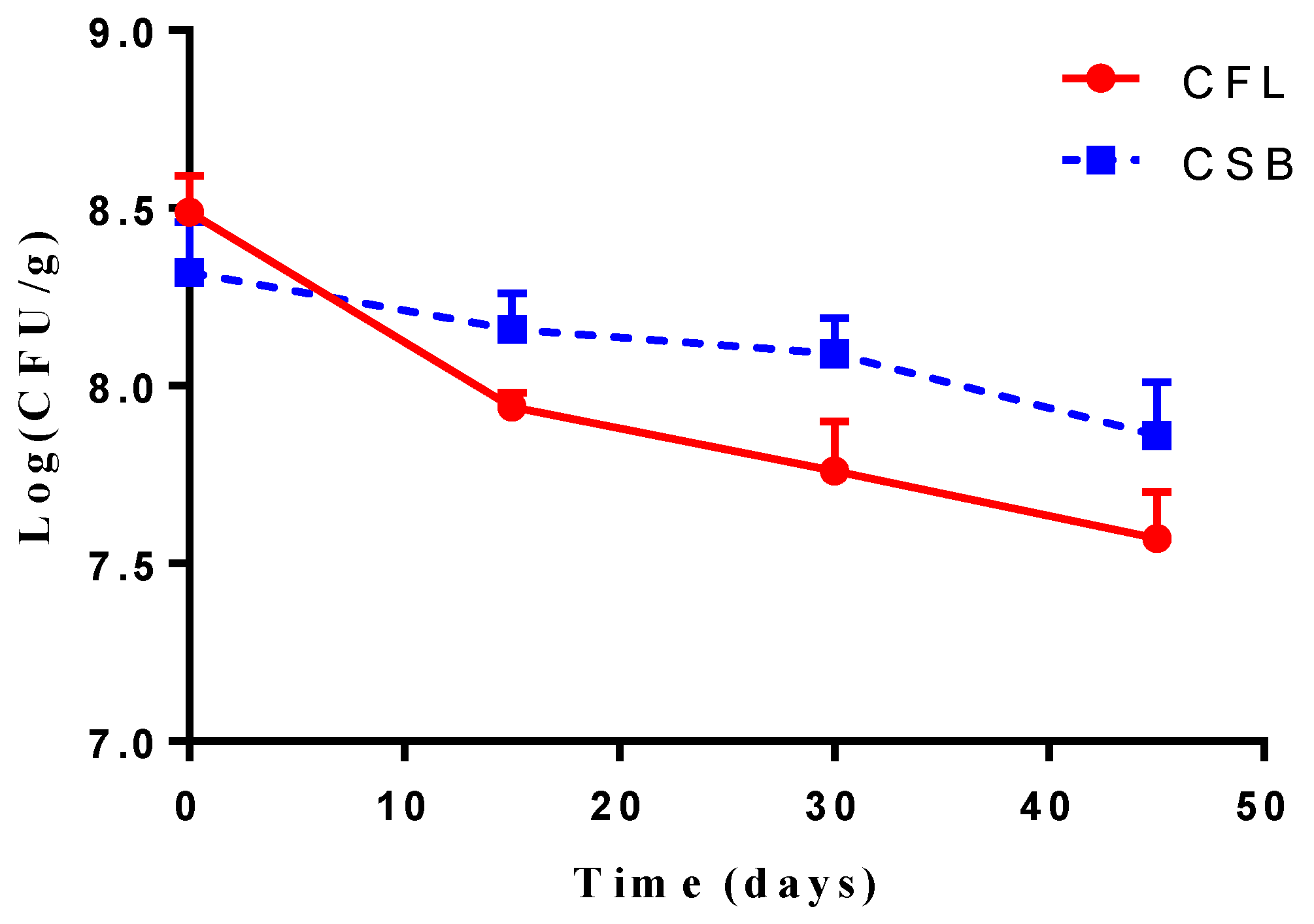
3.7. Microbiological Analysis of Cheese during Maturation and Storage
3.8. Volatile Aroma Compounds in Cheese
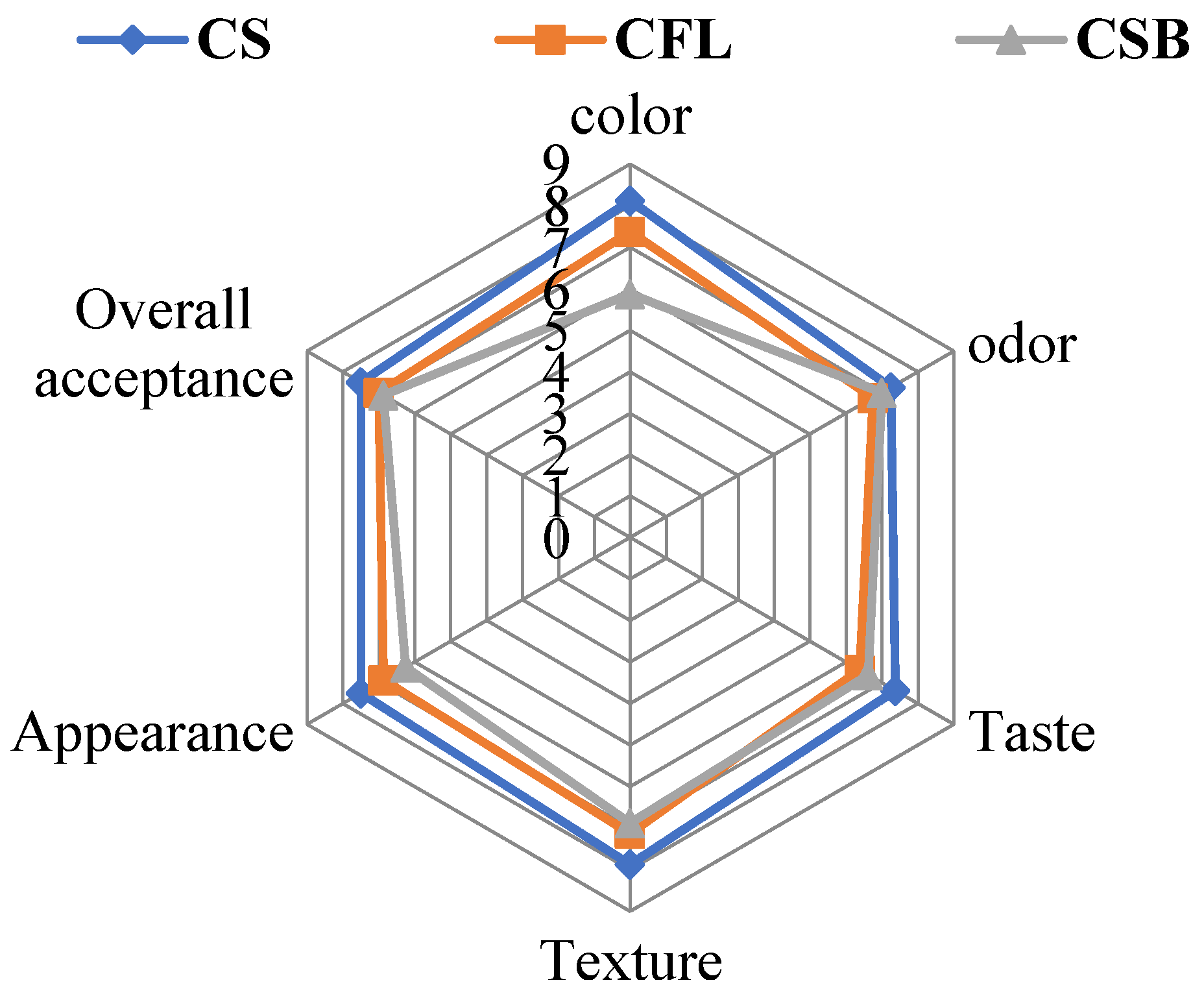
3.9. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; WHO. Evaluation of Health and Nutritional Properties of Probiotics in Food; Food and Agriculture Organization of the United Nations: Cordoba, Argentina; World Health Organization: Geneva, Switzerland, 2001; pp. 1–34. Available online: https://www.fao.org/3/a0512e/a0512e.pdf (accessed on 4 September 2022).
