Growth Performance and Biochemical Composition of Waste-Isolated Microalgae Consortia Grown on Nano-Filtered Pig Slurry and Cheese Whey under Mixotrophic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Nano-Filtered Permeate and Cheese Whey Sampling and Characterization
2.2. Microalgae Consortia and Preparation of Inoculum
2.3. Microalgae Consortia Molecular Characterization
2.4. Experimental Set-Up and Cultivation
2.5. Microalgae Consortia Growth Determination
2.6. Biochemical Analysis
2.7. Data Analysis
3. Results and Discussion
3.1. Subsection Characterization of Nano-Filtered Permeate (NFP) and Cheese Whey (CW)
3.2. Microalgae Consortia Growth
3.3. Nutrient Mass Balance
3.4. Biochemical Composition of AC Biomasses
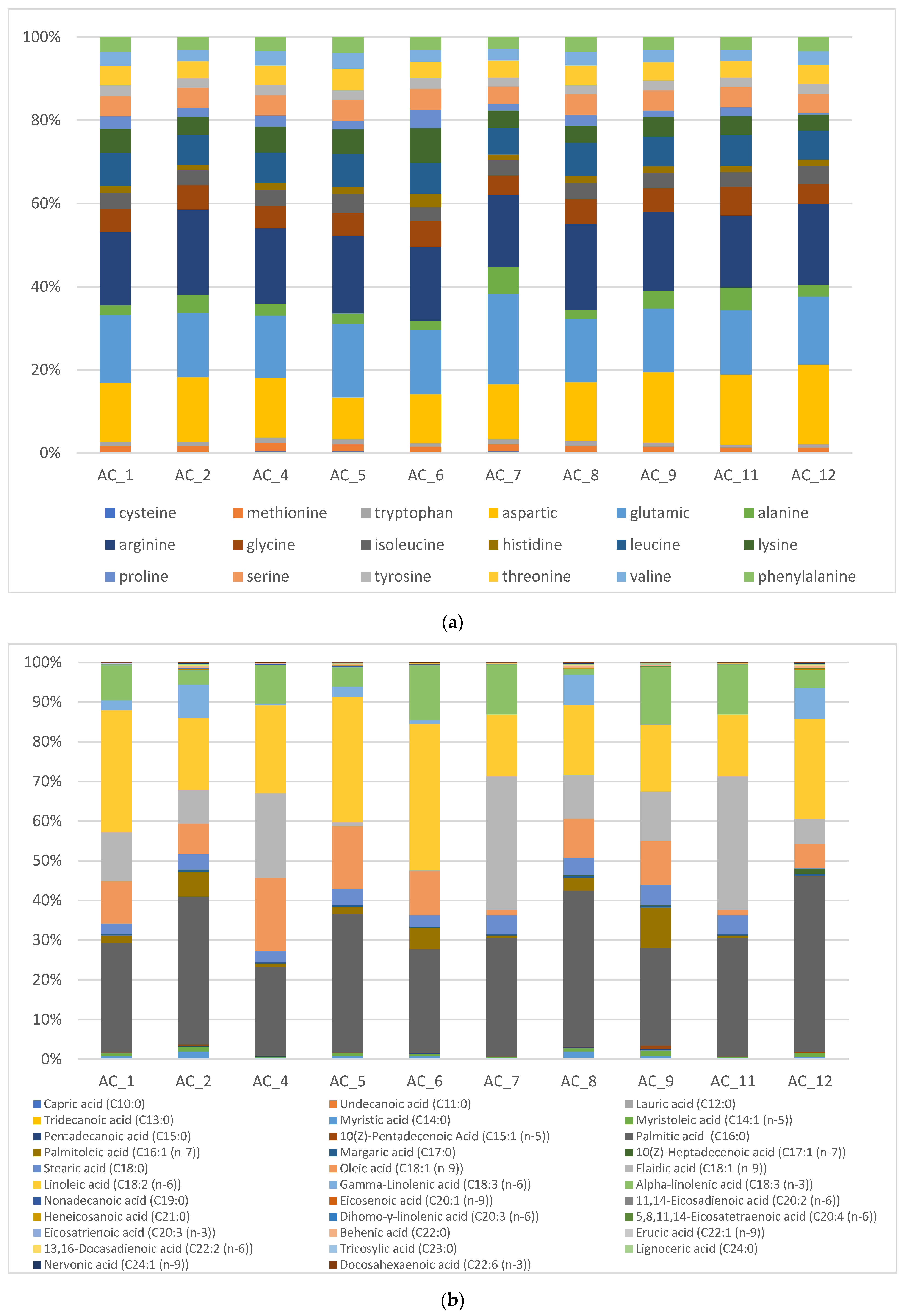
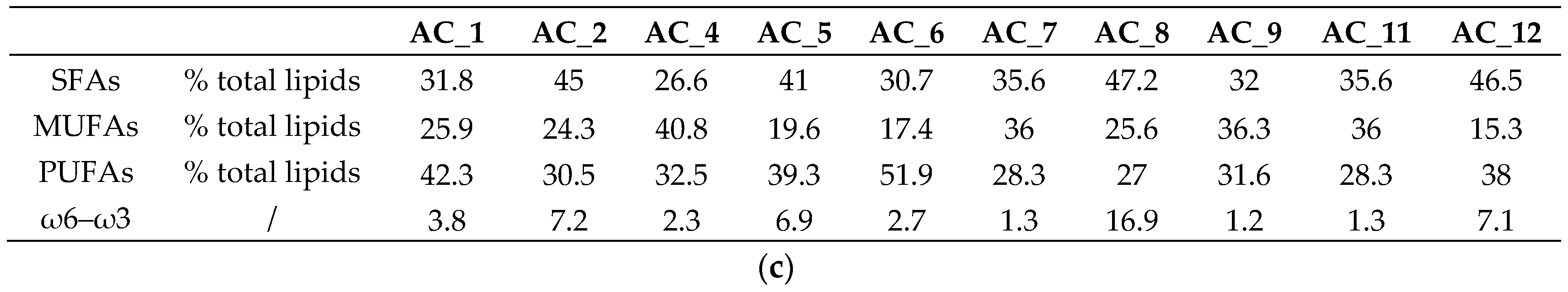
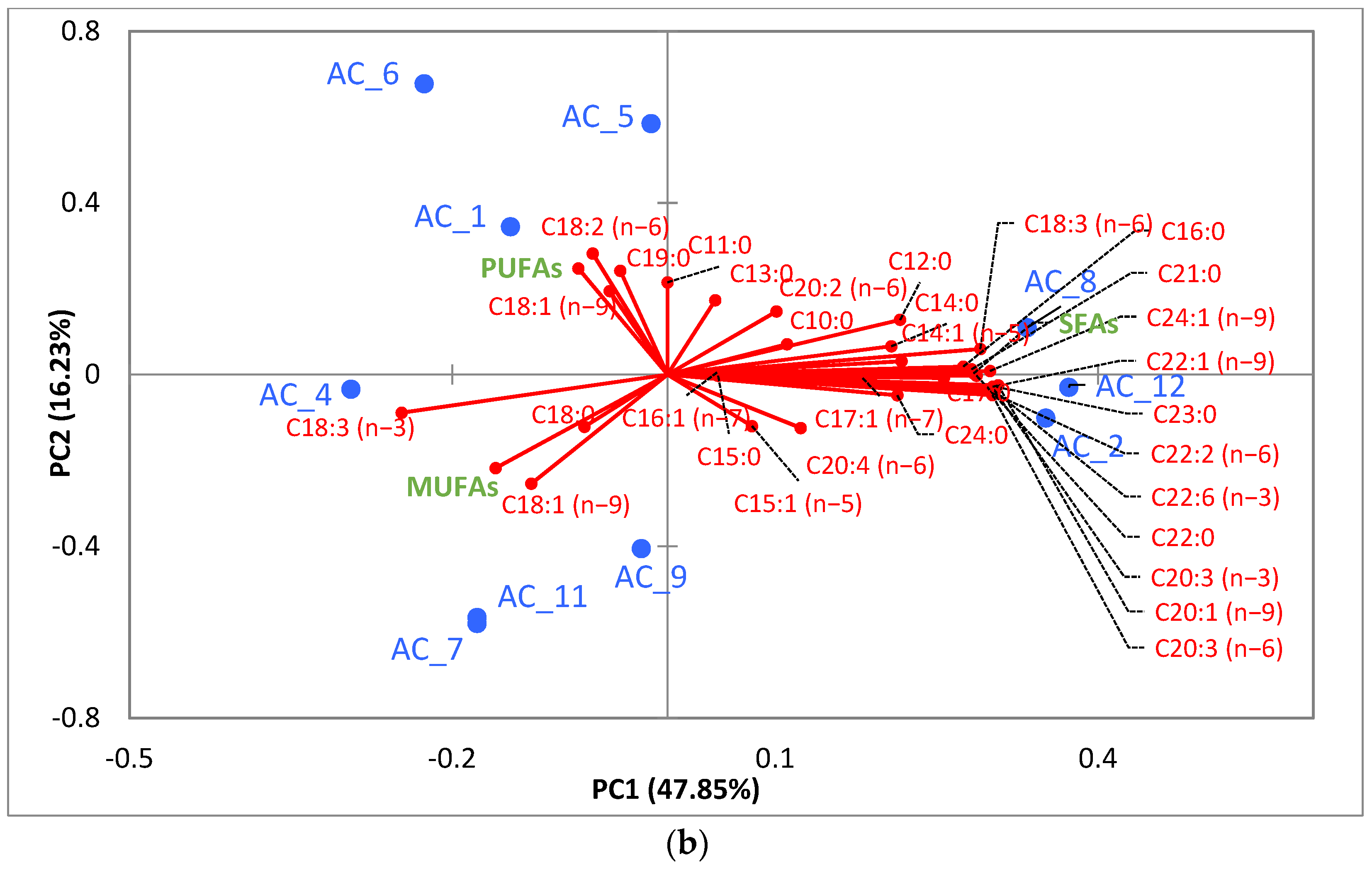
3.5. Amino Acids (AA) and Fatty Acids (FA) Speciation
3.5.1. Amino Acids (AA)
3.5.2. Fatty Acids (FA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SundarRajan, P.S.; Gopinath, K.P.; Greetham, D.; Antonysamy, A.J. A review on cleaner production of biofuel feedstock from integrated CO2 sequestration and wastewater treatment system. J. Clean. Prod. 2019, 210, 445–458. [Google Scholar] [CrossRef]
- Abreu, A.P.; Fernandes, B.; Vicente, A.A.; Teixeira, J.; Dragone, G. Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresour. Technol. 2012, 118, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Cecchin, M.; Benfatto, S.; Griggio, F.; Mori, A.; Cazzaniga, S.; Vitulo, N.; Delledonne, M.; Ballottari, M. Molecular basis of autotrophic vs mixotrophic growth in Chlorella sorokiniana. Sci. Rep. 2018, 8, 6465. [Google Scholar] [CrossRef]
- Salati, S.; D’Imporzano, G.; Menin, B.; Veronesi, D.; Scaglia, B.; Abbruscato, P.; Mariani, P.; Adani, F. Mixotrophic cultivation of Chlorella for local protein production using agro-food by-products. Bioresour. Technol. 2017, 230, 82–89. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Chinnasamy, S.; Singh, M.; Das, K.C. Renewable biomass production by mixotrophic algae in the presence of various carbon sources and wastewaters. Appl. Energy 2011, 88, 3425–3431. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef]
- Italy: Whey Powder from Cheese (Regions). Available online: https://www.clal.it/en/index.php?section=siero_regioni (accessed on 19 July 2022).
