Abstract
The pyrolytic conversion of lignocellulosic biomass into fuels and chemicals is a promising option for the valorization of agricultural and forestry residues. However, technological developments are still needed to maximize product recovery and carbon fixation of the pyrolysis process. The pyrolysis aqueous condensate (PAC), a pyrolysis by-product, has a high water content and is highly toxic, hampering its use. The anaerobic digestion of PAC from different biomasses has been proven a viable technology for PAC valorization and detoxification, but its toxicity limits the methanogenic potential. Alternatively, methanation or VFA production from syngas by anaerobic mixed cultures are technologies of scientific interest. This study investigates the potential of a two-stage process to convert the carbon and energy in syngas and PAC into L-malate. PAC and syngas were co-fermented by two mixed cultures at 37 and 55 °C, identifying kinetic inhibitions and the effects of increasing PAC concentrations on the product pool. The media from selected mixed culture fermentations were then inoculated with Aspergillus oryzae for L-malate production. The results show that mixed cultures can perform simultaneous syngas fermentation and PAC detoxification. While PAC concentrations above 2% completely inhibited methanogenesis, CO consumption was inhibited at PAC concentrations above 5%, regardless of the temperature. In fermentations where PAC inhibited methanation, the mixed cultures channelled the carbon and electrons from syngas and PAC to volatile fatty acids or acetate/H2 production, depending on the incubation temperature. Substantial detoxification of PAC was observed under PAC concentrations up to 10% independently of the rates of syngas metabolism. PAC detoxification enabled the further valorization of the acetate produced via syngas and PAC fermentations into L-malate, achieving yields up to 0.17 mM/mM. These results are promising for the development of an integrated process that simultaneously detoxifies and recovers value from gaseous and aqueous waste streams originating from pyrolysis.
1. Introduction
Growing concerns for the impact of anthropogenic activities on the environment are shifting the socio-economic interests from a fossil-based economy towards a more sustainable and circular one. According to the Intergovernmental Panel on Climate Change and the International Energy Agency, it is estimated that the total share of biofuels will double in the next decades [1]. The source of all the biomass required to meet the need of an increased bio industry is still an open debate [2]. The energy potential of biomass is enormous considering that the Earth’s net biomass production amounts approximately to 2000 EJ/y [3]. However, the diverting part of the energetic reservoir built up by plants towards uses defined by anthropocentric needs could cause undesirable impacts on the environment and on its natural distribution of resources [3]. Similarly, many biofuel crops are competing with food production, and the increasing demands for biofuel could exceed agricultural capacity [2]. The development of new technologies to maximize the energy recovery from wastes and residues of human activities is considered a key step towards carbon-neutrality [4].
The pyrolysis of lignocellulosic waste from municipal and agricultural activities could represent a great opportunity, contributing to meet the needs of a developing bio-based economy [5]. During pyrolysis, the biomass is thermochemically deconstructed at temperatures ranging between 350 and 600 °C in the absence of oxygen [6]. The products of pyrolysis are pyrolysis syngas (PS) (15–20 wt%), a viscous energy-rich pyrolysis organic fraction (POF) (20–30 wt%), an aqueous condensate (PAC) (20–30 wt%) and bio-char (10–30 wt%) [6,7]. Biochar and bio-oil can be either fed back into the pyrolysis reactor or used as fuels. On the other hand, the PAC’s use is limited by the high concentrations of various toxic compounds and the high water content [8]. Similarly, the release of PS into the atmosphere should be avoided, due to its high concentrations of greenhouse gases (GHGs). In general, PAC and PS represent about 45 wt% of the total biomass fed into the pyrolysis reactor [7] and up to 41% of the carbon balance [6]. Thus, it might be worth investing into bioprocessing technologies able to convert PAC and syngas into industrially relevant biochemicals.
Several works have already focused on the development of biological processes to valorize the constituents of the PAC. The ability of microorganisms in single culture fermentations to grow on PAC is species-specific due to their varying resistance to toxins contained in PAC [8]. Basaglia et al. [9] studied the toxicity of PAC from fir wood to a wide range of different microbial groups. Out of the 42 strains tested, only 4 fungal strains showed tolerance to pure PAC, whereas several PAC dilutions are required for many bacterial and yeast isolates [9]. However, it appears that PAC must undergo one or more pre-treatment steps to reduce the toxicity, before enabling its bioprocessing in pure culture fermentations [10,11,12,13,14,15,16,17].
Anaerobic digestion is an established technology for the treatment of agricultural residues and industrial wastewaters [18]. The degradation of the organic matter into CH4 follows four primarily metabolic steps (hydrolysis, acidogenesis, acetogenesis, and methanogenesis) and depends upon mutual and syntrophic interactions between various microorganisms and trophic groups [19]. The wide and diverse genetic spectrum and functional redundancy of thousands of microbial species in anaerobic digesters offer what pure cultures currently cannot achieve: a higher tolerance to environmental stresses and toxicity. Multiple parallel biochemical routes provide greater functional stability because of the potential distribution of the substrate to several populations [20], resulting in a higher community resilience to perturbations [21].
Many studies successfully established anaerobic digestion with pre-treated and raw PACs for biomethane production [22,23,24,25,26], proving how anaerobic mixed culture fermentation is a viable alternative to intricate physiochemical pre-treatments for PAC detoxification and valorization. For example, Zhou et al. [25] studied the tolerance of anaerobic digestion towards increasing concentrations of raw and overlimed PAC as the sole carbon source for biomethane production in batch processes and direct evolution studies, respectively. The batch tests showed that loadings of 3% raw PAC were inhibiting methanogenesis. Extensive studies have been conducted towards a complete integration of pyrolysis and anaerobic digestion for methane production where all the by-products of pyrolysis (PAC and PS included) are fed into an anaerobic digester [24,27,28]. During the anaerobic digestion of PAC derived from corn stalk pellets, volatile fatty acid (VFA) production was observed even though PAC severely inhibited methanogenesis [24]. Giwa et al. [29] evaluated the effects of a real PS generated from a two-stage pyrolysis process treating food waste on methanation rates. The process, designed to minimize POF and PAC, generated syngas with a high H2-to-CO ratio (60:20%). Methanation rates were enhanced, producing almost 100% more CH4 than the synthetic syngas control fermentations. The topic has been evaluated also from a techno-economical perspective [30,31,32]: pairing anaerobic digestion with pyrolysis allows for relevant energy savings in handling pyrolysis by-products and strongly reduces GHGs emissions [30]. Salman et al. [33] estimated a higher annual revenue for the integrated process compared to the sole incineration of green waste.
In anaerobic communities, syngas is commonly metabolized by methanogenic archaea, hydrogenogenic bacteria, acetogenic bacteria, and sulfate-reducing bacteria [34]. By manipulating the fermentation environmental conditions, it is possible to control the syngas conversion towards different catabolic routes [35,36,37,38]. Methane is often the primary metabolite having the lowest free energy content per electron, regardless of the temperature range [37]. On the other hand, when methanogenesis is inhibited and in mesophilic environments with high concentrations of reduced compounds such as ethanol and/or lactate, the mixed culture can elongate C1 compounds from syngas into medium-chain carboxylates (MCCs). Such a wide array of metabolic products at mesophilic temperatures is the result of an intricate metabolic network ultimately limited by thermodynamics [39]. Syngas-converting microbial communities at thermophilic temperatures show higher water–gas shift reaction (WGSR) kinetics than mesophilic ones. The high diversity of carboxydotrophic hydrogenogenic bacteria and the thermodynamics of H2-producing reactions in thermophilic environments favor higher CO conversion rates to produce primarily H2 and short-chain carboxylates [40]. Hydrogen or MCC production via mixed culture anaerobic fermentation are gaining more scientific and industrial interest [41,42]. However, the success of these technologies is linked to the identification of cheap and recoverable methane inhibitors [43,44].
A. oryzae belongs to the Ascomycetes group and its industrial application spans from food processing to commodity chemical production [45,46]. Several studies have evaluated the potential of producing biochemicals, biofuels or cell biomass (single-cell proteins) with A. oryzae from VFA rich waste streams or from acetate [47,48,49]. Moreover, the fungus was reported to tolerate small concentrations of pyrolysis oils and various PAC components [50] and to be able to grow on the acetate contained in pre-treated PAC from wheat straw [15].
To extend the knowledge about the integration of thermochemical and biochemical processes treating lignocellulose waste, this work evaluates a two-stage process where the products from the co-fermentation of PAC and syngas by anaerobic mixed cultures are fed to an aerobic fermentation to produce L-malate by A. oryzae. Several anaerobic mixed culture bottle fermentations were performed at 37 and 55 °C at increasing PAC concentrations in order to understand the effects of PAC on the metabolism of gaseous and liquid compounds. After the syngas fermentation stage, the media from selected mixed culture fermentations were inoculated with A. oryzae, focusing on the conversion of acetate from syngas and PAC metabolism into L-malate. Fungal growth, together with the quantification of the removal of selected PAC components, was used to prove the occurrence and the extent of PAC detoxification.