- Salminen, S.; Von Wright, A.; Morelli, L.; Marteau, P.; Brassart, D.; de Vos, W.M.; Fondén, R.; Saxelin, M.; Collins, K.; Mogensen, G.; et al. Demonstration of safety of probiotics—A review. Int. J. Food Microbiol. 1998, 20, 93–106. [Google Scholar] [CrossRef]
- Chiang, S.S.; Pan, T.M. Beneficial effects of L. paracasei subsp. Appl. Microbiol. Biotechnol. 2012, 93, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Kvakova, M.; Bertkova, I.; Stofilova, J.; Savidge, T.C. Co-encapsulated Synbiotics and immobilized probiotics in human health and gut microbiota modulation. Foods 2021, 10, 1297. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Nigam, P.S.; Biliaderis, C.G. Growth adaptation of probiotics in biopolymer-based coacervate structures to enhance cell viability. LWT 2017, 77, 282–289. [Google Scholar] [CrossRef]
- Shori, A.B. Influence of food matrix on the viability of probiotic bacteria: A review based on dairy and non-dairy beverages. Food Biosci. 2016, 13, 1–8. [Google Scholar] [CrossRef]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Iddir, M.; Brito, A.; Dingeo, G.; Fernandez Del Campo, S.S.F.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: Considerations during the COVID-19 crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- Ismael, N.M.M.; Shehata, M.G. Improvement of lipid profile and antioxidant of hyperlipidemic albino rats by functional Plantago psyllium cake. Curr. Res. Nutr. Food Sci. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Vasiliki, S.; Terpou, A.; Bosnea, L.; Kanellaki, M.; Nigam, P.S. Entrapment of Lactobacillus casei ATCC393 in the viscus matrix of Pistacia terebinthus resin for functional myzithra cheese manufacture. LWT 2018, 89, 441–448. [Google Scholar] [CrossRef]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of antifungal metabolites produced by novel lactic acid bacterium and their potential application as food biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Bosnea, L.; Kanellaki, M.; Ganatsios, V.; Koutinas, A.A. Wheat bran as prebiotic cell immobilisation carrier for industrial functional Feta-type cheese making: Chemical, microbial and sensory evaluation. Biocatal. Agric. Biotechnol. 2018, 13, 75–83. [Google Scholar] [CrossRef]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A. Novel antifungal bacteriocin from lactobacillus paracasei KC39 with anti-mycotoxigenic properties. Biosci. Res. 2018, 15, 4171–4183. [Google Scholar]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process Biochem. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K.; Kiers, G. Industrial manufacture of feta-type cheeses. In Brined Cheeses; Tamime, A., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2006; pp. 77–116. [Google Scholar] [CrossRef]
- AOAC. Official methods of analysis of AOAC International. In Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 1995; Available online: https://www.worldcat.org/title/official-methods-of-analysis-of-aoac-international/oclc/421897987 (accessed on 4 September 2022).
- Bourne, M.C. Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Elsevier Science & Technology Books: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Tharmaraj, N.; Shah, N.P. Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus, and propionibacteria. J Dairy Sci. 2003, 86, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Polychroniadou, A.; Michaelidou, A.; Paschaloudis, N. Effect of time, temperature and extraction method on the trichloroacetic acid-soluble nitrogen of cheese. Int. Dairy J. 1999, 9, 559–568. [Google Scholar] [CrossRef]
- Tharmaraj, N.; Shah, N.P. Survival of Lactobacillus acidophilus, Lactobacillus paracasei subsp. paracasei, Lactobacillus rhamnosus, Bifidobacterium animalis and Propionibacterium in cheese-based dips and the suitability of dips as effective carriers of probiotic bacteria. Int. Dairy J. 2004, 14, 1055–1066. [Google Scholar] [CrossRef]
- El-Sohaimy, A.A.; Masry, S.H.D.; Shehata, M.G.; Al-Kahtani, S.N.; Abdelwahab, T.E.; Abdelmotaleb, Y.A.T.; Nour, M.E. Isolation, identification and antimicrobial activity of unprecedented lactic acid bacterial isolates from honeybees. Pak. J. Biol. Sci. 2020, 23, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Cantoni, C.; Careri, M.; Chiesa, L.; Musci, M.; Pinna, A. Characterization of the aromatic profile for the authentication and differentiation of typical Italian dry-sausages. Talanta 2007, 72, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D.; Kourkoutas, Y.; Koutinas, A.A.; Kanellaki, M. Thermally dried immobilized kefir on casein as starter culture in dried whey cheese production. Food Microbiol. 2009, 26, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Izco, J.M.; Torre, P. Characterisation of volatile flavour compounds in Roncal cheese extracted by the ‘purge and trap’ method and analysed by GC–MS. Food Chem. 2000, 70, 409–417. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Windows; Version 23.0; IBM Corp.: Armonk, NY, USA, 2015; Available online: https://www.ibm.com/support/pages/how-cite-ibm-spss-statistics-or-earlier-versions-spss (accessed on 4 September 2022).
