Influence of Oat β-Glucan on the Survival and Proteolytic Activity of Lactobacillus rhamnosus GG in Milk Fermentation: Optimization by Response Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Obtaining and Preparation
2.2. Oats Grinding
2.3. Physicochemical Composition
2.4. β-Glucan Extraction and Quantification
2.5. Fermentation and Probiotic Growth
2.6. Free Amino Acid Groups Concentration Response (Proteolytic Activity of L. rhamnosus GG)
2.7. Optimization of Fermentation
2.7.1. Experimental Design
2.7.2. Experimental Design Validation
3. Results
3.1. Proximate Compositional Analysis and Concentration of β-Glucan in Oat Milling Fractions
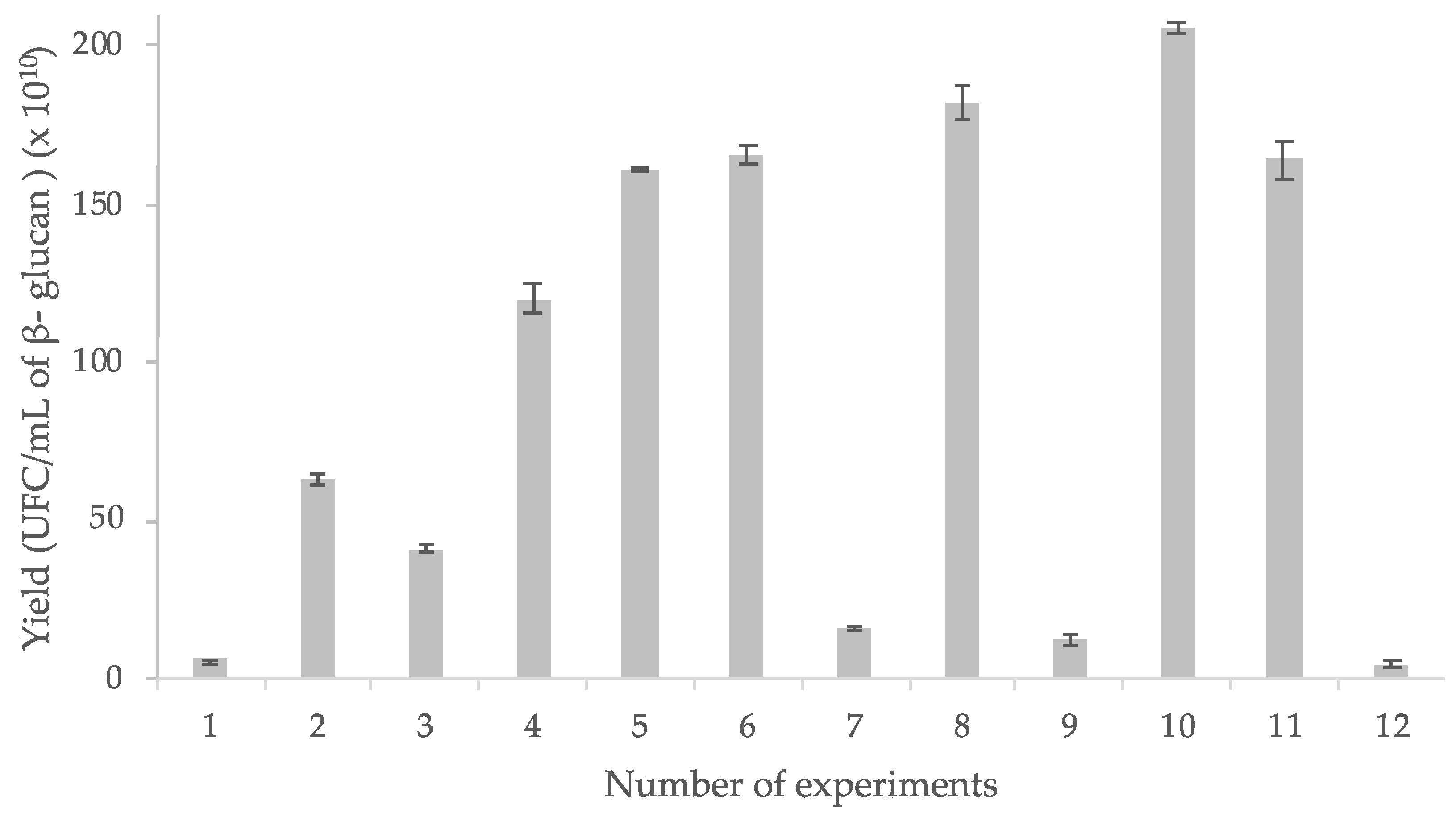
3.2. β-Glucan Extraction
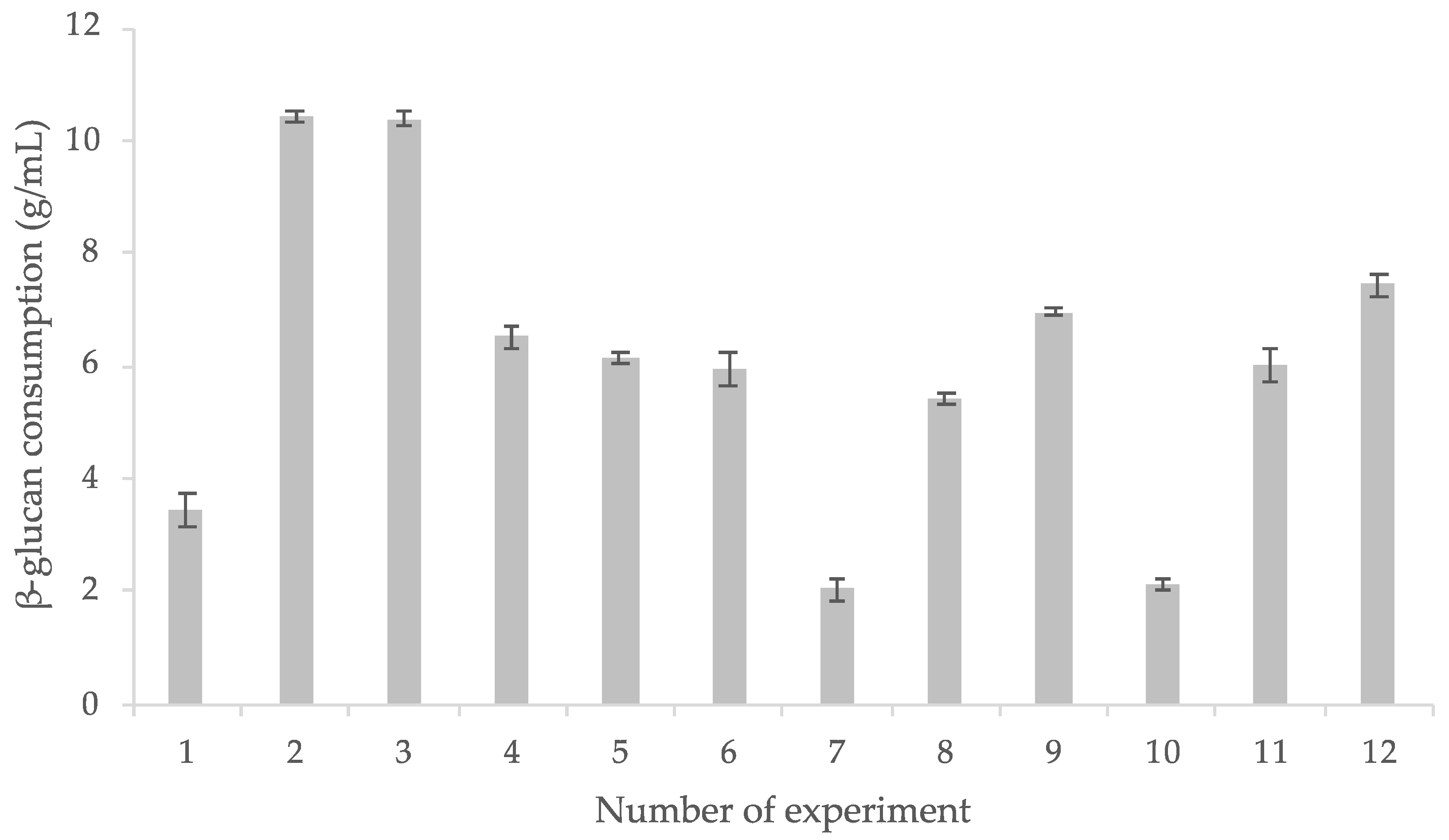
3.3. β-Glucan Intake and L. rhamnosus GG Survival
3.4. Analysis of the Proteolytic Capacity of L. rhamnosus GG
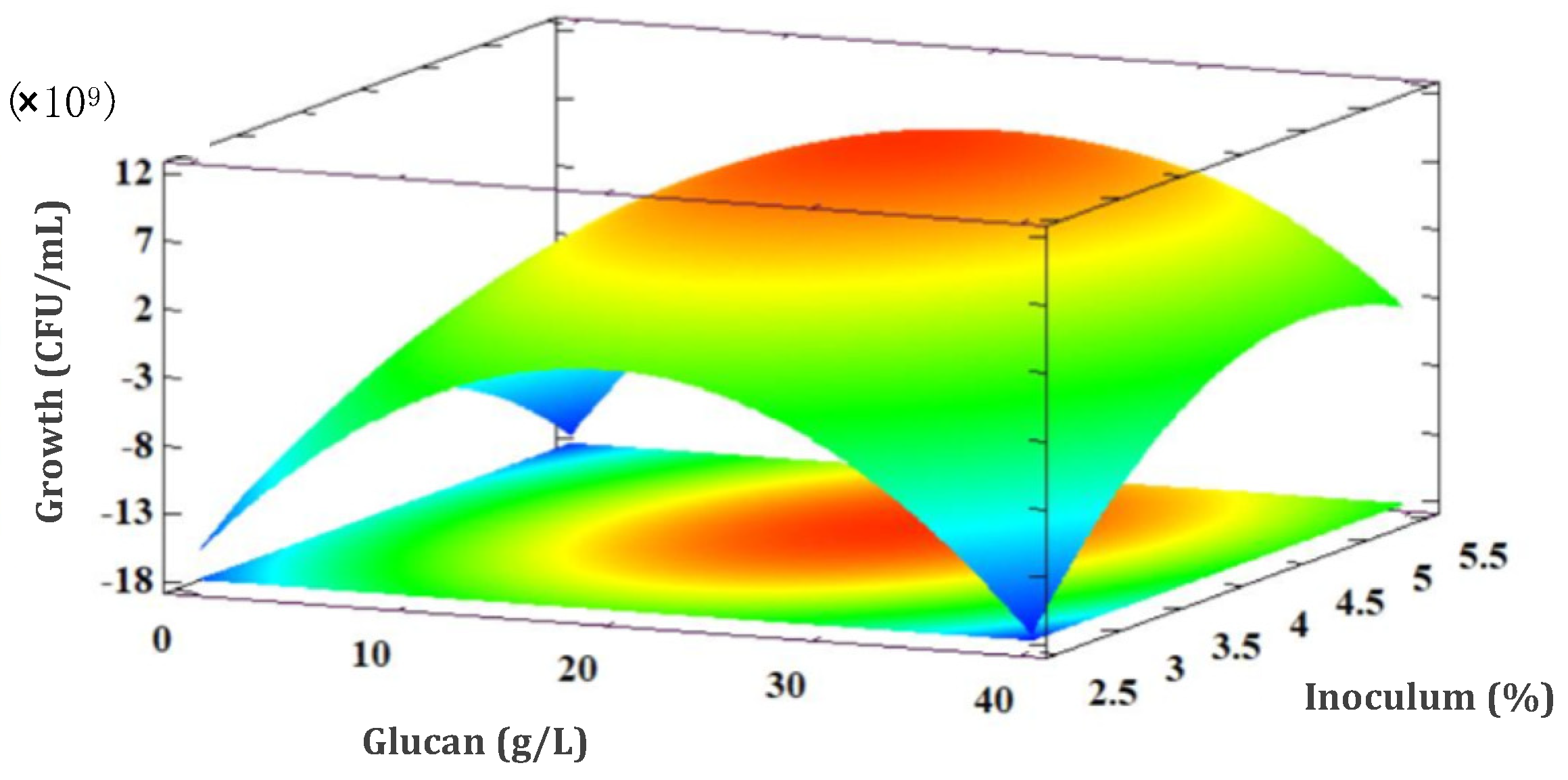
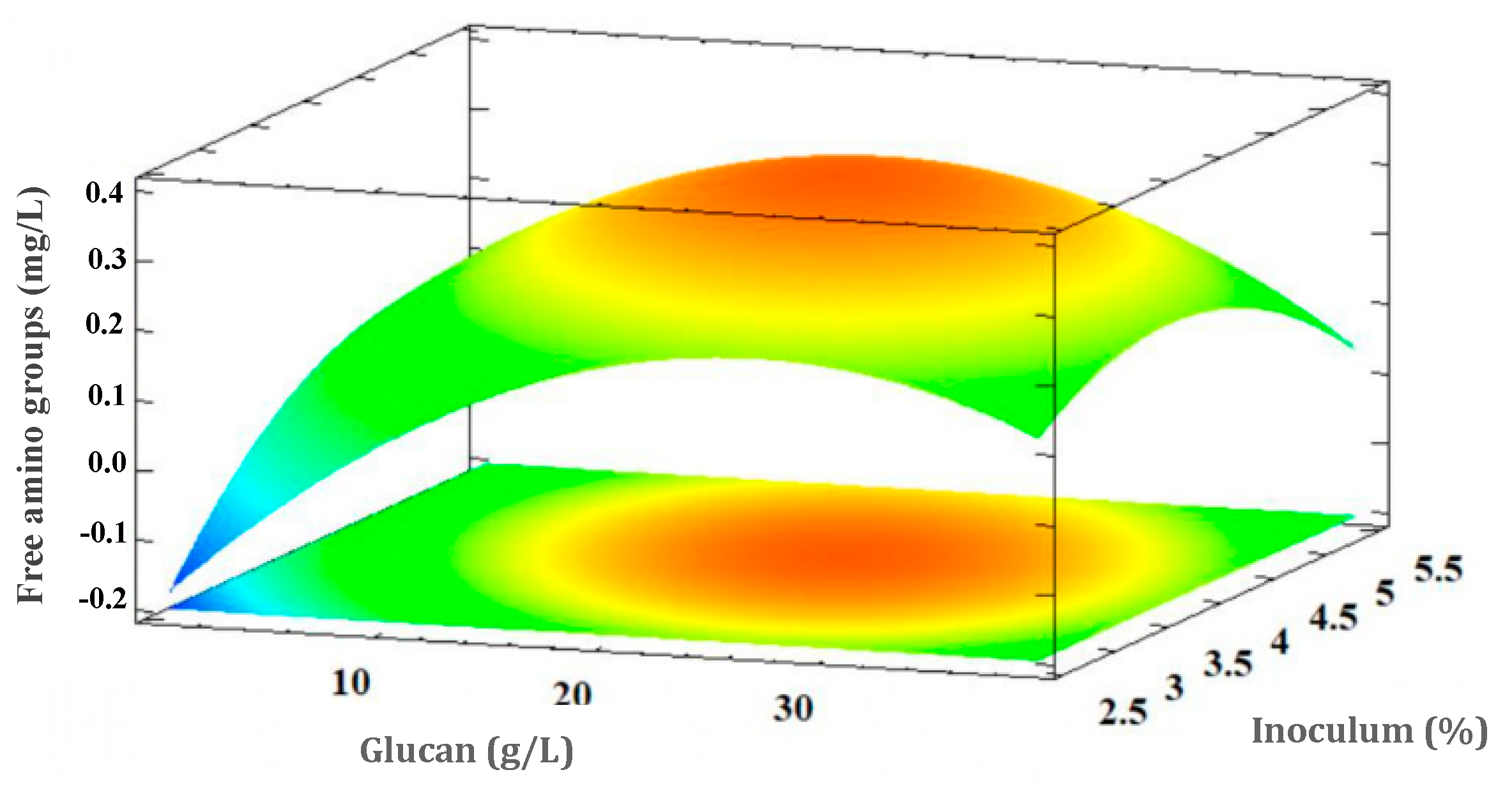
3.5. Experimental Design Results
3.5.1. L. rhamnosus GG Growth Response
3.5.2. Free Amino Acid Groups Concentration Response (Proteolytic Activity of L. rhamnosus GG)
3.5.3. Polynomial Model Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havrlentova, M.; Petrulakova, Z.; Burgarova, A.; Gago, F.; Hlinkova, A.; Šturdík, E. β-glucans and their significance for the preparation of functional foods—A review. Czech. J. Food Sci. 2011, 29, 1–14. [Google Scholar] [CrossRef]
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal B-Glucans: The Impact of Processing and How It Affects Physiological Responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Olveira, G.; Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Valcheva, R.; Dieleman, L.A. Prebiotics: Definition and protective mechanisms. Best. Pract. Res. Clin. Gastroenterol. 2016, 30, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Roberfroid, M.; Gibson, G.R.; Hoyles, L.; McCartney, A.L.; Rastall, R.; Rowland, I.; Wolvers, D.; Watzl, B.; Szajewska, H.; Stahl, B.; et al. Prebiotic effects: Metabolic and health benefits. Br. J. Nutr. 2010, 104, S1–S63. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheraji, S.H.; Ismail, A.; Manap, M.Y.; Mustafa, S.; Yusof, R.M.; Hassan, F.A. Prebiotics as functional foods: A review. J. Funct. Foods 2013, 5, 1542–1553. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Xu, B. A critical review on production and industrial applications of beta-glucans. Food Hydrocoll. 2016, 52, 275–288. [Google Scholar] [CrossRef]
- Dong, J.L.; Yu, X.; Dong, L.E.; Shen, R.L. In vitro fermentation of oat β-glucan and hydrolysates by fecal microbiota and selected probiotic strains. J. Sci. Food Agric. 2017, 97, 4198–4203. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Chung, S.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Bozbulut, R.; Sanlier, N. Promising effects of β-glucans on glyceamic control in diabetes. Trends Food Sci. Technol. 2019, 83, 159–166. [Google Scholar] [CrossRef]
- Sima, P.; Vannucci, L.; Vetvicka, V. β-glucans and cholesterol (Review). Int. J. Mol. Med. 2018, 41, 1799–1808. [Google Scholar] [CrossRef]
- Korolenko, T.A.; Bgatova, N.P.; Ovsyukova, M.V.; Shintyapina, A.; Vetvicka, V. Hypolipidemic Effects of β-Glucans, Mannans, and Fucoidans: Mechanism of Action and Their Prospects for Clinical Application. Molecules 2020, 25, 1819. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Rosales, L.-B.; Cruz-Guerrero, A.-E.; Ramírez-Moreno, E.; Quintero-Lira, A.; Contreras-López, E.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Añorve-Morga, J.; Calderón-Ramos, Z.-G.; Arias-Rico, J.; et al. Impact of the Gut Microbiota Balance on the Health–Disease Relationship: The Importance of Consuming Probiotics and Prebiotics. Foods 2021, 10, 1261. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Caggianiello, G.; Fiocco, D.; Russo, P.; Torelli, M.; Spano, G.; Capozzi, V. Barley β-glucans-containing food enhances probiotic performances of beneficial bacteria. Int. J. Mol. Sci. 2014, 15, 3025–3039. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; de Chiara, M.L.V.; Capozzi, V.; Arena, M.P.; Amodio, M.L.; Rascón, A.; Dueñas, M.T.; López, P.; Spano, G. Lactobacillus plantarum strains for multifunctional oat-based foods. LWT Food Sci. Technol. 2016, 68, 288–294. [Google Scholar] [CrossRef]