2. Materials and Methods
2.1. Growth Medium
All reagent-grade chemicals were purchased from Sigma-Aldrich (Schnelldorf, Germany) or Carl-Roth (Karlsruhe, Germany). The fermentation medium used in all serum-bottle and flasks experiments was a modified basal anaerobic medium (BA) composed of the following stock solutions: mineral salts solution (NH4Cl, 161.2 g/L; MgCl2 × 6H2O, 5.4 g/L; CaCl2 × 2H2O 6.5 g/L); phosphate buffer solution (KH2PO4, 136 g/L); vitamins solution (Biotin, 0.002 g/L; Folic Acid, 0.002 g/L; Pyridoxin, 0.01 g/L; Thiamin, 0.005 g/L; Riboflavin, 0.005 g/L; Nicotinic Acid, 0.005 g/L; Ca-Panthothenate, 0.005 g/L; Vitamin B12, 0.005 g/L; Aminobenzoic Acid, 0.005 g/L; Liponic Acid, 0.005 g/L); trace elements solution (FeCl2 × 4H2O, 1.5 g/L; MnCl2, 0.1 g/L; CoCl2 × 6H2O, 0.19 g/L; ZnCl2, 0.07 g/L; CuCl2 × 2H2O, 0.002 g/L; NiCl2 × 6H2O, 0.024 g/L; Na2MoO4 × 2H2O, 0.036 g/L; H3BO3, 0.006 g/L; Na2SeO3 × 5H2O, 0.003 g/L; Na2WO4 × 2H2O, 0.02 g/L); reducing agent solution (L-Cysteine, 100 g/L); resazurin solution (Resazurin sodium salt, 1 g/L). For each liter of medium added: 100 mL of mineral salt solution, 800 mL of phosphate buffer solution, 10 mL of vitamins solution, 10 mL of trace elements solution, 5 mL of resazurin solution and 3 mL of reducing agent solution. Once all the solutions were mixed, the pH was adjusted to 6 with 4M NaOH solution as pH-adjusting agent and sodium source. The remaining volume was filled with deionized water to 1 L.
2.2. Inocula and PAC
The anaerobic sludge was collected from an anaerobic digester treating cow manure (Alois & Simon Frey Biogas GbR, Bräunlingen, Germany). Due to the high content of straw residues, right after collection, the sludge was sieved down to 0.5 mm discarding the straw and the retained solids. The sludge was then poured into an anaerobic container and stored in a fridge at 4 °C until needed. The pH, the total suspended solids (TSS) and volatile suspended solids (VSS) concentration of the sieved sludge corresponded to 8.46, 41.36 ± 2.25 g/L and 12.27 ± 0.13 g/L, respectively. The TSS and VSS analytics were performed in triplicate and determined as described in [51].
Aspergillus oryzae DSM 1863 was obtained from the DSMZ strain collection (Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany). The cryo-stock of fungal conidia was prepared and stored as described by [49].
The PAC used in this experiment was produced during the fast pyrolysis of miscanthus at BioLiq plant (Karlsruhe Institute of Technology, Karlsruhe, Germany). The chemical oxygen demand (COD) and total organic carbon (TOC) were 253.25 ± 10.25 g/L and 118.58 ± 0.11 g/L, respectively. The total nitrogen (TN) was 140.25 ± 4.24 mg/L. The pH of raw PAC was 2.8, while acetate, propionate and n-butyrate concentrations were about 34, 5.07 and 0.5 g/L, respectively. The fast pyrolysis at the BioLiq plant is run as described in [7] and [52]: the flue gases (composed primarily of 20% CO, 25% CO2, 1.5% H2, alkanes and N2) coming from the combustion chamber pass through a hot cyclone to separate the biochar from the product gas stream. Then, the gaseous phase is sent through a series of two quench condensers at ~85–90 °C and at ~30 °C separated by an electrostatic precipitator. The PAC used in this study is the product of the second condensation step.
2.3. Bottle Preparation and Fermentation
Mesophilic and thermophilic experiments with (M-CTRL and T-CTRL) and without methanation (M-BES and T-BES) were run as controls to evaluate the metabolism and the performances of the inoculum grown on synthetic pyrolysis syngas. To inhibit methanogenesis, 50 mM of Sodium 2-bromoethanesulfonate (BES) were dissolved into the BA medium. All experiments not containing PAC were performed in triplicate. To test PAC inhibition, exponentially increasing concentrations ranging from 0.5 to 30% v/v were added in the M-PAC and T-PAC fermentations. As control, abiotic experiments with equal concentrations of PAC (M-PAC-AB and T-PAC-AB) were also prepared and run simultaneously to the corresponding experiments. The mixed culture fermentations and abiotic PAC incubations were performed in 250 mL serum bottles with 50 mL of active volume. Figure 1 and Table 1 summarize the experimental design.

Figure 1.
Schematic representation of the experimental design. Pyrolysis aqueous condensate and pyrolysis syngas were co-fermented by two mixed cultures at mesophilic and thermophilic temperatures. The media from selected mixed culture fermentations were centrifuged and inoculated with A. oryzae to convert acetate into L-malate.

Table 1.
Overview of experiments. MC is mixed culture; AB is abiotic; Asp is Aspergillus oryzae.
The liquid phase was composed of 5 mL of BA medium, increasing PAC concentrations depending on the experimental design and 4M NaOH as needed to re-adjust the pH of the medium back to 6 after PAC addition. The remaining volume was filled with deionized water up to 45 mL. The serum bottles were stored into an anaerobic tent (5% H2 in N2) to anaerobize overnight at room temperature. The bottles were then inoculated with 10% v/v anaerobic sludge and sealed with butyl rubber stoppers and aluminum rings. After sealing the flasks, the bottles were initially flushed and then pressurized with a synthetic pyrolysis gas mixture consisting of 6 kPa H2, 21 kPa CO, 26 kPa CO2 and N2 to a final pressure of 210 kPaabs. Bottle pressurization was performed using a precision pressure indicator GMH 3100 Series (Greisinger, Mainz, Germany) at room temperature.
A total of 3 mL of gas phase was sampled daily or depending on the rates of CO or H2 consumption. The ambient temperature and pressure and the gauge pressure of the bottles were recorded at each sampling, right after taking the bottle from the incubator. When the CO and/or H2 molar concentrations or the absolute pressure of the serum bottles were about zero or below 190 kPaabs, respectively, then the headspace of the bottle was re-pressurized with the synthetic pyrolysis gas mixture. The maximum possible theoretical uptake rate for CO was about 1.650 mmol/d, while for exogenous H2 it was 0.570 mmol/d. A total of 1 mL of liquid samples was withdrawn twice a week. The pH of the sample was measured, and the samples were then centrifuged at 17,000 × g and ambient temperature for 15 min. The resulting supernatant was filtered with 0.2 µm cellulose acetate syringe filters (Restek GmbH, Bad Homburg vor der Höhe, Germany) and stored in a freezer at −20 °C for later analytics. All bottles were incubated in the dark in shaker incubators (multitron incubator shaker, Infors, Bottmingen, Switzerland) at temperatures of 37 or 55 °C. The agitation was set to 200 rpm. All mixed culture fermentations and abiotic controls lasted 39 days of elapsed fermentation time (EFT).
The medium from selected mesophilic and thermophilic fermentations M-PAC and T-PAC (2.5%, 5%, 7.5%, 10%, 20%) and from the corresponding abiotic controls was centrifuged at 4700 × g for 8 h. The supernatant was collected, 9 mL of which together with 1 mL fresh BA medium were then poured into 100 mL baffled Erlenmeyer shake flasks. The shake flasks were inoculated with 0.1 mL of the A. oryzae conidia cryo-stock, with spore concentration of 3 × 107 spores/mL. The pH of the medium was not adjusted. All the shake flasks were incubated at 30 °C and 100 rpm. In total, 0.2 mL of liquid samples were taken every 24 h from inoculation for 5 consecutive days. The pH of the sample was measured, and the samples were then stored in a freezer at −20 °C for later analytics. All fermentations with A. oryzae were done in triplicate.
2.4. Analytical Methods and Data Processing
The concentration in the fermentation medium of linear and branched monocarboxylates C1-C8 (lactate, acetate, propionate, iso- and n-butyrate, iso- and n-valerate, iso- and n-caproate), of the normal alcohols (ethanol, propanol, butanol and pentanol) and of some selected PAC compounds (2-cyclopenten-1-one, furfural, phenol, guaiacol and o-,m-,p-cresol) were measured by a high-performance liquid chromatography (HPLC) device (Agilent 1100 Series, Agilent, Waldbronn, Germany) operated with an oven set at 55 °C equipped with a Rezex ROA organic acid H + (8%) column (300 by 7.8 mm, 8 µm; Phenomenex, Aschaffenburg, Germany) and a Rezex ROA organic acid H + (8%) guard column (50 by 7.8 mm). The mobile phase was 5 mM H2SO4 with a flow of 0.6 mL/min. Short- and medium-chain carboxylates and PAC compound detection was performed with a UV detector at 220 nm at 55 °C, while normal alcohols were detected with an RID detector at 50 °C.