- Charalampopoulos, D.; Pandiella, S.S.; Webb, C. Evaluation of the effect of malt, wheat and barley extracts on the viability of potentially probiotic lactic acid bacteria under acidic conditions. Int. J. Food Microbiol. 2003, 82, 133–141. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Nedovic, V.; Goyal, A.; Kourkoutas, Y. Immobilization technologies in probiotic food production. J. Nutr. Metab. 2013, 716861. [Google Scholar] [CrossRef]
- Bekatorou, A.; Plessas, S.; Mallouchos, A. Cell immobilization technologies for applications in alcoholic beverages. In Handbook of Microencapsulation and Controlled Release; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 933–955. [Google Scholar]
- Dimitrellou, D.; Kandylis, P.; Sidira, M.; Koutinas, A.A.; Kourkoutas, Y. Free and immobilized Lactobacillus casei ATCC 393 on whey protein as starter cultures for probiotic Feta-type cheese production. J. Dairy Sci. 2014, 97, 4675–4685. [Google Scholar] [CrossRef] [PubMed]
- Michaelidou, A.; Katsiari, M.C.; Kondyli, E.; Voutsinas, L.P.; Alichanidis, E. Effect of a commercial adjunct culture on proteolysis in low-fat Feta-type cheese. Int. Dairy J. 2003, 13, 179–189. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Mallouchos, A.; Komaitis, M.; Koutinas, A.A.; Kourkoutas, Y. Effect of freeze–dried kefir culture on proteolysis in feta-type and whey-cheeses. Food Chem. 2010, 119, 795–800. [Google Scholar] [CrossRef]
- Gomes da Cruz, A.; Alonso Buriti, F.C.; Batista de Souza, C.H.; Fonseca Faria, J.A.; Isay Saad, S.M. Probiotic cheese: Health benefits, technological and stability aspects. Trends Food Sci. Technol. 2009, 20, 344–354. [Google Scholar] [CrossRef]
- Litopoulou-Tzanetaki, E.; Tzanetakis, N. Microbiological study of white-brined cheese made from raw goat milk. Food Microbiol. 1992, 9, 13–19. [Google Scholar] [CrossRef]
- Cárdenas, N.; Calzada, J.; Peirotén, A.; Jiménez, E.; Escudero, R.; Rodríguez, J.M.; Medina, M.; Fernández, L. Development of a potential probiotic fresh cheese using two lactobacillus Salivarius strains isolated from human milk. BioMed Res. Int. 2014, 801918. [Google Scholar] [CrossRef]
- El-Shibiny, S.; Abd El-Gawad, M.A.M.; Assem, F.M.; Seleet, F.L.; Abou Dawood, S.A.; Elaaser, M. Preparation, composition and microbiological and rheological properties of functional processed cheese supplemented with rice bran. Res. J. Appl. Sci. 2013, 9, 4927–4934. [Google Scholar]
- Xue, X.; Wang, J.; Li, S.; Zhang, X.; Dong, J.; Gui, L.; Chang, Q. Effect of micronised oat bran by ultrafine grinding on dietary fibre, texture and rheological characteristic of soft cheese. Int. J. Food Sci. Technol. 2020, 55, 578–588. [Google Scholar] [CrossRef]
- Corcoran, B.M.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Survival of probiotic lactobacilli in acidic environments is enhanced in the presence of metabolizable sugars. Appl. Environ. Microbiol. 2005, 71, 3060–3067. [Google Scholar] [CrossRef]