- Sharma, P.; Trivedi, N.; Gat, Y. Development of functional fermented whey–oat-based product using probiotic bacteria. 3 Biotech 2017, 7, 272. [Google Scholar] [CrossRef]
- Jaimez-Ordaz, J.; Martinez-Ramirez, X.; Cruz-Guerrero, A.E.; Contreras-Lopez, E.; Ayala-Nino, A.; Castro-Rosas, J.; González-Olivares, L.G. Survival and proteolytic capacity of probiotics in a fermented milk enriched with agave juice and stored in refrigeration. Food Sci. Technol. 2019, 39, 188–194. [Google Scholar] [CrossRef]
- Rolim, F.R.; Neto, O.C.F.; Oliveira, M.E.G.; Oliveira, C.J.; Queiroga, R.C. Cheeses as food matrixes for probiotics: In vitro and in vivo tests. Trends Food Sci. Technol. 2020, 100, 138–154. [Google Scholar] [CrossRef]
- Rojas-Ronquillo, R.; Cruz-Guerrero, A.; Flores-Nájera, A.; Rodríguez-Serrano, G.; Gómez-Ruiz, L.; Reyes-Grajeda, J.P.; Jiménez-Guzmán, J.; García-Garibay, M. Antithrombotic and angiotensin-converting enzyme inhibitory properties of peptides released from bovine casein by Lactobacillus casei Shirota. Int. Dairy J. 2012, 26, 147–154. [Google Scholar] [CrossRef]
- de Lourdes, E.; de Lima, C.; Roberta, H. Optimized fermentation of goat cheese whey with Lactococcus lactis for production of antilisterial bacteriocin-like substances. LWT Food Sci. Technol. 2017, 84, 710–716. [Google Scholar] [CrossRef]
- González-Olivares, L.G.; López-Cuellar, Z.L.; Añorve-Morga, J.; Franco-Fernández, M.J.; Castañeda-Ovando, A.; Contreras-López, E.; Jaimez-Ordaz, J.; Rodríguez-Serrano, G.M. Viability and proteolytic capacity of Lactobacillus bulgaricus 2772 and Lactobacillus rhamnosus GG during cheese ripening. J. Biosci. Med. 2014, 2014, 7–12. [Google Scholar]