The gas phase samples were analyzed with an Inficon 3000 Micro GC System with a Thermal Conductivity Detector (TCD) equipped with a CP-Molsieve 5 Å column and a PoraPLOT Q column at 80 °C using argon and helium as carrier gases, respectively. The molar composition of the headspace gas of the bottles was computed assuming the ideal gas law after subtracting any air contamination caused by sampling. The accumulation or consumption of each gas was first corrected by a factor accounting for the pressure lost by sampling withdrawal and then cumulated.
The yields and recoveries (in terms of carbon (C-mol) and electron (e-mol) equivalents) for control experiments were calculated using only CO/CO2 and CO/H2 as substrates, respectively, as described by Grimalt-Alemany et al. [53]. For M-PAC and T-PAC experiments, CO was accounted as the sole carbon source while CO and H2 were assumed as electron donors. The multitude of compounds present in PAC interfered with the identification of other metabolites beyond acetate, propionate, and n-butyrate. Therefore, only these three acids as well as CO2, CH4 and H2 were accounted as products. The IC50 value was adopted from Zhou et al. [25], indicating the toxicant concentration that causes 50% reduction in cumulative CO consumption or CH4 production over a fixed period of exposure time. Acetate selectivity is the ratio between acetate and metabolites with carbon atom number greater than 2.
3. Results and Discussion
3.1. Mesophilic and Thermophilic Anaerobic Mixed Microbial Cultures Grown on Pyrolysis Synthetic Syngas
The first set of experiments aimed to understand whether the synthetic pyrolysis syngas used in this study is a suitable carbon and electron source for production of methane, short- and medium-chain carboxylates as well as solvents with mixed microbial cultures. M-CTRL and T-CTRL are bottle fermentations incubated at 37 and 55 °C, respectively, performing syngas methanation. M-BES and T-BES are bottle fermentations at 37 and 55 °C with the addition of 50 mM BES as methanogenesis inhibitor. The metabolism of the communities under M-CTRL, T-CTRL, M-BES and T-BES conditions were characterized and later used as a reference for comparison with the fermentations in the presence of PAC. The initial pH of all control bottles after inoculation was 6.7 ± 0.2. Figure 2 shows C-mol recovery and e-mol recovery from all control experiments.
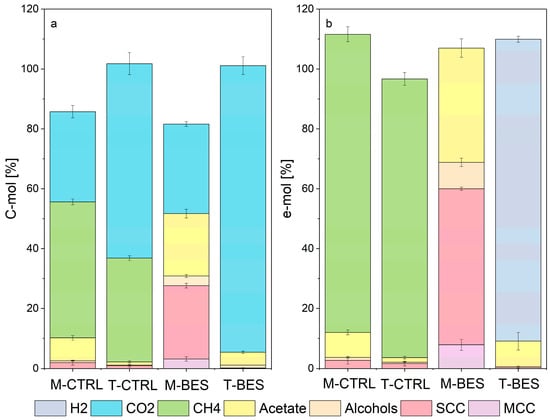
Figure 2.
C-mol (a) and e-mol (b) balances for experiments M-CTRL, T-CTRL, M-BES and T-BES. Conversion factors for electron balances are available in the supplementary materials (Table S1). CO, CO2 and H2 were considered as the sole carbon and/or electron donors for all experiments but for T-BES, where CO was the only carbon and electron donor. Alcohols are ethanol, propanol and butanol. Short-chain carboxylates C3- C5 (SCCs) are lactate, iso- and n-butyrate, propionate and iso- and n-valerate. Medium-chain carboxylates (MCCs) are iso- and n-caproate. The productivities of alcohols, some SCCs and MCCs are available in the supplementary materials, Table S2.
During syngas methanation at mesophilic range (M-CTRL), the mixed culture produced primarily CH4 (45.5 ± 1%) and CO2 (30.1 ± 2.1%), while 7.6 ± 0.1% of the total carbon metabolized was fixed into acetate. Acetate accounted for 83.7 ± 1.7% of the total C2–C6 metabolites detected in the liquid phase. The carbon stored in carboxylates other than acetate was about 2.6%. The average CO and H2 uptake rates were 0.34 ± 0.02 mmol/d and 0.28 ± 0.02 mmol/d, while CH4 was produced at a rate of 0.15 ± 0.01 mmol/d.
From about 20 days EFT, methanogenic rates increased concomitantly to homoacetogenic/hydrogenotrophic activity from exogenous CO2 and H2 consumption (Supplementary materials, Figures S1–S4). Simultaneously, decreasing acetate concentrations in the bottles might indicate acetoclastic methanation. However, acetoclastic methanogenesis appears to have barely contributed to the methanation yield. At 37 °C, pH 5.5, and 100 mM acetate hydrogenotrophic methanogenesis has more favorable thermodynamics than acetoclastic methanogenesis [38]. Considering that CO and H2/CO2 metabolisms have been reported to have similar kinetics [54], changes in the rates of gases uptake or production might be attributed to shifts within the composition of the microbial population. With the progression of M-CTRL experiments, CO uptake rates lowered the CO partial pressures favoring acetogenic/methanogenic hydrogenotrophism. High CO partial pressures are known to be inhibiting cellular hydrogenase and H2 uptake [55,56] and might have contributed to the delayed start of H2/CO2 metabolism. Liu et al. [57] detected a two-phased process characterized by an initial CO consumption followed by the onset of H2/CO2 metabolism to acetate attributed to homoacetogenic microorganisms while performing CO biomethanation with anaerobic granular sludge. In M-CTRL bottles, carboxidotrophic methanation, if any, had a limited contribution towards methane production. Carboxydotrophic methanogens are expected to be easily outcompeted by carboxydotrophic acetogens and hydrogenogens, as the few species that are capable of directly converting CO into CH4 do so at very low reaction rates [58,59].
The thermophilic syngas methanation (T-CTRL) occurred at higher kinetics but lower yield when compared to M-CTRL. In total, 34.6 ± 0.8% of the carbon from CO was converted into CH4 while CO2 accounted for 65 ± 3.7%. Acetate accounted for about 1% for the total carbon from CO and the acetate selectivity was 63 ± 3.51%. The average CO and H2 uptake rates were 1.48 ± 0.05 mmol/d and 0.56 ± 0.01 mmol/d, respectively. CO2 and CH4 were produced at 0.98 ± 0.05 mmol/d and 0.515 ± 0.01 mmol/d, respectively. T-CTRL bottles have been performing primarily carboxidotrophic hydrogenogenesis via the WGSR followed by hydrogenotrophic methane generation, as also described by other studies [34,36,39].
Mesophilic and thermophilic metabolic rates calculated in this study correspond to those reported by Sipma et al. [60], who tested several mesophilic anaerobic sludges from wastewater treatment reactors to convert CO at 30 and 55 °C. The sludges were incubated at 30 °C in serum bottles with 50 mL initial active volume and produced primarily CH4 and/or acetate. Incubation at 55 °C resulted in the formation of mainly CH4 and/or H2 [60]. Sipma et al. detected CO conversion rates ranging between 0.14 and 0.62 mmol/d for the cultures incubated at 30 °C, while thermophilic CO depletion rates varied between 0.73 and 1.32 mmol/d.
The BES addition inhibited all methanogenic pathways in both control mesophilic syngas (M-BES) and control thermophilic syngas (T-BES) fermentations. M-BES fermentations consumed CO at a rate of 0.36 ± 0.03 mmol/d, a similar value to what was calculated for M-CTRL. H2 uptake rate was 0.03 ± 0.01 mmol/d and CO2 production rate was 0.11 ± 0.01 mmol/d. HPLC analytics showed that M-BES cultures have been chain elongating CO to n-caproate with a net exogenous H2 consumption to a final caproate concentration of 2.18 ± 0.47 mM. About 60% of the e-mol recovery was accounted for metabolites with a carbon atom number higher than two. CO2 (29.9 ± 0.8%) and acetate (20.8 ± 1.5%) were the two major carbon sinks.
T-BES experiments showed greater CO consumption kinetics then M-BES. The mixed culture performed almost solely WGSR, generating 1.04 ± 0.33 mmol/d of CO2 and 1.05 ± 0.31 mmol/d H2, while the average CO uptake rate was 1.18 ± 0.09 mmol/d. CO2 accounted for more than 95% of the total carbon fed while acetate was only about 5%. Acetate was the primary metabolite produced by the consortium with selectivities higher than 80%. More than 99% of the e-mol recovery was molecular H2. These results are corroborated by the work carried out by other research groups. Grimalt-Alemany et al. [39] characterized the conversion of CO by a thermophilic enriched consortium in the presence of BES, resulting in the production of H2 and acetate as primary metabolites. Slepova et al. [61] traced 14CO to study the metabolism of mixed cultures collected from three pH-neutral hot springs of Uzon Caldera (Kamchatka) under temperatures from 60 to 90 °C. A major part of 14CO was oxidized to 14CO2. Samples from the spring with a temperature of 60 °C converted less than 5% of the CO into carboxylates and only 1% in springs with higher temperatures [61]. High acetate selectivities were also reported by Wang et al. [62], showing a 99% acetate selectivity at the end of their thermophilic (55 °C) enrichment process with H2 and CO2 as substrates. Shen et al. [63] achieved final acetate selectivity of 96.7% and 96.3% in two hollow fiber membrane bioreactors after 60 days EFT starting from an inoculum from an anaerobic digester. Alves et al. [35] tested different enrichment strategies in bottle experiments at 55 °C and obtained syngas-converting communities able to fix approximately 97% of product recovery into acetate from CO2 and H2.