- Shori, A.B. The potential applications of probiotics on dairy and non-dairy foods focusing on viability during storage. Biocatal. Agric. Biotechnol. 2015, 4, 423–431. [Google Scholar] [CrossRef]
- Hashem, N.M.; Shehata, M.G. Antioxidant and antimicrobial activity of Cleome droserifolia (Forssk.) Del. and Its biological effects on redox status, immunity, and gut microflora. Animals 2021, 11, 1929. [Google Scholar] [CrossRef] [PubMed]
- Terpou, A.; Gialleli, A.I.; Bosnea, L.; Kanellaki, M.; Koutinas, A.A.; Castro, G.R. Novel cheese production by incorporation of sea buckthorn berries (Hippophae rhamnoides L.) supported probiotic cells. LWT 2017, 79, 616–624. [Google Scholar] [CrossRef]
- Guidone, A.; Braghieri, A.; Cioffi, S.; Claps, S.; Genovese, F.; Morone, G.; Napolitano, F.; Parente, E. Effect of adjuncts on microbiological and chemical properties of Scamorza cheese. J. Dairy Sci. 2015, 98, 1467–1478. [Google Scholar] [CrossRef] [PubMed]
- Masoumikia, R.; Ganbarov, K. Antagonistic activity of probiotic lactobacilli against human enteropathogenic bacteria in homemade tvorog curd cheese from Azerbaijan. BioImpacts 2015, 5, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y. Effect of cooling rate, freeze-drying, and storage on survival of free and immobilized Lactobacillus casei ATCC 393. LWT 2016, 69, 468–473. [Google Scholar] [CrossRef]
- Viljoen, B.C. The interaction between yeasts and bacteria in dairy environments. Int. J. Food Microbiol. 2001, 69, 37–44. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kourkoutas, Y.; Banat, I.M.; Marchant, R.; Koutinas, A.A. Whey cheese production using freeze-dried kefir culture as a starter. J. Appl. Microbiol. 2007, 103, 1170–1183. [Google Scholar] [CrossRef] [PubMed]
- El Soda, M.; Law, J.; Tsakalidou, E.; Kalantzopoulos, G. Lipolytic activity of cheese related microorganisms and its impact on cheese flavour. Dev. Food Sci. 1995, 37, 1823–1847. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Cakmakci, S.; Brechany, E.Y.; Deegan, K.C.; McSweeney, P.L.H. Microbiology, biochemistry, and volatile composition of Tulum cheese ripened in goat’s skin or plastic bags. J. Dairy Sci. 2007, 90, 1102–1121. [Google Scholar] [CrossRef]
- Hayaloglu, A.A.; Brechany, E.Y.; Deegan, K.C.; McSweeney, P.L.H. Characterization of the chemistry, biochemistry and volatile profile of Kuflu cheese, a mould-ripened variety. LWT 2008, 41, 1323–1334. [Google Scholar] [CrossRef]
- Sahingil, D.; Hayaloglu, A.A.; Simsek, O.; Ozer, B. Changes in volatile composition, proteolysis and textural and sensory properties of white-brined cheese: Effects of ripening temperature and adjunct culture. Dairy Sci. Technol. 2014, 94, 603–623. [Google Scholar] [CrossRef]