- Aguilar-Toalá, J.E.; Santiago-López, L.; Peres, C.M.; Peres, C.; Garcia, H.S.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Assessment of multifunctional activity of bioactive peptides derived from fermented milk by specific Lactobacillus plantarum strains. J. Dairy Sci. 2017, 100, 65–75. [Google Scholar] [CrossRef]
- Pérez-Escalante, E.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Contreras-López, E.; Añorve-Morga, J.; González-Olivares, L.G. Antithrombotic activity of milk protein hydrolysates by lactic acid bacteria isolated from commercial fermented milks. Braz. Archiv. Biol. Technol. 2018, 61, e18180132. [Google Scholar] [CrossRef]
- Fuentes, F.; Poblete, C.; Huerta, M.; Palape, I. Evaluación de la producción y calidad nutritiva de avena como forraje verde hidropónico en condiciones de desierto. Idesia 2011, 29, 75–81. [Google Scholar] [CrossRef][Green Version]
- Pontieri, P.; Pepe, G.; Campiglia, P.; Meriai, F.; Basilicata, M.G.; Smolensky, D.; Calcagnile, M.; Troisi, J.; Romano, R.; Del Giudice, F.; et al. Comparision of content in phenolic compounds and antioxidant capacity in grains of white, red and black sorghum varieties grown in the Mediterranean area. ACS Food Sci. Technol. 2021, 1, 1109–1119. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MA, USA, 2005. [Google Scholar]
- Benito-Román, O.; Alonso, E.; Lucas, S. Optimization of the β-glucan extraction conditions from different waxy barley cultivars. J. Cereal Sci. 2011, 53, 271–276. [Google Scholar] [CrossRef]
- Beer, M.U.; Arrigoni, E.; Amado, R. Extraction of oat gum from oat bran: Effects of process on yield, molecular weight distribution, viscosity and (1→3)(1→4)-b-D-glucan content of the gum. Cereal Chem. 1996, 73, 58–62. [Google Scholar]
- Cruz-Guerrero, A.; Hernández-Sánchez, H.; Rodríguez-Serrano, G.; Gómez- Ruiz, L.; García-Garibay, M.; Figueroa-González, I. Commercial probiotic bacteria and prebiotic carbohydrates: A fundamental study on prebiotics uptake, antimicrobials production and inhibition of pathogens. J. Sci. Food Agric. 2014, 94, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Herigstad, B.; Hamilton, M.; Heersink, J. How to optimize the drop plate method for enumerating bacteria. J. Microbiol. Methods 2001, 44, 121–129. [Google Scholar] [CrossRef]