- Acién, F.G.; Fernández, J.M.; Magán, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Vo Hoang Nhat, P.; Ngo, H.H.; Guo, W.S.; Chang, S.W.; Nguyen, D.D.; Nguyen, P.D.; Bui, X.T.; Zhang, X.B.; Guo, J.B. Can algae-based technologies be an affordable green process for biofuel production and wastewater remediation? Bioresour. Technol. 2018, 256, 491–501. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of nutrients from wastewaters using microalgae. Front. Sustain. Food Syst. 2018, 2, 59. [Google Scholar] [CrossRef]
- Su, M.; Dell’orto, M.; Scaglia, B.; D’imporzano, G.; Bani, A.; Adani, F. Growth performance, biochemical composition and nutrient recovery ability of twelve microalgae consortia isolated from various local organic wastes grown on nano-filtered pig slurry. Molecules 2022, 27, 422. [Google Scholar] [CrossRef]
- Devi, N.D.; Sun, X.; Ding, L.; Goud, V.V.; Hu, B. Mixotrophic growth regime of novel strain Scenedesmus sp. DDVG I in municipal wastewater for concomitant bioremediation and valorization of biomass. J. Clean. Prod. 2022, 365, 132834. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae biotechnology for industrial wastewater treatment, bioenergy production, and high-value bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef]
- Ferreira, A.; Molnar Jazić, J.; Gouveia, L.; Maletić, S.; Tomić, M.; Agbaba, J.; Vladić, J. Valorisation of microalga Tetradesmus obliquus grown in brewery wastewater using subcritical water extraction towards zero waste. Chem. Eng. J. 2022, 437, 135324. [Google Scholar] [CrossRef]
- Wang, S.; Yin, C.; Yang, Z.; Hu, X.; Liu, Z.; Song, W. Assessing the potential of Chlorella sp. for treatment and resource utilization of brewery wastewater coupled with bioproduct production. J. Clean. Prod. 2022, 367, 132939. [Google Scholar] [CrossRef]
- Su, M.; Dell’Orto, M.; D’Imporzano, G.; Bani, A.; Dumbrell, A.J.; Adani, F. The structure and diversity of microalgae-microbial consortia isolated from various local organic wastes. Bioresour. Technol. 2022, 347, 126416. [Google Scholar] [CrossRef] [PubMed]
- Subashchandrabose, S.R.; Ramakrishnan, B.; Megharaj, M.; Venkateswarlu, K.; Naidu, R. Consortia of cyanobacteria/microalgae and bacteria: Biotechnological potential. Biotechnol. Adv. 2011, 29, 896–907. [Google Scholar] [CrossRef] [PubMed]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Travieso, L.; Benítez, F.; Sánchez, E.; Borja, R.; Martín, A.; Colmenarejo, M.F. Batch mixed culture of Chlorella vulgaris using settled and diluted piggery waste. Ecol. Eng. 2006, 28, 158–165. [Google Scholar] [CrossRef]
- Sforza, E.; Cipriani, R.; Morosinotto, T.; Bertucco, A.; Giacometti, G.M. Excess CO2 supply inhibits mixotrophic growth of Chlorella protothecoides and Nannochloropsis salina. Bioresour. Technol. 2012, 104, 523–529. [Google Scholar] [CrossRef]
- Cerón García, M.C.; Sánchez Mirón, A.; Fernández Sevilla, J.M.; Molina Grima, E.; García Camacho, F. Mixotrophic growth of the microalga phaeodactylum tricornutum: Influence of different nitrogen and organic carbon sources on productivity and biomass composition. Process Biochem. 2005, 40, 297–305. [Google Scholar] [CrossRef]
- Silva-Benavides, A.M.; Torzillo, G. Nitrogen and phosphorus removal through laboratory batch cultures of microalga Chlorella vulgaris and cyanobacterium Planktothrix isothrix grown as monoalgal and as co-cultures. J. Appl. Phycol. 2012, 24, 267–276. [Google Scholar] [CrossRef]
- Mišurcová, L.; Buňka, F.; Vávra Ambrožová, J.; Machů, L.; Samek, D.; Kráčmar, S. Amino acid composition of algal products and its contribution to RDI. Food Chem. 2014, 151, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, A.C.; Mulbry, W.W. Recovery of dairy manure nutrients by benthic freshwater algae. Elsevier 2002, 84, 81–91. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Philos. 1990, 20, 215–240. [Google Scholar]
