Immunomodulatory Effect of Sasa quelpaertensis Leaves Fermentation Products in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Sasa quelpaertensis Leaves Fermentation Products
2.2. β-glucan Contents of Three Mushroom Fermented Sasa quelpaertensis Leaves Extracts
2.3. Animal and Experiment
2.4. Isolation of PBMCs, Splenocytes, and Peritoneal Macrophage
2.5. Analysis of T Cells and Macrophage by Flow Cytometry
2.6. Cytokines Production in Splenocytes
2.7. Nitric Oxide Production in Peritoneal Macrophage
2.8. Statistical Analysis
3. Results
3.1. Extraction of Sasa quelpaertensis Leaves Fermentation Products
3.2. Glucan Content of the Extracts of Sasa quelpaertensis and Mushroom Fermented Sasa quelpaertensis Extracts
3.3. T Cell Population in PBMCs
3.4. Macrophage Population in Peritoneal Cavity
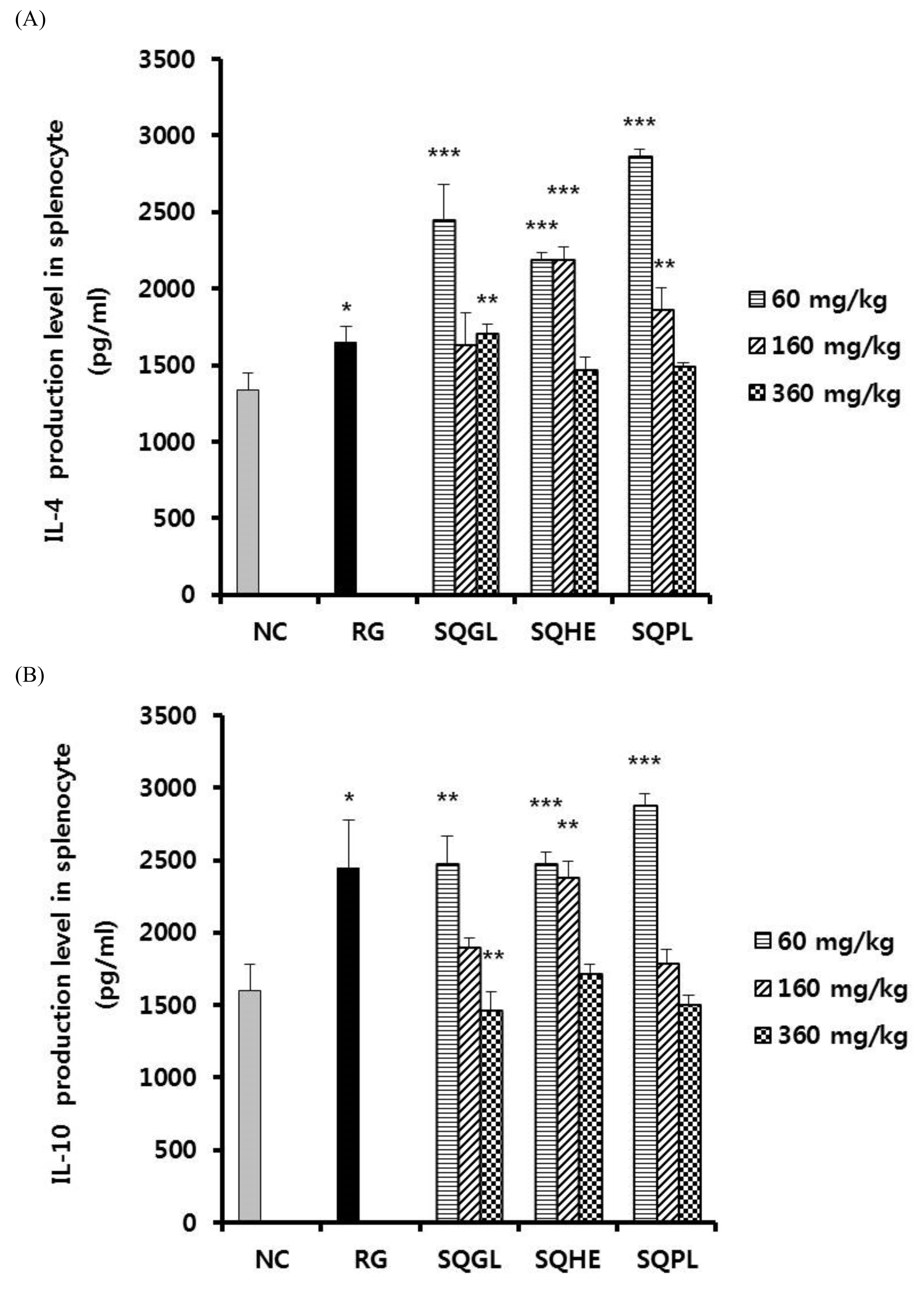
3.5. Effects of Sasa quelpaertensis Leaves Fermentation Products on Cytokine Production
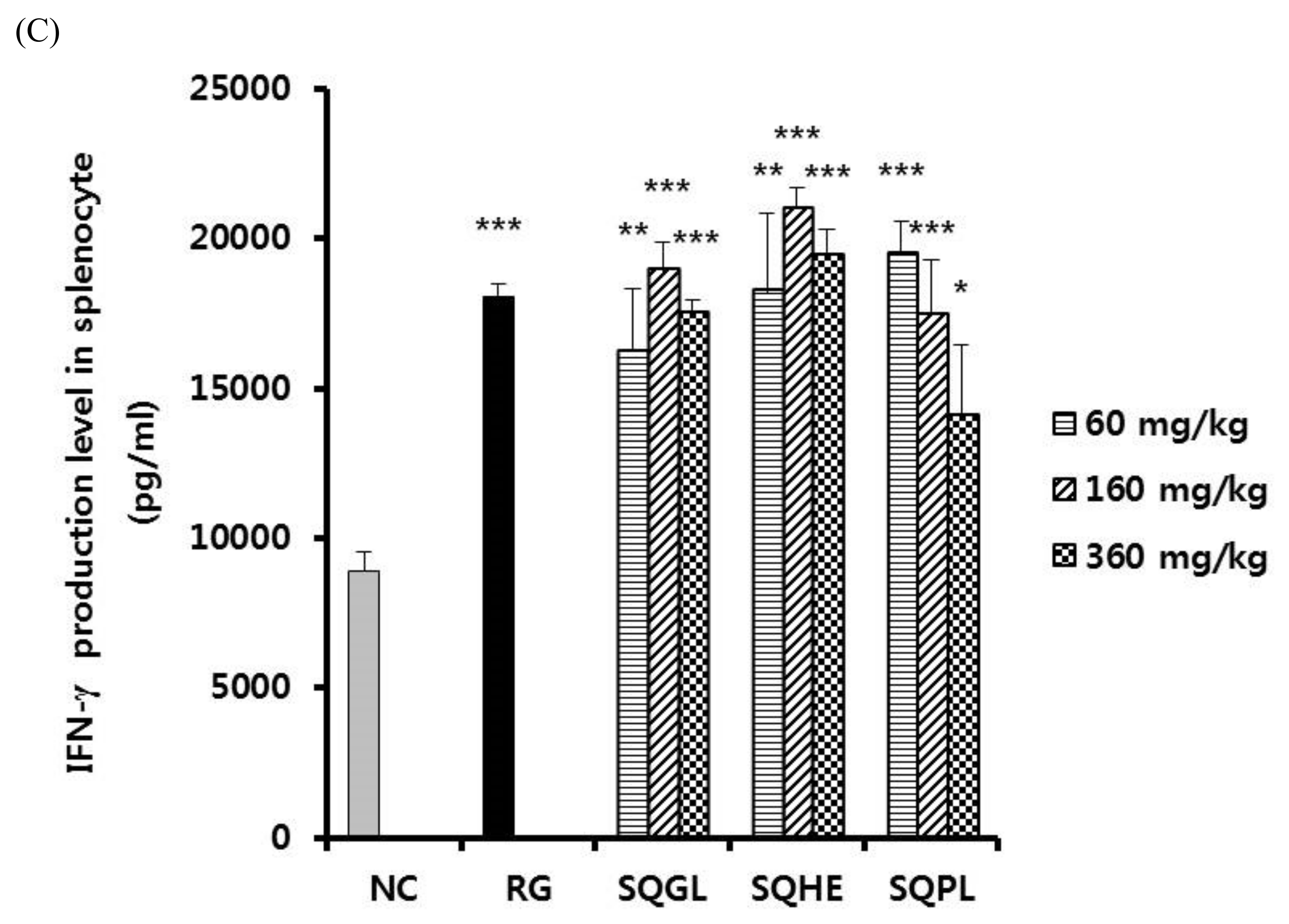
3.6. Effects of Sasa quelpaertensis Leaves Fermentation Products on Nitric Oxide Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kindt, T.; Osborne, B.A.; Goldsby, R.A.; Kuby, J. Kuby Immunology; W.H. Freeman: New York, NY, USA, 2007. [Google Scholar]