- Alder-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzensulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Ren, Y.; Li, Y.; Fan, M.; Qian, H.; Wang, L.; Wu, G.; Zhang, H.; Qi, X.; Xu, M.; et al. Physiological functionalities and mechanisms of β-glucans. Trends Food Sci. Technol. 2019, 88, 57–66. [Google Scholar] [CrossRef]
- Wood, P.J. Cereal β-glucans in diet and health. J. Cereal Sci. 2007, 46, 230–238. [Google Scholar] [CrossRef]
- Hernandez-Campuzano, A.V.; Martínez-Rueda, C.G.; Estrada-Campuzano, G.; Dominguez-López, A. Effect of genotype and nitrogen fertilization on the yield, nitrogen content and β-glucans in the grain of oats (Avena sativa L.). Rev. Investig. Agropecu. 2018, 44, 88–95. [Google Scholar]
- Yoo, H.U.; Ko, M.J.; Chung, M.S. Hydrolysis of beta-glucan in oat flour during subcritical-water extraction. Food Chem. 2020, 308, 125670. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, N.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Optimisation of yield and molecular weight of β-glucan from barley flour using response surface methodology. J. Cereal Sci. 2015, 62, 38–44. [Google Scholar] [CrossRef]
- Arora, T.; Sharma, R.K. Prebiotic effectiveness of galactooligosaccharides and β-glucan in stimulation of growth of Lactobacillus acidophilus NCDC 13 in vitro. Curr. Top. Nutr. Res. 2011, 9, 67. [Google Scholar]
- Figueroa-Gonzalez, I.; Rodríguez-Serrano, G.; Gomez-Ruiz, L.; Garcia-Garibay, M.; Cruz-Guerrero, A. Prebiotic effect of commercial saccharides on probiotic bacteria isolated from commercial products. Food Sci. Technol. 2019, 39, 747–753. [Google Scholar] [CrossRef]
- Kocková, M.; Mendel, J.; Medveďová, A.; Šturdík, E.; Valík, Ľ. Cereals and pseudocereals as substrates for growth and metabolism of a probiotic strain Lactobacillus rhamnosus GG. J. Food Nutr. Res. 2013, 52, 25–36. [Google Scholar]