- Kim, S.; eun Park, J.; Cho, Y.B.; Hwang, S.J. Growth rate, organic carbon and nutrient removal rates of Chlorella sorokiniana in autotrophic, heterotrophic and mixotrophic conditions. Bioresour. Technol. 2013, 144, 8–13. [Google Scholar] [CrossRef]
- Patel, A.K.; Joun, J.M.; Hong, M.E.; Sim, S.J. Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresour. Technol. 2019, 282, 245–253. [Google Scholar] [CrossRef]
- Subramanian, G.; Yadav, G.; Sen, R. Rationally Leveraging mixotrophic growth of microalgae in different photobioreactor configurations for reducing the carbon footprint of an algal biorefinery: A techno-economic perspective. RSC Adv. 2016, 6, 72897–72904. [Google Scholar] [CrossRef]
- Nicodemou, A.; Kallis, M.; Agapiou, A.; Markidou, A.; Koutinas, M. The effect of trophic modes on biomass and lipid production of five microalgal strains. Water 2022, 14, 240. [Google Scholar] [CrossRef]
- Prasad, R.; Gupta, S.K.; Shabnam, N.; Oliveira, C.Y.B.; Nema, A.K.; Ansari, F.A.; Bux, F. Role of microalgae in global CO2 sequestration : Physiological mechanism, recent development, challenges, and future prospective. Sustainability 2021, 13, 23. [Google Scholar] [CrossRef]
- Park, K.C.; Whitney, C.; Mcnichol, J.C.; Dickinson, K.E.; Macquarrie, S.; Skrupski, B.P.; Zou, J.; Wilson, K.E.; Leary, S.J.B.O.; Mcginn, P.J. Mixotrophic and photoautotrophic cultivation of 14 microalgae isolates from Saskatchewan, Canada : Potential applications for wastewater remediation for biofuel production. J. Appl. Phycol. 2012, 24, 339–348. [Google Scholar] [CrossRef]
- Combres, C.; Laliberté, G.; Reyssac, J.S.; de la Noüe, J. Effect of acetate on growth and ammonium uptake in the microalga Scenedesmus obliquus. Physiol. Plant. 1994, 91, 729–734. [Google Scholar] [CrossRef]
- D’Imporzano, G.; Veronesi, D.; Salati, S.; Adani, F. Carbon and nutrient recovery in the cultivation of Chlorella vulgaris: A life cycle assessment approach to comparing environmental performance. J. Clean. Prod. 2018, 194, 685–694. [Google Scholar] [CrossRef]
- Wan, M.; Liu, P.; Xia, J.; Rosenberg, J.N.; Oyler, G.A.; Betenbaugh, M.J.; Nie, Z.; Qiu, G. The Effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl. Microbiol. Biotechnol. 2011, 91, 835–844. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors : An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Andruleviciute, V.; Makareviciene, V.; Skorupskaite, V.; Gumbyte, M. Biomass and oil content of Chlorella sp., Haematococcus sp., Nannochloris sp. and Scenedesmus sp. under mixotrophic growth conditions in the presence of technical glycerol. J. Appl. Phycol. 2014, 26, 83–90. [Google Scholar] [CrossRef]
- Kong, W.B.; Yang, H.; Cao, Y.T.; Song, H.; Hua, S.F.; Xia, C.G. Effect of glycerol and glucose on the enhancement of biomass, lipid and soluble carbohydrate production by Chlorella vulgaris in mixotrophic culture. Food Technol. Biotechnol. 2013, 51, 62–69. [Google Scholar]
- Perez-Garcia, O.; de-Bashan, L.E.; Hernandez, J.P.; Bashan, Y. Efficiency of growth and nutrient uptake from wastewater by heterotrophic, autotrophic, and mixotrophic cultivation of Chlorella vulgaris immobilized with azospirillum brasilense. J. Phycol. 2010, 46, 800–812. [Google Scholar] [CrossRef]
- Gao, F.; Yang, H.L.; Li, C.; Peng, Y.Y.; Lu, M.M.; Jin, W.H.; Bao, J.J.; Guo, Y.M. Effect of organic carbon to nitrogen ratio in wastewater on growth, nutrient uptake and lipid accumulation of a mixotrophic microalgae Chlorella sp. Bioresour. Technol. 2019, 282, 118–124. [Google Scholar] [CrossRef]
- Babaei, A.; Mehrnia, M.R.; Shayegan, J.; Sarrafzadeh, M.H.; Amini, E. Evaluation of nutrient removal and biomass production through mixotrophic, heterotrophic, and photoautotrophic cultivation of Chlorella in nitrate and ammonium wastewater. Int. J. Environ. Res. 2018, 12, 167–178. [Google Scholar] [CrossRef]