- Gallegos, J.; Garrido-Delgado, R.; Arce, L.; Medina, L.M. Volatile metabolites of goat cheeses determined by ion mobility spectrometry. Potential applications in quality control. Food Anal. Methods 2015, 8, 1699–1709. [Google Scholar] [CrossRef]
- Starr, G.; Petersen, M.A.; Jespersen, B.M.; Hansen, Å.S. Variation of volatile compounds among wheat varieties and landraces. Food Chem. 2015, 174, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.A. Invited review: Sensory analysis of dairy foods. J. Dairy Sci. 2007, 90, 4925–4937. [Google Scholar] [CrossRef] [PubMed]



| Ingredients | Unit | Quantity |
|---|---|---|
| Skim Milk Powder | g | 145 |
| Milk Protein concentrate | g | 145 |
| Butter | g | 70 |
| Stabilizer | mL | 2.5 |
| Salt | g | 15 |
| Calcium chloride | g | 2 |
| Rennet (1%) | mL | 10 |
| Water added | mL | 620 |
| Time (Months) | Wet Culture | Freeze-Dried Culture | ||
|---|---|---|---|---|
| Free Cell | Immobilized on Wheat Bran | Free Cells | Immobilized on Wheat Bran | |
| 0 | 8.91 ± 0.25 aA | 8.75 ± 0.30 aA | 9.06 ± 0.33 aA | 8.74 ± 0.61 aA |
| 1 | 8.19 ± 0.32 aB | 8.44 ± 0.14 aAB | 8.40 ± 0.34 aB | 8.55 ± 0.29 aA |
| 2 | 7.28 ± 0.19 bC | 7.73 ± 0.50 abB | 8.11 ± 0.29 aB | 8.27 ± 0.45 aA |
| 3 | 5.28 ± 0.24 cD | 6.75 ± 0.51 bC | 7.34 ± 0.36 abC | 7.89 ± 0.49 aA |
| Parameters | CS | CFL | CSB |
|---|---|---|---|
| Chemical composition | |||
| pH | 5.21 ± 0.01 a | 5.13 ± 0.01 b | 5.08 ± 0.03 c |
| Acidity% | 0.54 ± 0.005 c | 0.58 ± 0.02 b | 0.64 ± 0.02 a |
| Moisture% | 64.15 ± 2.20 a | 65.36 ± 1.00 a | 64.30 ± 0.95 a |
| Fiber% | 0.50 ± 0.01 b | 0.58 ± 0.08 b | 1.12 ± 0.06 a |
| Protein% | 8.42 ± 0.05 b | 8.45 ± 0.05 b | 8.96 ± 0.20 a |
| Fat% | 6.65 ± 0.15 a | 6.44 ± 0.36 a | 6.21 ± 0.11 a |
| Fat/DM% | 18.55 a | 18.59 a | 17.39 b |
| Color analysis | |||
| L* | 91.82 ± 0.74 a | 90.42 ± 0.41 ab | 88.840 ± 0.79 b |
| a* | −0.24 ± 0.02 a | −0.29 ± 0.06 a | −0.286 ± 0.05 a |
| b* | 4.00 ± 0.11 b | 5.14 ± 0.22 a | 5.096 ± 0.17 a |
| Texture Parameters | Unit | CS | CFL | CSB |
|---|---|---|---|---|
| Hardness Cycle 1 | g | 2648 ± 1.23 a | 1761 ± 1.17 c | 2341 ± 1.18 b |
| Adhesive Force | g | 156 ± 0.27 b | 191 ± 0.13 a | 90 ± 0.32 c |
| Adhesiveness | mJ | 1.6 ± 0.02 b | 2.7 ± 0.05 a | 0.7 ± 0.03 c |
| Hardness Cycle 2 | g | 2689 ± 1.16 a | 1445 ± 1.22 c | 2075 ± 1.16 b |
| Hardness Work Cycle 2 | mJ | 66.6 ± 0.01 a | 38.4 ± 0.02 c | 53.7 ± 0.07 b |
| Springiness | mm | 6.44 ± 0.02 b | 7.29 ± 0.01 a | 6.66 ± 0.02 b |
| Springiness Index | - | 0.92 ± 0.04 b | 1.04 ± 0.03 a | 0.95 ± 0.03 b |
| Stages | Free Freeze-Dried Lc. paracasei Cells (FFDC) | Soft White Cheeses with Free Lc. paracasei Cells (CFL) | Soft White Cheeses with Freeze-Dried Symbiotic Biocatalyst (CSB) |
|---|---|---|---|
| Oral phase | 9.39 ± 0.15 a | 9.14 ± 0.23 a | 9.10 ± 0.22 a |
| Gastric phase | 7.87 ± 0.33 b | 8.48 ± 0.14 a | 8.55 ± 0.27 a |
| Intestinal phase | 7.28 ± 0.14 b | 7.80 ± 0.30 ab | 8.28 ± 0.28 a |