- Oh, N.S.; Lee, J.Y.; Kim, Y. The growth kinetics and metabolic and antioxidant activities of the functional synbiotic combination of Lactobacillus gasseri 505 and Cudrania tricuspidata leaf extract. Appl. Microbiol. Biotechnol. 2016, 100, 10095–10106. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Álvarez, M.M.; Aguirre-Ezkauriatza, E.J.; Ramírez-Medrano, A.; Rodríguez-Sánchez, Á. Kinetic analysis and mathematical modeling of growth and lactic acid production of Lactobacillus casei var. rhamnosus in milk whey. J. Dairy Sci. 2010, 93, 5552–5560. [Google Scholar] [CrossRef]
- Begunova, A.V.; Savinova, O.S.; Glazunova, O.A.; Moiseenko, K.V.; Rozhkova, I.V.; Fedorova, T.V. Development of antioxidant and antihypertensive properties during growth of Lactobacillus helveticus, Lactobacillus rhamnosus and Lactobacillus reuteri on Cow’s Milk: Fermentation and peptidomics study. Foods 2021, 10, 17. [Google Scholar] [CrossRef]
- Habibi Najafi, M.B.; Fatemizadeh, S.S.; Tavakoli, M. Effect of fat percentage and prebiotic composition on proteolysis, ACE-inhibitory and antioxidant activity of probiotic yogurt. Int. J. Nutr. Food Eng. 2017, 11, 622–628. [Google Scholar] [CrossRef]
- Buntin, N.; Hongpattarakere, T.; Ritari, J.; Douillard, F.P.; Paulin, L.; Boeren, S.; Shetty, A.; de Vos, W.M. An inducible operon is involved in inulin utilization in Lactobacillus plantarum strains, as revealed by comparative proteogenomics and metabolic profiling. Appl. Environ. Microbiol. 2017, 83, e02402-16. [Google Scholar] [CrossRef]
- Cui, Y.; Qu, X. Genetic mechanisms of prebiotic carbohydrate metabolism in lactic acid bacteria: Emphasis on Lacticaseibacillus casei and Lacticaseibacillus paracasei as flexible, diverse and outstanding prebiotic carbohydrate starters. Trends Food Sci. Technol 2021, 115, 486–499. [Google Scholar] [CrossRef]