- Massa, M.; Buono, S.; Langellotti, A.L.; Castaldo, L.; Martello, A.; Paduano, A.; Sacchi, R.; Fogliano, V. Evaluation of anaerobic digestates from different feedstocks as growth media for Tetradesmus obliquus, Botryococcus braunii, Phaeodactylum tricornutum and Arthrospira maxima. N. Biotechnol. 2017, 36, 8–16. [Google Scholar] [CrossRef]
- Gupta, S.K.; Ansari, F.A.; Shriwastav, A.; Sahoo, N.K.; Rawat, I.; Bux, F. Dual role of Chlorella sorokiniana and Scenedesmus obliquus for comprehensive wastewater treatment and biomass production for bio-fuels. J. Clean. Prod. 2016, 115, 255–264. [Google Scholar] [CrossRef]
- Scarponi, P.; Ghirardini, A.M.V.; Bravi, M.; Cavinato, C. Evaluation of Chlorella vulgaris and Scenedesmus obliquus growth on pretreated organic solid waste digestate. Waste Manag. 2021, 119, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Yun, H.; Park, Y.; Kabra, A.N.; Oh, I.; Choi, J. Mixotrophic cultivation of a microalga Scenedesmus obliquus in municipal wastewater supplemented with food wastewater and flue gas CO2 for biomass production. J. Environ. Manag. 2015, 159, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Dahmani, S.; Zerrouki, D.; Ramanna, L.; Rawat, I.; Bux, F. Cultivation of Chlorella pyrenoidosa in outdoor open raceway pond using domestic wastewater as medium in arid desert region. Bioresour. Technol. 2016, 219, 749–752. [Google Scholar] [CrossRef]
- Kang, C.D.; Lee, J.S.; Park, T.H.; Sim, S.J. Complementary limiting factors of astaxanthin synthesis during photoautotrophic induction of haematococcus pluvialis: C/N ratio and light intensity. Appl. Microbiol. Biotechnol. 2007, 74, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Nzayisenga, J.C.; Eriksson, K.; Sellstedt, A. Mixotrophic and heterotrophic production of lipids and carbohydrates by a locally isolated microalga using wastewater as a growth medium. Bioresour. Technol. 2018, 257, 260–265. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Konyukhov, I.V.; Pogosyan, S.I.; Lobakova, E.S.; Gorelova, O.A. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Feng, Y.; Li, C.; Zhang, D. Lipid production of Chlorella vulgaris cultured in artificial wastewater medium. Bioresour. Technol. 2011, 102, 101–105. [Google Scholar] [CrossRef]
- Prathima Devi, M.; Venkata Subhash, G.; Venkata Mohan, S. Heterotrophic cultivation of mixed microalgae for lipid accumulation and wastewater treatment during sequential growth and starvation phases: Effect of nutrient supplementation. Renew. Energy 2012, 43, 276–283. [Google Scholar] [CrossRef]
- Lowrey, J.; Brooks, M.S.; McGinn, P.J. Heterotrophic and mixotrophic cultivation of microalgae for biodiesel production in agricultural wastewaters and associated challenges—A critical review. J. Appl. Phycol. 2015, 27, 1485–1498. [Google Scholar] [CrossRef]
- Markou, G. Effect of various colors of light-emitting diodes (leds) on the biomass composition of arthrospira platensis cultivated in semi-continuous mode. Appl. Biochem. Biotechnol. 2014, 172, 2758–2768. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Nawrocka, A.; Wiącek, D.; Krzemińska, I. Agro-industrial by-product in photoheterotrophic and mixotrophic culture of Tetradesmus obliquus: Production of ω3 and ω6 essential fatty acids with biotechnological importance. Sci. Rep. 2020, 10, 6411. [Google Scholar] [CrossRef]
- Gomaa, M.; Ali, M.M.A. Biomass and bioenergy enhancement of microalgal biomass, lipid production and biodiesel characteristics by mixotrophic cultivation using enzymatically hydrolyzed chitin waste. Biomass Bioenergy 2021, 154, 106251. [Google Scholar] [CrossRef]
- de Melo, R.G.; de Andrade, A.F.; Bezerra, R.P.; Correia, D.S.; de Souza, V.C.; Brasileiro-Vidal, A.C.; Viana Marques, D.d.A.; Porto, A.L.F. Chlorella vulgaris mixotrophic growth enhanced biomass productivity and reduced toxicity from agro-industrial by-products. Chemosphere 2018, 204, 344–350. [Google Scholar] [CrossRef]