3.2. Co-Fermentation of Syngas and PAC
The effects of increasing PAC concentrations were evaluated on two mixed microbial cultures growing on pyrolysis gas at 37 and 55 °C. The aim was to identify kinetic inhibition and changes in metabolites production patterns of syngas metabolism caused by PAC. Additional interest was to test the PAC detoxification potential of the microbial cultures.
3.2.1. Impact of PAC on the Syngas Metabolism of the Anaerobic Mixed Culture at 37 °C and 55 °C
Figure 3 reports the rates of syngas metabolism at increasing PAC concentration both at mesophilic (37 °C) and thermophilic (55 °C) temperatures. Similar to the control experiments, the initial pH of all M-PAC and T-PAC experiments was 6.7 ± 0.2 after inoculation.
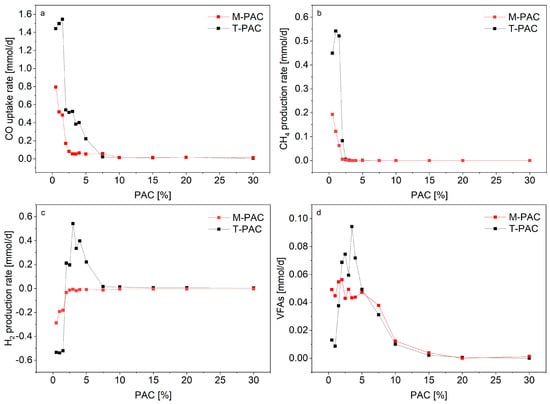
Figure 3.
Rates of consumption and/or production of CO (a), CH4 (b) and H2 (c) at increasing PAC loadings at mesophilic (37 °C) and thermophilic (55 °C) temperatures. Negative production rates for H2 indicate consumption. Volatile fatty acids (VFAs) (d) are acetate, propionate and n-butyrate. Productivities for all experiments are available in the Supplementary materials Tables S3 and S4.
The CO consumption rates for mesophilic fermentations M-PAC at PAC concentrations of 0.5, 1 and 1.5 were all above 0.4 mmol/d. For PAC concentrations higher than 5%, the rates of CO consumption rapidly decreased towards zero. Exogenous H2 consumption was detected in all M-PAC bottles. Additionally, CO2 production rates were 60% lower than the stoichiometry of the WGSR, suggesting that the mesophilic mixed culture co-fermented CO and H2/CO2. While the methane production rates quickly dropped to zero for concentrations above 1.5% PAC, the VFA daily production decreased only from PAC concentrations above 7.5%.
At thermophilic range, PAC concentrations below 1.5% did not significantly affect CO consumption (Figure 3a). The average CO consumption rates at 55 °C with PAC concentrations from 0.5 to 1.5% were all above 1.4 mmol/d, similar to what was achieved in the control experiments T-CTRL. Above 5% PAC, the kinetics of CO consumption rapidly decreased towards zero. At thermophilic range, methanogenesis was detected for PAC concentrations from 0.5 to 2.5% PAC. The highest CH4 production rate was 0.54 mmol/d for bottles containing 1% PAC. In T-PAC fermentations with 1.5, 2, 2.5% PAC, the methane production showed a delayed start of about 6 days when compared to T-CTRL (Supplementary materials Figures S5–S8). In Figure 3c, H2 was consumed to generate methane via hydrogenothropic methanogenesis under conditions with up to 1.5% PAC. At higher PAC loadings, net H2 production occurred concomitantly to the inhibition of the methanogenic activity. The highest H2 production rate was detected at 3% PAC with values of 0.54 mmol/d, but it decreased at rates equivalent to CO consumption for higher PAC percentages. Similar to mesophilic bottles, the VFA production rates were low under low PAC loadings and peaked at 3.5% PAC when no methane production was detected.
The kinetics of syngas metabolism for thermophilic PAC fermentations were consistently higher than at mesophilic range, a result consistent with the kinetics of the control experiments. However, Figure 4a shows that, when normalizing M-PAC and T-PAC CO uptake rates to the correponding rates of M-CTRL and T-CTRL, the overall effects of PAC toxicity did not differ between mesophilic and thermophilic experiments. Thus, thermodynamic limitations and different gas solubilities at different temperatures were likely the dominant factors affecting the kinetics of syngas metabolism.
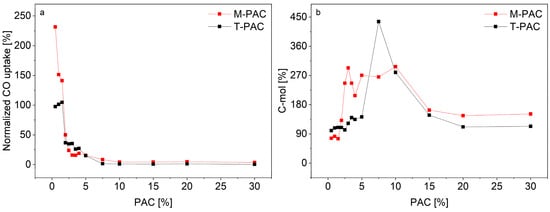
Figure 4.
(a) M-PAC and T-PAC CO uptake rates normalized to control experiments M-CTRL and T-CTRL, respectively. (b) C-mol balances for M-PAC and T-PAC experiments.
Additionally, Figure 4a shows that M-PAC bottles with low PAC concentrations (0.5 to 1.5% PAC) had at least 40% higher CO consumption rates compared to the respective M-CTRL values, peaking at 231% at 0.5% PAC. For bottles with 0.5 to 1.5% M-PAC, from about 20 days EFT, CO oxidation rates higher than 0.36 mmol/d (average CO uptake for M-BES) were detected, matching those of T-CTRL experiments rather than M-CTRL or M-BES (supplementary materials, Figures S1–S4). Factors such as CO and PAC toxicity probably contributed to hinder acetogenic and methanogenic activity at early fermentation stages and the high CO uptake rates might be the result of changes in microbial population consequently to PAC detoxification. However, contrarily to M-CTRL fermentations, the higher kinetics of the WGSR provided enough endogenous CO2 to all metabolic routes resulting in a net CO2 production (supplementary materials, Figures S1–S3).
3.2.2. Different PAC Tolerance of Different Trophic Groups
Methane production was inhibited by lower PAC concentrations than CO consumption in both M-PAC and T-PAC cultures. The IC50 values for CO uptake rates at mesophilic range correspond to 2% PAC. Methane production, on the other hand, is halved at PAC concentrations between 1 and 1.5%. At thermophilic range, the IC50 values for CO uptake rates fell within the 2 to 3% PAC range. Regarding methane, the IC50 was found to be between 1.5 and 2% PAC. Zhou et al. [25] reported that the IC50 of mesophilic biomethane potential tests of overlimed PAC was 4.8% PAC. Even though Zhou et al. [25] did not report the IC50 for raw PAC, it could be assumed that the higher tolerance of methanogens towards PAC achieved in their study was the result of the synchrony of the pre-treatment and a lower specific PAC availability, as both factors are known to affect methanation rates [64]. Here, raw PAC loading rates that severely inhibited methanogenesis were 0.41 gCOD/gVSS (2% PAC) at both the mesophilic and thermophilic range, respectively (supplementary materials, Table S4).
When comparing methanogenic versus carboxydotrophic/homoacetogenic activity under PAC influence, homoacetogenesis had a higher tolerance to PAC than methanogenesis. Compounds present in PAC such as furfural, phenol and phenolic compounds can be produced also from the hydrolysis of lignocellulosic matter [8,65,66]. Acetogens are involved in synthropic interactions with other microorganisms during the anaerobic degradation of compounds deriving from the degradation of lignin. Synthetic co-cultures with Pelobacter acidigallici, Acetobacterium woodii, and Methanosarcina barkeri have been reported to convert phenylmethylethers to CH4 and CO2 [67]. A. woodii metabolizes phenylmethylethers to yield acetate and phenols [68]. Phenols can be degraded to acetate by P. acidigallici [69]. In another work studying the degradation of lignin-derived monoaromatic compounds, the initial step was catalyzed by Sporomusa spp. to generate acetate via O-demethylation of the methoxylated aromatics. The demethoxylated aromatics were then metabolized into acetate, H2 and CO2 by Firmicutes. Finally, methane was generated from acetate and H2/CO2 by acetoclastic and hydrogenotrophic methanogens, respectively [70]. The latter examples represent interactions between microorganisms that might have occurred in the inoculum in the presence of PAC. Methanogens work at the end of the chain of syntrophic interactions resulting in the production of CH4 as the primary end-product of the fermentative process. Thus, methanogenic activity is highly influenced by the degradation of those compounds that would otherwise be inhibitory. Low concentrations of lignin derivatives with aldehyde groups or apolar substituents are known to be highly toxic to methanogens [71]. Aromatic carboxylates, on the other hand, were reported to be only mildly toxic. Phenols and their derivatives are known for being methanogenic inhibitors [64,72,73]; however, phenolic compounds have been already proven to be degraded to CH4 [74,75].