- Ramírez-Godínez, J.; Gutiérrez-Rodríguez, J.F.; Contreras-López, E.; Rodríguez-Serrano, G.M.; Castañeda-Ovando, A.; Jaimez-Ordaz, J.; González-Olivares, L.G. Agave Juice improves survival and proteolytic activity of Lactobacillus rhamnosus GG during ripening of semi-ripened Mexican cheese. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Habibi-Najafi, M.B.; Sadat-Fatemizadeh, S.; Tavakoli, M. Release of proteolysis products with ACE-inhibitory and antioxidant activities in probiotic yogurt containing different levels of fat and prebiotics. Int. J. Pept. Res. Ther. 2019, 25, 367–377. [Google Scholar] [CrossRef]
- Pinto, G.; Picariello, G.; Addeo, F.; Chianese, L.; Scaloni, A.; Caira, S. Proteolysis and process-induced modifications in symbiotic yogurt investigated by peptidomics and phosphopeptidomics. J. Agric. Food Chem. 2020, 68, 8744–8754. [Google Scholar] [CrossRef] [PubMed]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic system of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.; Lamarque, M.; Bissardon, I.; Atlan, D.; Galinier, A. Autoregulation of the biosynthesis of the CcpA-like protein, PepR1 in Lactobacillus delbrueckii subsp bulgaricus. J. Mol. Microbiol. Biotechnol. 2001, 3, 63–66. [Google Scholar]
- Rodríguez-Serrano, G.M.; Garcia-Garibay, J.M.; Cruz-Guerrero, A.E.; Gomez-Ruiz, L.D.C.; Ayala-Nino, A.; Castañeda-Ovando, A.; González-Olivares, L.G. Proteolytic System of Streptococcus thermophilus. J. Microbiol. Biotechnol. 2018, 28, 1581–1588. [Google Scholar] [CrossRef] [PubMed]


| Experimental Factor | Level | ||||
|---|---|---|---|---|---|
| −α | −1 | 0 | 1 | +α | |
| x1 | 5.85 | 10 | 20 | 30 | 34.41 |
| x2 | 2.58 | 3 | 4 | 5 | 5.41 |
| Number of experiment | Factor | ||||
| x1 | x2 | ||||
| 1 | −1 | −1 | |||
| 2 | 1 | 1 | |||
| 3 | +α | 0 | |||
| 4 | 0 | +α | |||
| 5 | 0 | 0 | |||
| 6 | 0 | 0 | |||
| 7 | −α | 0 | |||
| 8 | 0 | 0 | |||
| 9 | 1 | −1 | |||
| 10 | −1 | 1 | |||
| 11 | 0 | 0 | |||
| 12 | 0 | −α | |||
| Parameters (g/100 g of Sample) | Husk | Semolina | Flour |
|---|---|---|---|
| Moisture | 20.02 ±0.62 | 21.02 ± 0.14 | 19.05 ± 0.18 |
| Ash | 5.24 ± 0.37 | 2.12 ± 0.16 | 1.16 ± 0.01 |
| Fiber | 5.24 ± 0.37 | 4.21 ± 0.07 | 0.29 ± 0.03 |
| Protein | 4.51 ± 1.35 | 11.73 ± 0.11 | 8.94 ± 0.42 |
| Fat | 3.23 ± 0.15 | 6.58 ± 0.40 | 6.90 ± 0.11 |