| ID | Compound Name | R. Time (min) | Identification Method 1 | Classification | Cheese Samples | ||
|---|---|---|---|---|---|---|---|
| CS | CFL | CSB | |||||
| 1 | Ethyl butanoate | 10.0 | RT, KI, MS | Esters | + | ND | ND |
| 2 | 3-methylbutyl acetate | 10.4 | RT, MS | Esters | + | ND | ND |
| 3 | Ethyl hexanoate | 11.0 | RT, KI, MS | Esters | + | + | ND |
| 4 | Hexyl acetate | 12.0 | RT, KI, MS | Esters | + | + | ND |
| 5 | Ethyl dodecanoate | 12.4 | RT, MS | Esters | + | + | + |
| 6 | Hexanal | 12.8 | RT, MS | Aldehydes | + | + | + |
| 7 | Terpene | 13.0 | RT, MS | Terpenes | + | + | + |
| 8 | 1,4-p-Menthadiene | 13.4 | RT, MS | Terpenes | + | ND | ND |
| 9 | Ethylbenzene | 13.5 | RT, MS | Aromatic hydrocarbons | + | ND | ND |
| 10 | Isopentyl acetate | 13.8 | RT, MS | Esters | + | + | + |
| 11 | 2-Methyl 3-pentanone | 13.9 | RT, MS | Carbonyl compounds | ND | ND | + |
| 12 | 2-Pentanol | 14.0 | RT, MS | Alcohols | ND | + | + |
| 13 | 1-Penten-3-ol | 14.4 | RT, MS | Alcohols | + | + | + |
| 14 | 3-Methyl-1-butanol | 14.8 | RT, KI, MS | Alcohols | + | ND | ND |
| 15 | 1-Hexanol | 15.0 | RT, KI, MS | Alcohols | + | ND | ND |
| 16 | 1-heptanol | 15.3 | RT, KI, MS | Alcohols | + | + | + |
| 17 | Hexyl butanoate | 15.4 | RT, MS | Esters | + | + | + |
| 18 | Ethyl tetradecanoate | 15.5 | RT, MS | Esters | + | + | + |
| 19 | Ethyl octanoate | 15.6 | RT, KI, MS | Esters | ND | + | + |
| 20 | Ethyl decanoate | 15.7 | RT, KI, MS | Esters | ND | + | + |
| 21 | 2-Phenylethyl acetate | 16.5 | RT, KI, MS | Esters | ND | + | + |
| 22 | Hexanoic acid | 17.5 | RT, MS | organic acid | + | + | + |
| 23 | Octanoic acid | 18.6 | RT, MS | organic acid | ND | + | + |
| 24 | decanoic acid | 20.0 | RT, MS | organic acid | ND | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shehata, M.G.; Abd El-Aziz, N.M.; Darwish, A.G.; El-Sohaimy, S.A. Lacticaseibacillus paracasei KC39 Immobilized on Prebiotic Wheat Bran to Manufacture Functional Soft White Cheese. Fermentation 2022, 8, 496. https://doi.org/10.3390/fermentation8100496
Shehata MG, Abd El-Aziz NM, Darwish AG, El-Sohaimy SA. Lacticaseibacillus paracasei KC39 Immobilized on Prebiotic Wheat Bran to Manufacture Functional Soft White Cheese. Fermentation. 2022; 8(10):496. https://doi.org/10.3390/fermentation8100496
Chicago/Turabian StyleShehata, Mohamed G., Nourhan M. Abd El-Aziz, Amira G. Darwish, and Sobhy A. El-Sohaimy. 2022. "Lacticaseibacillus paracasei KC39 Immobilized on Prebiotic Wheat Bran to Manufacture Functional Soft White Cheese" Fermentation 8, no. 10: 496. https://doi.org/10.3390/fermentation8100496
APA StyleShehata, M. G., Abd El-Aziz, N. M., Darwish, A. G., & El-Sohaimy, S. A. (2022). Lacticaseibacillus paracasei KC39 Immobilized on Prebiotic Wheat Bran to Manufacture Functional Soft White Cheese. Fermentation, 8(10), 496. https://doi.org/10.3390/fermentation8100496