Hübner et al. [22] reported longer lag phases at increasing initial PAC concentrations in anaerobic digestion experiments. PAC extended the lag phase of methanogenesis from a few days to some weeks, indicating temporary inhibition [22]. Inhibition of anaerobic digestion by PAC from corn stalk was also observed by Torri and Fabbri [27]. Longer lag-phases at increasing PAC loadings were also detected in this work at mesophilic and thermophilic range for both carboxidotrophism and methanogenesis (Supplementary materials Figures S4 and S8). In general, an extended lag-phase could be related to a lack of acclimatization of the inoculum to an inhibiting organic compounds hard to degrade, therefore requiring enrichment of the microbial community [73]. Alternatively, the inoculation/bioaugmentation of fermentations with cultures collected from particular ecosystems could be a strategy to increase the performances of biological processes [76,77,78,79].
3.2.3. PAC Detoxification
The C-mol and e-mol recoveries for bottles with 2.5 to 10% PAC at both temperatures showed balances much higher than 100% (Figure 4b). Most of the VFAs (primariliy acetate) produced in those bottles were not the result of syngas metabolism but from the degradation of aromatc compounds, as proven in other works [67,70,80]. This is also supported by the detoxification efficacy of selected PAC compounds (Figure 5) where high degradation efficacies were recorded at low PAC concentrations.
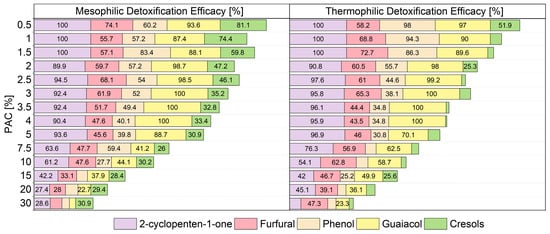
Figure 5.
Removal efficacies for some selected PAC compounds after syngas mixed culture fermentations performed at 37 and 55 °C. Numeric values of the removal efficacies below 25% are now shown.
For PAC concentrations above 5%, the efficacy of degradation decreased both at the thermophilic and mesophilic range. A work performed by Fedorak and Hrudey [81] reporting high removal of phenol and m- and p-cresol from a wastewater of a coal liquefaction plant during anaerobic batch culture experiments supports what was detected here. Hübner and Mumme [22] suggested that low cresols degradation efficacies might be accounting for cresols production via phenol degradation, as cresols and guaiacol are phenol derivates.
Considering that bottles with low CO consumption rates showed high PAC detoxifications efficacies, it can be assumed that PAC detoxification was independent from syngas metabolism and it occurred at concentrations inhibiting carboxidotrophism and homoacetogenesis. On the other hand, the longer lag phases at increasing PAC concentration might suggest that syngas metabolism was dependent on the detoxification of toxins in PAC and it recovered once the concentration of some PAC components fell below toxic levels.
3.3. A. oryzae Cultivation on Acetate Derived from Syngas Fermentation and PAC Detoxification
To further test the degree of PAC detoxification and to valorize the carboxylates from the M-PAC and T-PAC experiments, the media from some selected bottles were centrifuged and the resulting supernatant inoculated with A. oryzae.
No fungal growth was detected in the media containing the broth from syngas abiotic control experiments with syngas, M-PAC-AB-Asp and T-PAC-AB-Asp (Figure 6). Thus, abiotic incubation over an extensive amount of time did not lower the toxicity levels of PAC towards A. oryzae. On the contrary, A. oryzae growth was detected in all fermentations up to M-PAC-Asp 10% and T-PAC-Asp 10%. Inhibitory effects of pyrolysis products of wheat straw on A. oryzae growth were previously elucidated by Dörsam et al. [50], who studied the toxicity of some selected PAC components. Phenolic compounds such as phenol, o-, m-, p-cresol and guaiacol resulted in a strong inhibition of A. oryzae growth even at low concentrations. Although it is known that A. oryzae has genes encoding for enzymes enabling the degradation of cresols, it only tolerates cresol in very low concentrations [82]. Additionally, 2-cyclopenten-1-one was reported to be the most toxic compound among the tested ones [50].
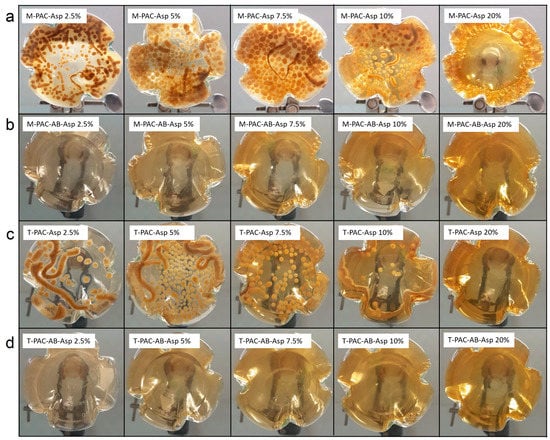
Figure 6.
Growth of A. oryzae in aerobic flasks containing medium from syngas fermentations and abiotic controls. Rows (a) and (c) show fungal growth in medium from mesophilic and thermophilic syngas culture fermentations, respectively. Rows (b) and (d) show the results of fungal growth in medium from the abiotic incubation of PAC and BA medium.
Malate Production from Acetate by A. oryzae
The ability of A. oryzae to convert glucose and VFAs from various sources into L-malate or biomass has been studied in previous works [49,83,84,85,86]. Here, the acetate detected at the start of the A. oryzae fermentations derived from different sources: syngas fermentation; acetate originally contained in the PAC; and PAC detoxification.
Complete acetate consumption was recorded in all flasks containing medium from bottle fermentations with up to 10% PAC (Figure 7a,c). L-malate production was detected in all bottles alongside acetate consumption. For both A-M-PAC 20% and A-T-PAC 20%, no acetate consumption nor L-malate production were detected.
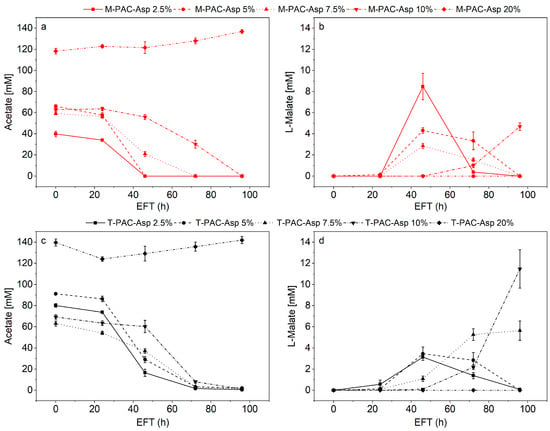
Figure 7.
Acetate and L-malate from A. oryzae fermentations in the medium from mesophilic syngas fermentations (a,b) and thermophilic syngas fermentations (c,d).
For the medium from mesophilic syngas fermentations, the highest amount and yield of malate from acetate of 8.47 ± 0.21 mM and 0.21 mM/mM, respectively, were obtained in M-PAC-Asp 2.5%. Overall, L-malate yields decreased at increasing PAC concentrations for M-PAC-Asp fermentations. On the other hand, when considering the medium from thermophilic syngas fermentations, the highest amount of L-malate produced was detected for T-PAC-Asp 10% at 11.46 ± 0.16 mM with the highest yield of 0.17 mM/mM. Contrarily to M-PAC-Asp fermentations, L-malate yields increased at increasing PAC concentrations. Process optimization for L-malate production exceeded the scope of this work; however, the highest malate yields detected in this study are comparable to the 0.20 g of malic acid per gram of acetate for concentrations of 40 g/L of acetate reported by Kövilein et al. [49]. Kövilein et al. [49] tested acetate concentrations between 10 and 55 g/L for malate production in A. oryzae shake flasks cultures. Malate production was reported to be highly dependent on acetate concentration with the highest yield for concentrations of up to 40 g/L [49]. Similarly, Uwineza et al. [84] grew A. oryzae on VFAs from the anaerobic digestion of food waste with maximum concentrations of acetate of 9 g/L yielding 0.29 gCDW/gVFAs. Higher concentrations of acetate did not affect the yield. Oswald et al. [83] presented a process concept, in which malate was produced from acetate generated from syngas fermentation by C. ljungdahlii. Malate production by A. oryzae in the medium from the syngas fermentations with acetate as sole carbon source reached yields of 0.33 g of malate per gram of acetate [83]. The overall conversion of CO and H2 into malate was calculated to be 0.22 g malate per gram of syngas [83]. The high malate yields achieved in this work, as already hypothesized by Oswald et al. [83], might be linked to the richness in micronutrients of the medium from the previous fermentations.