| Carbohydrate | 25.70 ± 1.75 | 54.35 ± 0.23 | 63.67 ± 0.58 |
| β-glucan | 3.35 ± 0.05 | 1.24 ± 0.06 | 0.12 ± 0.08 |
| Variable | Predicted | Experimental | P (α = 0.05) |
|---|---|---|---|
| Free amino groups (mg/L) | 0.33 | 0.33 ± 0.02 | 0.098 |
| Growth (UFC/mL) × 109 | 10.44 | 9.87 ± 0.07 | 0.059 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chávez de la Vega, M.I.; Alatorre-Santamaría, S.; Gómez-Ruiz, L.; García-Garibay, M.; Guzmán-Rodríguez, F.; González-Olivares, L.G.; Cruz-Guerrero, A.E.; Rodríguez-Serrano, G.M. Influence of Oat β-Glucan on the Survival and Proteolytic Activity of Lactobacillus rhamnosus GG in Milk Fermentation: Optimization by Response Surface. Fermentation 2021, 7, 210. https://doi.org/10.3390/fermentation7040210
Chávez de la Vega MI, Alatorre-Santamaría S, Gómez-Ruiz L, García-Garibay M, Guzmán-Rodríguez F, González-Olivares LG, Cruz-Guerrero AE, Rodríguez-Serrano GM. Influence of Oat β-Glucan on the Survival and Proteolytic Activity of Lactobacillus rhamnosus GG in Milk Fermentation: Optimization by Response Surface. Fermentation. 2021; 7(4):210. https://doi.org/10.3390/fermentation7040210
Chicago/Turabian StyleChávez de la Vega, María Isabel, Sergio Alatorre-Santamaría, Lorena Gómez-Ruiz, Mariano García-Garibay, Francisco Guzmán-Rodríguez, Luis Guillermo González-Olivares, Alma Elizabeth Cruz-Guerrero, and Gabriela Mariana Rodríguez-Serrano. 2021. "Influence of Oat β-Glucan on the Survival and Proteolytic Activity of Lactobacillus rhamnosus GG in Milk Fermentation: Optimization by Response Surface" Fermentation 7, no. 4: 210. https://doi.org/10.3390/fermentation7040210
APA StyleChávez de la Vega, M. I., Alatorre-Santamaría, S., Gómez-Ruiz, L., García-Garibay, M., Guzmán-Rodríguez, F., González-Olivares, L. G., Cruz-Guerrero, A. E., & Rodríguez-Serrano, G. M. (2021). Influence of Oat β-Glucan on the Survival and Proteolytic Activity of Lactobacillus rhamnosus GG in Milk Fermentation: Optimization by Response Surface. Fermentation, 7(4), 210. https://doi.org/10.3390/fermentation7040210