- Kadkhodaei, S.; Abbasiliasi, S.; Shun, T.J.; Fard Masoumi, H.R.; Mohamed, M.S.; Movahedi, A.; Rahim, R.; Ariff, A.B. Enhancement of protein production by microalgae Dunaliella salina under mixotrophic conditions using response surface methodology. RSC Adv. 2015, 5, 38141–38151. [Google Scholar] [CrossRef]
- Heredia-Arroyo, T.; Wei, W.; Ruan, R.; Hu, B. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 2011, 35, 2245–2253. [Google Scholar] [CrossRef]
- Kong, W.; Song, H.; Cao, Y.; Yang, H.; Hua, S.; Xia, C. The characteristics of biomass production, lipid accumulation and chlorophyll biosynthesis of Chlorella vulgaris under mixotrophic cultivation. Afr. J. Biotechnol. 2011, 10, 11620–11630. [Google Scholar] [CrossRef]
- Ruiz-marin, A.; Canedo-lópez, Y.; Narvaez-garcía, A.; Robles-, J.C.; Zavala-loria, J.C. Productivity and biodiesel quality of fatty acids contents from Scenedesmus obliquus in domestic wastewater using phototrophic and mixotrophic cultivation systems. Open Biotechnol. J. 2018, 12, 229–240. [Google Scholar] [CrossRef]
- González-González, L.M.; De-Bashan, L.E. Toward the enhancement of microalgal metabolite production through microalgae–bacteria consortia. Biology 2021, 10, 282. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Energy and Protein; World Health Organization & Food and Agriculture Organization of the United Nations: Geneva, Switerland, 1973. [Google Scholar]
- Miao, M.S.; Yao, X.D.; Shu, L.; Yan, Y.J.; Wang, Z.; Li, N.; Cui, X.T.; Lin, Y.M.; Kong, Q. Mixotrophic growth and biochemical analysis of Chlorella vulgaris cultivated with synthetic domestic wastewater. Int. Biodeterior. Biodegrad. 2016, 113, 120–125. [Google Scholar] [CrossRef]
- Grosse, J.; Brussaard, C.P.D.; Boschker, H.T.S. Nutrient limitation driven dynamics of amino acids and fatty acids in coastal phytoplankton. Limnol. Oceanogr. 2019, 64, 302–316. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Eladel, H.M.; Battah, M.G.; Abd-Elal, S.M. Effect of different nitrogen sources on growth and biochemical composition of the green microalgae Scenedesmus obliquus and Chlorella kessleri. Int. Confer. Biol. Sci. 2004, 3, 419–432. [Google Scholar]
- Wang, Y.; Tibbetts, S.M.; Berrue, F.; McGinn, P.J.; MacQuarrie, S.P.; Puttaswamy, A.; Patelakis, S.; Schmidt, D.; Melanson, R.; MacKenzie, S.E. A rat study to evaluate the protein quality of three green microalgal species and the impact of mechanical cell wall disruption. Foods 2020, 9, 1531. [Google Scholar] [CrossRef] [PubMed]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation; Food and Agriculture Organization: Rome, Italy, 2013. [Google Scholar]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Li, Y.R.; Tsai, W.T.; Hsu, Y.C.; Xie, M.Z.; Chen, J.J. Comparison of autotrophic and mixotrophic cultivation of green microalgal for biodiesel production. Energy Procedia 2014, 52, 371–376. [Google Scholar] [CrossRef]
- Choi, H.J.; Yu, S.W. Influence of crude glycerol on the biomass and lipid content of microalgae. Biotechnol. Biotechnol. Equip. 2015, 29, 506–513. [Google Scholar] [CrossRef]
- Gross, M.; Wen, Z. Yearlong evaluation of performance and durability of a pilot-scale revolving algal biofilm (rab) cultivation system. Bioresour. Technol. 2014, 171, 50–58. [Google Scholar] [CrossRef]
- Rattanapoltee, P.; Kaewkannetra, P. Cultivation of microalga, chlorella vulgaris under different auto-hetero-mixo trophic growths as a raw material during biodiesel production and cost evaluation. Energy 2014, 78, 4–8. [Google Scholar] [CrossRef]