4. Conclusions
In this study, PAC and syngas were co-fermented by mesophilic and thermophilic mixed cultures and the effects of increasing concentrations of PAC were evaluated. PAC could be used effectively to inhibit methanogenesis and steer microbial metabolism towards other metabolites. Fermenting PAC and syngas in the mesophilic range led to acetate, propionate and n-butyrate accumulation in the fermentation broth with net H2 consumption, whereas fermentations at the thermophilic range produced primarily acetate and H2. These results show that the mixed cultures performed the dual task of fixing C1 compounds from syngas and detoxifying PAC. Treating PAC together with syngas enabled carboxylates valorization to platform chemicals such as L-malate by A. oryzae via a sequential secondary fermentation stage. Mesophilic carboxylate production and thermophilic biohydrogen production via mixed culture syngas fermentations are becoming the center of extensive interest for biochemical or biofuel production. Thus, exploring alternative and effective methods for the inhibition of methanogenesis is still necessary, and inhibitors, such as PAC, are ideal candidates. This work contributes towards a better understanding of the efficient integration of thermochemical processes and mixed culture anaerobic fermentations. Further studies should test the feasibility of this work in continuous bioreactors, aiming to gain a better understanding of the microbial interactions that are contributing to the PAC degradation and syngas metabolism.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation8100512/s1. Table S1: Conversion factors for carbon and electron balances; Table S2: Productivities (mM/d) of selected metabolites calculated at 39 days EFT for bottles of the control experiments M-CTRL, T-CTRL, M-BES, T-BES; Table S3: Acetate, propionate and n-butyrate productivities for all bottles of M-PAC and T-PAC experiments; Table S4: Productivities of CO, CH4, H2 CO2 and VFAs in mM/d at increasing PAC concentrations and different temperatures. Negative productivity indicates consumption; Figure S1: Cumulative CO uptake rate in mmol for experiments M-BES, M-CTRL and M-PAC; Figure S2: Cumulative H2 uptake rate in mmol for experiments M-BES, M-CTRL and M-PAC; Figure S3: Cumulative CO2 uptake rate in mmol for experiments M-BES, M-CTRL and M-PAC. Negative values mean consumption; Figure S4: Cumulative CH4 uptake rate in mmol for experiments M-BES, M-CTRL and M-PAC; Figure S5: Cumulative CO uptake rate in mmol for experiments T-BES, T-CTRL and T-PAC; Figure S6: Cumulative H2 uptake rate in mmol for experiments T-BES, T-CTRL and T-PAC. A negative uptake means production; Figure S7: Cumulative CO2 uptake rate in mmol for experiments T-BES, T-CTRL and T-PAC. A negative uptake means production; Figure S8: Cumulative CH4 uptake rate in mmol for experiments T-BES, T-CTRL and T-PAC.
Author Contributions
Conceptualization, A.R., C.K., F.C.F.B., K.O. and A.N.; methodology, A.R., C.K. and F.C.F.B.; formal analysis, A.R.; investigation, A.R., C.W. and C.K.; resources, A.R. and A.N.; data curation, A.R.; writing—original draft preparation, A.R.; writing—review and editing, C.K., F.C.F.B., K.O., A.N.; visualization, A.R.; supervision, A.N.; project administration, A.N.; funding acquisition, A.N. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Helmholtz Research Program “Materials and Technologies for the Energy Transition (MTET), Topic 3: Chemical Energy Carriers” and the support from the KIT-Publication Fund of the Karlsruhe Institute of Technology. Open access funding enabled and organized by Projekt DEAL.
Institutional Review Board Statement
Not applicable.
Acknowledgments
The authors acknowledge Institute of Catalysis Research & Technology, Karlsruhe Institute of Technology, for providing the PAC, Habibu Aliyu for mentoring and the technical staff at Institute of Process Engineering in Life Sciences 2: Technical Biology, Karlsruhe Institute of Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cherubini, F.; Ulgiati, S. Crop residues as raw materials for biorefinery systems—A LCA case study. Appl. Energy 2010, 87, 47–57. [Google Scholar] [CrossRef]
- Rathmann, R.; Szklo, A.; Schaeffer, R. Land use competition for production of food and liquid biofuels: An analysis of the arguments in the current debate. Renew. Energy 2010, 35, 14–22. [Google Scholar] [CrossRef]
- Popp, J.; Lakner, Z.; Harangi-Rákos, M.; Fári, M. The effect of bioenergy expansion: Food, energy, and environment. Renew. Sustain. Energy Rev. 2014, 32, 559–578. [Google Scholar] [CrossRef]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Gil, A. Current insights into lignocellulose related waste valorization. Chem. Eng. J. Adv. 2021, 8, 100186. [Google Scholar] [CrossRef]
- Funke, A.; Morgano, M.T.; Dahmen, N.; Leibold, H. Experimental comparison of two bench scale units for fast and intermediate pyrolysis. J. Anal. Appl. Pyrolysis 2017, 124, 504–514. [Google Scholar] [CrossRef]
- Niebel, A.; Funke, A.; Pfitzer, C.; Dahmen, N.; Weih, N.; Richter, D.; Zimmerlin, B. Fast Pyrolysis of Wheat Straw—Improvements of Operational Stability in 10 Years of Bioliq Pilot Plant Operation. Energy Fuels 2021, 35, 11333–11345. [Google Scholar] [CrossRef]
- Leng, L.; Yang, L.; Chen, J.; Hu, Y.; Li, H.; Li, H.; Jiang, S.; Peng, H.; Yuan, X.; Huang, H. Valorization of the aqueous phase produced from wet and dry thermochemical processing biomass: A review. J. Clean. Prod. 2021, 294, 126238. [Google Scholar] [CrossRef]
- Basaglia, M.; Favaro, L.; Torri, C.; Casella, S. Is pyrolysis bio-oil prone to microbial conversion into added-value products? Renew. Energy 2021, 163, 783–791. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, X.; Chi, Z.; Rover, M.; Johnston, P.; Brown, R.; Jarboe, L.; Wen, Z. Utilization of acetic acid-rich pyrolytic bio-oil by microalga Chlamydomonas reinhardtii: Reducing bio-oil toxicity and enhancing algal toxicity tolerance. Bioresour. Technol. 2013, 133, 500–506. [Google Scholar] [CrossRef]
- Lange, J.; Müller, F.; Bernecker, K.; Dahmen, N.; Takors, R.; Blombach, B. Valorization of pyrolysis water: A biorefinery side stream, for 1,2-propanediol production with engineered Corynebacterium glutamicum. Biotechnol. Biofuels 2017, 10, 277. [Google Scholar] [CrossRef]
- Arnold, S.; Moss, K.; Dahmen, N.; Henkel, M.; Hausmann, R. Pretreatment strategies for microbial valorization of bio-oil fractions produced by fast pyrolysis of ash-rich lignocellulosic biomass. GCB Bioenergy 2019, 11, 181–190. [Google Scholar] [CrossRef]
- Lian, J.; Garcia-Perez, M.; Coates, R.; Wu, H.; Chen, S. Yeast fermentation of carboxylic acids obtained from pyrolytic aqueous phases for lipid production. Bioresour. Technol. 2012, 118, 177–186. [Google Scholar] [CrossRef]
- Lian, J.; Chen, S.; Zhou, S.; Wang, Z.; O’Fallon, J.; Li, C.-Z.; Garcia-Perez, M. Separation, hydrolysis and fermentation of pyrolytic sugars to produce ethanol and lipids. Bioresour. Technol. 2010, 101, 9688–9699. [Google Scholar] [CrossRef]
- Kubisch, C.; Ochsenreither, K. Detoxification of a pyrolytic aqueous condensate from wheat straw for utilization as substrate in Aspergillus oryzae DSM 1863 cultivations. Biotechnol. Biofuels Bioprod. 2022, 15, 18. [Google Scholar] [CrossRef]
- Arnold, S.; Henkel, M.; Wanger, J.; Wittgens, A.; Rosenau, F.; Hausmann, R. Heterologous rhamnolipid biosynthesis by P. putida KT2440 on bio-oil derived small organic acids and fractions. AMB Express 2019, 9, 80. [Google Scholar] [CrossRef]
- Arnold, S.; Tews, T.; Kiefer, M.; Henkel, M.; Hausmann, R. Evaluation of small organic acids present in fast pyrolysis bio-oil from lignocellulose as feedstocks for bacterial bioconversion. GCB Bioenergy 2019, 11, 1159–1172. [Google Scholar] [CrossRef]
- Vítězová, M.; Kohoutová, A.; Vítěz, T.; Hanišáková, N.; Kushkevych, I. Methanogenic microorganisms in industrial wastewater anaerobic treatment. Processes 2020, 8, 1546. [Google Scholar] [CrossRef]
- Anukam, A.; Mohammadi, A.; Naqvi, M.; Granström, K. A Review of the Chemistry of Anaerobic Digestion: Methods of accelerating and optimizing process efficiency. Processes 2019, 7, 504. [Google Scholar] [CrossRef]
- Hashsham, S.A.; Fernandez, A.S.; Dollhopf, S.L.; Dazzo, F.B.; Hickey, R.F.; Tiedje, J.M.; Criddle, C.S. Parallel processing of substrate correlates with greater functional stability in methanogenic bioreactor communities perturbed by glucose. Appl. Environ. Microbiol. 2000, 66, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.J.; Knights, D.; Garcia, M.L.; Scalfone, N.B.; Smith, S.; Yarasheski, K.; Cummings, T.A.; Beers, A.R.; Knight, R.; Angenent, L.T. Bacterial community structures are unique and resilient in full-scale bioenergy systems. Proc. Natl. Acad. Sci. USA 2011, 108, 4158–4163. [Google Scholar] [CrossRef]
- Hübner, T.; Mumme, J. Integration of pyrolysis and anaerobic digestion—Use of aqueous liquor from digestate pyrolysis for biogas production. Bioresour. Technol. 2015, 183, 86–92. [Google Scholar] [CrossRef]
- Wen, C.; Moreira, C.M.; Rehmann, L.; Berruti, F. Feasibility of anaerobic digestion as a treatment for the aqueous pyrolysis condensate (APC) of birch bark. Bioresour. Technol. 2020, 307, 123199. [Google Scholar] [CrossRef] [PubMed]
- Torri, C.; Fabbri, D. Biochar enables anaerobic digestion of aqueous phase from intermediate pyrolysis of biomass. Bioresour. Technol. 2014, 172, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Brown, R.C.; Wen, Z. Anaerobic digestion of aqueous phase from pyrolysis of biomass: Reducing toxicity and improving microbial tolerance. Bioresour. Technol. 2019, 292, 121976. [Google Scholar] [CrossRef] [PubMed]
- Seyedi, S.; Venkiteshwaran, K.; Zitomer, D. Toxicity of various pyrolysis liquids from biosolids on methane production yield’. Front. Energy Res. 2019, 7, 1–12. [Google Scholar] [CrossRef]
- Fabbri, D.; Torri, C. Linking pyrolysis and anaerobic digestion (Py-AD) for the conversion of lignocellulosic biomass. Curr. Opin. Biotechnol. 2016, 38, 167–173. [Google Scholar] [CrossRef]
- Torri, C.; Pambieri, G.; Gualandi, C.; Piraccini, M.; Rombolà, A.G.; Fabbri, D. Evaluation of the potential performance of hyphenated pyrolysis-anaerobic digestion (Py-AD) process for carbon negative fuels from woody biomass. Renew. Energy 2020, 148, 1190–1199. [Google Scholar] [CrossRef]
- Giwa, A.S.; Chang, F.; Xu, H.; Zhang, X.; Huang, B.; Li, Y.; Wu, J.; Wang, B.; Vakili, M.; Wang, K. Pyrolysis of difficult biodegradable fractions and the real syngas bio-methanation performance. J. Clean. Prod. 2019, 233, 711–719. [Google Scholar] [CrossRef]
- Righi, S.; Bandini, V.; Marazza, D.; Baioli, F.; Torri, C.; Contin, A. Life Cycle Assessment of high ligno-cellulosic biomass pyrolysis coupled with anaerobic digestion. Bioresour. Technol. 2016, 212, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, N.; Monlau, F.; Sambusiti, C.; Ficara, E.; Barakat, A.; Zabaniotou, A. Contribution to Circular Economy options of mixed agricultural wastes management: Coupling anaerobic digestion with gasification for enhanced energy and material recovery. J. Clean. Prod. 2019, 209, 505–514. [Google Scholar] [CrossRef]
- Funke, A.; Mumme, J.; Koon, M.; Diakité, M. Cascaded production of biogas and hydrochar from wheat straw: Energetic potential and recovery of carbon and plant nutrients. Biomass Bioenergy 2013, 58, 229–237. [Google Scholar] [CrossRef]
- Salman, C.A.; Schwede, S.; Thorin, E.; Yan, J. Enhancing biomethane production by integrating pyrolysis and anaerobic digestion processes. Appl. Energy 2017, 204, 1074–1083. [Google Scholar] [CrossRef]
- Navarro, S.S.; Cimpoia, R.; Bruant, G.; Guiot, S.R. Biomethanation of syngas using anaerobic sludge: Shift in the catabolic routes with the CO partial pressure increase. Front. Microbiol. 2016, 7, 1188. [Google Scholar] [CrossRef]
- Alves, J.I.; Stams, A.J.M.; Plugge, C.M.; Alves, M.M.; Sousa, D.Z. Enrichment of anaerobic syngas-converting bacteria from thermophilic bioreactor sludge. FEMS Microbiol. Ecol. 2013, 86, 590–597. [Google Scholar] [CrossRef]
- Liu, C.; Luo, G.; Wang, W.; He, Y.; Zhang, R.; Liu, G. The effects of pH and temperature on the acetate production and microbial community compositions by syngas fermentation. Fuel 2018, 224, 537–544. [Google Scholar] [CrossRef]
- Angenent, L.T.; Richter, H.; Buckel, W.; Spirito, C.M.; Steinbusch, K.J.J.; Plugge, C.M.; Strik, D.P.B.T.B.; Grootscholten, T.I.M.; Buisman, C.J.N.; Hamelers, H.V.M. Chain Elongation with Reactor Microbiomes: Open-Culture Biotechnology to Produce Biochemicals. Environ. Sci. Technol. 2016, 50, 2796–2810. [Google Scholar] [CrossRef]
- Baleeiro, F.C.F. Syngas-aided anaerobic fermentation for medium-chain carboxylate and alcohol production: The case for microbial communities. Appl. Microbiol. Biotechnol. 2019, 103, 8689–8709. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Łężyk, M.; Kennes-Veiga, D.M.; Skiadas, I.V.; Gavala, H.N. Enrichment of Mesophilic and Thermophilic Mixed Microbial Consortia for Syngas Biomethanation: The Role of Kinetic and Thermodynamic Competition. Waste Biomass Valorization 2020, 11, 465–481. [Google Scholar] [CrossRef]
- Conrad, R.; Wetter, B. Influence of temperature on energetics of hydrogen metabolism in homoacetogenic, methanogenic, and other anaerobic bacteria. Arch. Microbiol. 1990, 155, 94–98. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- de Groof, V.; Coma, M.; Arnot, T.; Leak, D.J.; Lanham, A.B. Medium chain carboxylic acids from complex organic feedstocks by mixed culture fermentation. Molecules 2019, 24, 398. [Google Scholar] [CrossRef]
- Zhang, F.; Ding, J.; Zhang, Y.; Chen, M.; Ding, Z.-W.; van Loosdrecht, M.C.; Zeng, R.J. Fatty acids production from hydrogen and carbon dioxide by mixed culture in the membrane biofilm reactor. Water Res. 2013, 47, 6122–6129. [Google Scholar] [CrossRef] [PubMed]
- Baleeiro, F.C.F.; Kleinsteuber, S.; Sträuber, H. Recirculation of H2, CO2, and Ethylene Improves Carbon Fixation and Carboxylate Yields in Anaerobic Fermentation. ACS Sustain. Chem. Eng. 2022, 10, 4073–4081. [Google Scholar] [CrossRef]
- Gibbs, P.A.; Seviour, R.J.; Schmid, F. Growth of filamentous fungi in submerged culture: Problems and possible solutions. Crit. Rev. Biotechnol. 2000, 20, 17–48. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Mahboubi, A.; Lennartsson, P.R.; Taherzadeh, M.J. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresour. Technol. 2016, 215, 334–345. [Google Scholar] [CrossRef]
- Uwineza, C.; Sar, T.; Mahboubi, A.; Taherzadeh, M.J. Evaluation of the cultivation of aspergillus oryzae on organic waste-derived vfa effluents and its potential application as alternative sustainable nutrient source for animal feed. Sustainability 2021, 13, 12489. [Google Scholar] [CrossRef]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Value-added products from dairy waste using edible fungi. Waste Manag. 2017, 59, 518–525. [Google Scholar] [CrossRef]
- Kövilein, A.; Umpfenbach, J.; Ochsenreither, K. Acetate as substrate for l-malic acid production with Aspergillus oryzae DSM 1863. Biotechnol. Biofuels 2021, 14, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Dörsam, S.; Kirchhoff, J.; Bigalke, M.; Dahmen, N.; Syldatk, C.; Ochsenreither, K. Evaluation of pyrolysis oil as carbon source for fungal fermentation. Front. Microbiol. 2016, 7, 2059. [Google Scholar] [CrossRef] [PubMed]
- Telliard, W.A. Method1684 Total, Fixed, and Volatile Solids in Water, Solids, and Biosolids; Draft January 2001; U.S. Environmental Protection Agency Office of Water Office of Science and Technology Engineering and Analysis Division (4303 )U.S. EPA’: Washington, DC, USA, 2001; pp. 1–13. [Google Scholar]