- Rincon, S.M.; Romero, H.M.; Aframehr, W.M.; Beyenal, H. Biomass production in Chlorella vulgaris biofilm cultivated under mixotrophic growth conditions. Algal Res. 2017, 26, 153–160. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Chanakya, H.N.; Ramachandra, T.V. Bioremediation and lipid synthesis through mixotrophic algal consortia in municipal wastewater. Bioresour. Technol. 2014, 168, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Alagawany, M.; Elnesr, S.S.; Farag, M.R.; Abd El-Hack, M.E.; Khafaga, A.F.; Taha, A.E.; Tiwari, R.; Iqbal Yatoo, M.; Bhatt, P.; Khurana, S.K.; et al. Omega-3 and Omega-6 fatty acids in poultry nutrition: Effect on production performance and health. Animals 2019, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.A.; Custódio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K.M. Alternative sources of n-3 long-chain polyunsaturated fatty acids in marine microalgae. Mar. Drugs 2013, 11, 2259–2281. [Google Scholar] [CrossRef] [PubMed]
- Arbib, Z.; Marín, D.; Cano, R.; Saúco, C.; Fernandez, M.; Lara, E. Chapter 9—Large-scale demonstration of microalgae-based wastewater biorefineries. In Integrated Wastewater Management and Valorization Using Algal Cultures; Elsevier: Amsterdam, The Netherlands, 2022; pp. 215–234. [Google Scholar]

| NFP | CW | BG-11 | ||
|---|---|---|---|---|
| pH | 8.5 ± 0.0 | 5.9 ± 0.0 | 7.4 | |
| TN | mg L−1 | 136 ± 0 | 601 ± 43 | 247 |
| NH4-N | mg L−1 | 132 ± 2 | 54 ± 4 | 19 |
| COD | g L−1 O2 | 0.1 ± 0.0 | 62 ± 2 | - |
| P | mg L−1 | 0.5 ± 0.0 | 502 ± 45 | 7.11 |
| Na | mg L−1 | 250 ± 3 | 2641 ± 87 | 414 |
| Mg | mg L−1 | 5.4 ± 0.1 | 69 ± 2 | 7.4 |
| K | mg L−1 | 188 ± 8 | 1491 ± 40 | 17.9 |
| Ca | mg L−1 | 9.7 ± 0.2 | 343 ± 12 | 9.8 |
| Fe | mg L−1 | u.d.l a | 1.8 ± 0.2 | 1.4 |
| B | mg L−1 | 0.5 ± 0.1 | u.d.l | 0.5 |
| Al | mg L−1 | 0.6 ± 0.0 | u.d.l | n.p. c |
| Cr | μg L−1 | 4.7 ± 0.6 | u.d.l | n.p. |
| Co | μg L−1 | 4.8 ± 1.2 | u.d.l | 10 |
| Cu | μg L−1 | 30.7 ± 0.4 | 359 ± 94 | 30 |
| Zn | μg L−1 | 57 ± 16 | 212 ± 27 | 50 |
| Se | μg L−1 | 5.2 ± 0.4 | u.d.l | n.p. |
| Mo | μg L−1 | 19 ± 5 | 70 ± 3 | 150 |
| Cd | μg L−1 | 7 b | u.d.l | n.p. |
| Pb | μg L−1 | 6.5 ± 2.0 | u.d.l | n.p. |
| As | μg L−1 | u.d.l | u.d.l | n.p. |
| Mn | μg L−1 | u.d.l | u.d.l | 500 |
| Ni | μg L−1 | u.d.l | 292 ± 25 | n.p. |
| Eukaryotic Genus | Prokaryotic Genus | µ (Auto) | µ (Mixo) | |||
|---|---|---|---|---|---|---|
| Algae % a,g | Other Eukaryotes % a,g | Algae % b,h | Other Prokaryotes % b,h | d−1 | d−1 | |
| AC_1 | Chlorella 99.1% | n.f. c | n.f. | Paludisphaera 36% (Planctomycetota) | 0.55 ± 0.04 a f | 0.53 ± 0.07 a |
| AC_2 | Chlorella 8.4% | Nuclearia 40.6%; Vahlkampfia 30.7%; Colpoda 15.6% | Synechocystis 35.9% | Truepera 21% (Deinococcata) | 0.22 ± 0.03 b | 0.47 ± 0.01 a * |
| AC_3 | Chlorella 85% | - | Synechocystis 19.6% | SM1A02 36.8% (Planctomycetota) | 0.25 ± 0.04 b | - e |
| AC_4 | Chlorella 76.4% | Colpoda 10.3% | Synechocystis 27.9% | SM1A02 34.5% (Planctomycetota) | 0.31 ± 0.12 b | 0.48 ± 0.05 a |
| AC_5 | Chlorella 30.6% | Colpoda 36.1%; Nuclearia 17.7% | Synechocystis 84.8% | n.f. | 0.29 ± 0.04 b | 0.43 ± 0.03 a |
| AC_6 | Tetradesmus 85.4% | Colpoda 9% | n.f. | Others d 61% | 0.31 ± 0.02 b | 0.43 ± 0.03 a * |
| AC_7 | Tetradesmus 42.6% | Colpoda 34.8% | Synechocystis 21.4% | Chloronema (Chloroflexi) 22.9% | 0.24 ± 0.02 b | 0.57 ± 0.01 a * |
| AC_8 | Scenedesmus 8.1%; Chlorella 6.3% | Colpoda 69.3% | n.f. | SM1A02 42.5% (Planctomycetota) | 0.28 ± 0.08 b | 0.40 ± 0.01 a |
| AC_9 | Chlorella 82.3% | Vermamoeba 11.9% | Synechocystis 35.4% | SM1A02 34.3% (Planctomycetota) | 0.31 ± 0.02 b | 0.61 ± 0.13 a |
| AC_10 | Tetradesmus 98.4% | n.f. | Synechocystis 54.2% | n.f. | 0.52 ± 0.06 a | - e |
| AC_11 | Chlorella 34.5% | Cyclidium 34.1% | Synechocystis 9.2% | SM1A02 57.7% (Planctomycetota) | 0.18 ± 0.01 b | 0.51 a |
| AC_12 | Chlorella 39.6%; Tetradesmus 32.6% | Vermamoeba 9.4% | Synechocystis 3.6% | Sandaracinus 29.8% (Proteobacteria); Others c 52.5% | 0.58 ± 0.06 a | 0.56 ± 0.11 a |