- Pfitzer, C.; Dahmen, N.; Tröger, N.; Weirich, F.; Sauer, J.; Günther, A.; Müller-Hagedorn, M. Fast Pyrolysis of Wheat Straw in the Bioliq Pilot Plant. Energy Fuels 2016, 30, 8047–8054. [Google Scholar] [CrossRef]
- Grimalt-Alemany, A.; Łȩzyk, M.; Lange, L.; Skiadas, I.V.; Gavala, H.N. Enrichment of syngas-converting mixed microbial consortia for ethanol production and thermodynamics-based design of enrichment strategies. Biotechnol. Biofuels 2018, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Grimalt-Alemany, A.; Skiadas, I.V.; Gavala, H.N. Syngas biomethanation: State-of-the-art review and perspectives. Biofuels Bioprod. Biorefining 2018, 12, 139–158. [Google Scholar] [CrossRef]
- Mahamkali, V.; Valgepea, K.; Lemgruber, R.D.S.P.; Plan, M.; Tappel, R.; Köpke, M.; Simpson, S.D.; Nielsen, L.K.; Marcellin, E. Redox controls metabolic robustness in the gas-fermenting acetogen Clostridium autoethanogenum. Proc. Natl. Acad. Sci. USA 2020, 117, 13168–13175. [Google Scholar] [CrossRef]
- Wang, S.; Huang, H.; Kahnt, H.H.; Mueller, A.P.; Köpke, M.; Thauer, R.K. NADP-Specific electron-bifurcating [FeFe]-hydrogenase in a functional complex with formate dehydrogenase in clostridium autoethanogenum grown on CO. J. Bacteriol. 2013, 195, 4373–4386. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, J.; Han, S.; Zhang, S.; Luo, G. Selective conversion of carbon monoxide to hydrogen by anaerobic mixed culture. Bioresour. Technol. 2016, 202, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Daniels, L.; Fuchs, G.; Thauer, R.K.; Zeikus, J.G. Carbon monoxide oxidation by methanogenic bacteria. J. Bacteriol. 1977, 132, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Rother, M.; Metcalf, W.W. Anaerobic growth of Methanosarcina acetivorans C2A on carbon monoxide: An unusual way of life for a methanogenic archaeon’. Proc. Natl. Acad. Sci. USA 2004, 101, 16929–16934. [Google Scholar] [CrossRef]
- Sipma, J.; Lens, P.N.L.; Stams, A.J.M.; Lettinga, G. Carbon monoxide conversion by anaerobic bioreactor sludges. FEMS Microbiol. Ecol. 2003, 44, 271–277. [Google Scholar] [CrossRef]
- Slepova, T.V.; Rusanov, I.I.; Sokolova, T.G.; Bonch-Osmolovskaya, E.A.; Pimenov, N.V. Radioisotopic tracing of carbon monoxide conversion by anaerobic thermophilic prokaryotes. Microbiology 2007, 76, 523–529. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Yu, S.J.; Zhang, F.; Xia, X.Y.; Zeng, R.J. Enhancement of acetate productivity in a thermophilic (55 °C) hollow-fiber membrane biofilm reactor with mixed culture syngas (H2/CO2) fermentation. Appl. Microbiol. Biotechnol. 2017, 101, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Dai, K.; Xia, X.Y.; Zeng, R.J.; Zhang, F. Conversion of syngas (CO and H2) to biochemicals by mixed culture fermentation in mesophilic and thermophilic hollow-fiber membrane biofilm reactors. J. Clean. Prod. 2018, 202, 536–542. [Google Scholar] [CrossRef]
- Liu, H.; Wang, J.; Wang, A.; Chen, J. Chemical inhibitors of methanogenesis and putative applications. Appl. Microbiol. Biotechnol. 2011, 89, 1333–1340. [Google Scholar] [CrossRef]
- Thompson, M.A.; Mohajeri, A.; Mirkouei, A. Comparison of pyrolysis and hydrolysis processes for furfural production from sugar beet pulp: A case study in southern Idaho, USA. J. Clean. Prod. 2021, 311, 127695. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotecnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Ljungdahl, L.G. Acetate Synthesis in Acetogenic Bacteria. Annu. Rev. Microbiol. 1986, 40, 415–450. [Google Scholar] [CrossRef] [PubMed]
- Bache, R.; Pfennig, N. Selective isolation of Acetobacterium woodii on methoxylated aromatic acids and determination of growth yields. Arch. Microbiol. 1981, 130, 255–261. [Google Scholar] [CrossRef]
- Schink, B.; Pfennig, N. Fermentation of trihydroxybenzenes by Pelobacter acidigallici gen. nov. sp. nov., a new strictly anaerobic, non-sporeforming bacterium. Arch. Microbiol. 1982, 133, 195–201. [Google Scholar] [CrossRef]
- Kato, S.; Chino, K.; Kamimura, N.; Masai, E.; Yumoto, I.; Kamagata, Y. Methanogenic degradation of lignin-derived monoaromatic compounds by microbial enrichments from rice paddy field soil. Sci. Rep. 2015, 5, 14295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Fedorak, P.M.; Hrudey, S.E. The effects of phenol and some alkyl phenolics on batch anaerobic methanogenesis. Water Res. 1984, 18, 361–367. [Google Scholar] [CrossRef]
- Battersby, N.S.; Wilson, V. Survey of the anaerobic biodegradation potential of organic chemicals in digesting sludge. Appl. Environ. Microbiol. 1989, 55, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Schink, B.Y.; Philipp, B.; Müller, J. Anaerobic Degradation of Phenolic Compounds. Naturwissenschaften 2000, 87, 12–23. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. The inhibition of anaerobic digestion by model phenolic compounds representative of those from Sargassum muticum. J. Appl. Phycol. 2019, 31, 779–786. [Google Scholar] [CrossRef]
- Kato, K.; Kozaki, S.; Sakuranaga, M. Degradation of ligning compounds by bacteria from termite guts. Biotechnol. Lett. 1998, 20, 459–462. [Google Scholar] [CrossRef]
- Huang, X.-F.; Santhanam, N.; Badri, D.V.; Hunter, W.J.; Manter, D.K.; Decker, S.R.; Vivanco, J.M.; Reardon, K.F. Isolation and characterization of lignin-degrading bacteria from rainforest soils. Biotechnol. Bioeng. 2013, 110, 1616–1626. [Google Scholar] [CrossRef]
- Ozbayram, E.G.; Kleinsteuber, S.; Nikolausz, M.; Ince, B.; Ince, O. Bioaugmentation of anaerobic digesters treating lignocellulosic feedstock by enriched microbial consortia. Eng. Life Sci. 2018, 18, 440–446. [Google Scholar] [CrossRef]
- Tuesorn, S.; Wongwilaiwalin, S.; Champreda, V.; Leethochawalit, M.; Nopharatana, A.; Techkarnjanaruk, S.; Chaiprasert, P. Enhancement of biogas production from swine manure by a lignocellulolytic microbial consortium. Bioresour. Technol. 2013, 144, 579–586. [Google Scholar] [CrossRef]
- Qiu, Y.L.; Hanada, S.; Ohashi, A.; Harada, H.; Kamagata, Y.; Sekiguchi, Y. Syntrophorhabdus aromaticivorans gen. nov., sp. nov., the first cultured anaerobe capable of degrading phenol to acetate in obligate syntrophic associations with a hydrogenotrophic methanogen. Appl. Environ. Microbiol. 2008, 74, 2051–2058. [Google Scholar] [CrossRef]
- Fedorak, P.M.; Hrudey, S.E. Inhibition of anaerobic degradation of phenolics and methanogenesis by coal coking wastewater. Water Sci. Technol. 1987, 19, 219–228. [Google Scholar] [CrossRef]
- Machida, M.; Asai, K.; Sano, M.; Tanaka, T.; Kumagai, T.; Terai, G.; Kusumoto, K.-I.; Arima, T.; Akita, O.; Kashiwagi, Y.; et al. Genome sequencing and analysis of Aspergillus oryzae. Nature 2005, 438, 1157–1161. [Google Scholar] [CrossRef]
- Oswald, F.; Dörsam, S.; Veith, N.; Zwick, M.; Neumann, A.; Ochsenreither, K.; Syldatk, C. Sequential mixed cultures: From syngas to malic acid. Front. Microbiol. 2016, 7, 891. [Google Scholar] [CrossRef]
- Uwineza, C.; Mahboubi, A.; Atmowidjojo, A.; Ramadhani, A.; Wainaina, S.; Millati, R.; Wikandari, R.; Niklasson, C.; Taherzadeh, M.J. Cultivation of edible filamentous fungus Aspergillus oryzae on volatile fatty acids derived from anaerobic digestion of food waste and cow manure. Bioresour. Technol. 2021, 337, 125410. [Google Scholar] [CrossRef]
- Kövilein, A.; Aschmann, V.; Hohmann, S.; Ochsenreither, K. Immobilization of Aspergillus oryzae DSM 1863 for l-Malic Acid Production. Fermentation 2022, 8, 26. [Google Scholar] [CrossRef]
- Schmitt, V.; Derenbach, L.; Ochsenreither, K. Enhanced l-Malic Acid Production by Aspergillus oryzae DSM 1863 Using Repeated-Batch Cultivation. Front. Bioeng. Biotechnol. 2022, 9, 1–15. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).