| AC | TNinitial a | TNfinal b | Nbiomass c | N Uptaken by Biomass | Pinitial d | Pfinal e | P Uptaken by Biomass | Cinitial f | Cfinal g | TOC Removal |
|---|---|---|---|---|---|---|---|---|---|---|
| mg L−1 | mg L−1 | g kg−1 DM i | % TNinitial a | mg L−1 | mg L−1 | % Pinitial d | mg L−1 | mg L−1 | % TOCinitial | |
| AC_1 | 169 ± 4 | 17 ± 2 | 61 ± 0 | 87 ± 2 | 51 ± 5 | 2 ± 1 | 96 ± 2 | 1962 ± 63 | 171 ± 21 | 91 ± 2 |
| AC_2 | 169 ± 4 | 30 ± 5 | 69 ± 1 | 87 ± 1 | 51 ± 5 | 1 ± 0 | 98 ± 1 | 1962 ± 63 | 261 ± 39 | 87 ± 3 |
| AC_3 | - h | |||||||||
| AC_4 | 169 ± 4 | 10 ± 1 | 44 ± 0 | 87 ± 1 | 51 ± 5 | 1 ± 0 | 99 ± 0 | 1962 ± 63 | 267 ± 31 | 86 ± 2 |
| AC_5 | 169 ± 4 | 14 ± 2 | 47 ± 0 | 79 ± 1 | 51 ± 5 | 1 ± 0 | 97 ± 1 | 1962 ± 63 | 295 ± 17 | 85 ± 1 |
| AC_6 | 169 ± 4 | 22 ± 1 | 76 ± 1 | 84 ± 3 | 51 ± 5 | 5 ± 0 | 91 ± 1 | 1962 ± 63 | 233 ± 48 | 88 ± 3 |
| AC_7 | 169 ± 4 | 22 ± 10 | 48 ± 2 | 95 ± 4 | 51 ± 5 | 9± 1 | 81 ± 1 | 1962 ± 63 | 242 ± 4 | 88 ± 0 |
| AC_8 | 169 ± 4 | 11 ± 1 | 63 ± 1 | 94 ± 3 | 51 ± 5 | 2 ± 0 | 97 ± 0 | 1962 ± 63 | 139 ± 19 | 93 ± 1 |
| AC_9 | 169 ± 4 | 33 ± 0 | 70 ± 2 | 67 ± 1 | 51 ± 5 | 2 ± 0 | 96 ± 1 | 1962 ± 63 | 260 ± 36 | 89 ± 3 |
| AC_10 | - h | |||||||||
| AC_11 j | 169 ± 4 | 39 | 85 | 71 | 51 ± 5 | 9 | 82 | 1962 ± 63 | 175 | 91 |
| AC_12 | 169 ± 4 | 51 ± 19 | 65 ± 1 | 74 ± 2 | 51 ± 5 | 9 ± 1 | 81 ± 1 | 1962 ± 63 | 395 ± 24 | 80 ± 17 |
| AC | Autotrophy | Mixotrophy | ||||
|---|---|---|---|---|---|---|
| Proteins | Lipids | Carbohydrates | Proteins | Lipids | Carbohydrates | |
| g kg−1 DM | g kg−1 DM | g kg−1 DM | g kg−1 DM | g kg−1 DM | g kg−1 DM | |
| AC_1 | 257 ± 0 g b | 119 ± 1 fg | 596 ± 4 a | 381 ± 1 e * | 121 ± 7 bcd | 470 ± 7 cde |
| AC_2 | 460 ± 4 b | 105 ± 7 g | 405 ± 9 ef | 430 ± 4 c | 75 ± 6 e * | 469 ± 8 de |
| AC_3 | 561 ± 1 a | 152 ± 10 cde | 254 ± 11 g | - a | ||
| AC_4 | 422 ± 3 c | 177 ± 3 b | 359 ± 6 f | 276 ± 1 g * | 118 ± 5 cd * | 573 ± 5 a |
| AC_5 | 266 ± 0 g | 153 ± 9 bcde | 565 ± 9 ab | 295 ± 1 fg * | 134 ± 1 b | 536 ± 2 ab |
| AC_6 | 305 ± 5 f | 173 ± 11 bc | 486 ± 12 cd | 474 ± 1 b * | 106 ± 7 d * | 392 ± 7 f |
| AC_7 | 334 ± 4 e | 128 ± 8 efg | 512 ± 9 bc | 301 ± 14 f | 151 ± 7 a * | 527 ± 16 abc |
| AC_8 | 420 ± 2 c | 177 ± 9 b | 369 ± 10 f | 394 ± 8 de | 124 ± 6 bc * | 447 ± 11 e |
| AC_9 | 398 ± 2 cd | 178 ± 8 b | 406 ± 8 ef | 436 ± 3 c * | 68 ± 5 e * | 460 ± 6 de |
| AC_10 | 382 ± 7 d | 156 ± 9 bcd | 425 ± 9 def | - a | ||
| AC_11 | 341 ± 2 e | 135 ± 4 def | 494 ± 5 c | 534 ± 5 a * | 77 ± 3 e * | 348 ± 6 f |
| AC_12 | 273 ± 22 g | 230 ± 15 a | 472 ± 27 cde | 405 ± 2 d | 63 ± 4 e * | 501 ± 4 bcd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.; Dell’Orto, M.; Scaglia, B.; D’Imporzano, G.; Adani, F. Growth Performance and Biochemical Composition of Waste-Isolated Microalgae Consortia Grown on Nano-Filtered Pig Slurry and Cheese Whey under Mixotrophic Conditions. Fermentation 2022, 8, 474. https://doi.org/10.3390/fermentation8100474
Su M, Dell’Orto M, Scaglia B, D’Imporzano G, Adani F. Growth Performance and Biochemical Composition of Waste-Isolated Microalgae Consortia Grown on Nano-Filtered Pig Slurry and Cheese Whey under Mixotrophic Conditions. Fermentation. 2022; 8(10):474. https://doi.org/10.3390/fermentation8100474
Chicago/Turabian StyleSu, Min, Marta Dell’Orto, Barbara Scaglia, Giuliana D’Imporzano, and Fabrizio Adani. 2022. "Growth Performance and Biochemical Composition of Waste-Isolated Microalgae Consortia Grown on Nano-Filtered Pig Slurry and Cheese Whey under Mixotrophic Conditions" Fermentation 8, no. 10: 474. https://doi.org/10.3390/fermentation8100474
APA StyleSu, M., Dell’Orto, M., Scaglia, B., D’Imporzano, G., & Adani, F. (2022). Growth Performance and Biochemical Composition of Waste-Isolated Microalgae Consortia Grown on Nano-Filtered Pig Slurry and Cheese Whey under Mixotrophic Conditions. Fermentation, 8(10), 474. https://doi.org/10.3390/fermentation8100474