- Biron, C.A.; Byron, K.S.; Sullivan, J.L. Severe Herpesvirus Infections in an Adolescent without Natural Killer Cells. N. Engl. J. Med. 1989, 320, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Daha, M.R. Grand Challenges in Molecular Innate Immunity. Front. Immunol. 2011, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Okabe, S.; Takeuchi, K.; Takagi, K.; Shibata, M. Stimulatry Effect of the Water Extract of Bamboo Grass (Fol1n Solution) on Gastric Acid Secretion in Pylorus-Ligated Rats. Jpn. J. Pharmacol. 1975, 25, 608–609. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.-H.; Lim, H.-S.; Heo, Y.-R. Sasa borealis leaves extract improves insulin resistance by modulating inflammatory cytokine secretion in high fat diet-induced obese C57/BL6J mice. Nutr. Res. Pract. 2010, 4, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Reilly, R.T.; Sacchi, N. Sasa health exerts a protective effect on Her2/NeuN mammary tumorigenesis. Anticancer. Res. 2004, 24, 2879–2884. [Google Scholar]
- Kang, S.-I.; Shin, H.-S.; Kim, H.-M.; Hong, Y.-S.; Yoon, S.-A.; Kang, S.-W.; Kim, J.-H.; Ko, H.-C.; Kim, S.-J. Anti-Obesity Properties of a Sasa quelpaertensis Extract in High-Fat Diet-Induced Obese Mice. Biosci. Biotechnol. Biochem. 2012, 76, 755–761. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Tanaka, A.; Hosoda, A.; Takano, F.; Ohta, T. Antioxidant C-glycosyl flavones from the leaves of Sasa kurilensis var. gigantea. Phytochemistry 2008, 69, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, X.; Zhang, Y.; Wang, J.; Sun, H.; Wang, J.; Cao, X.; Ye, Y. Isolation and prebiotic activity of water-soluble polysaccharides fractions from the bamboo shoots (Phyllostachys praecox). Carbohydr. Polym. 2016, 151, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-J.; Ran, C.-X.; Li, C.-F.; Xiong, Z.-W.; Ma, L.-Z. Comparisons of prebiotic activity of polysaccharides from shoot residues of bamboo (Chimonobambusa quadrangularis) via different ethanol concentrations. J. Food Biochem. 2020, 44, e13171. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and Anti-inflammatory Effect of p-Coumaric Acid, a Common Dietary Polyphenol on Experimental Inflammation in Rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Kwon, H.-Y.; Choi, S.-I.; Han, X.; Men, X.; Jang, G.-W.; Choi, Y.-E.; Kim, S.-H.; Kang, J.-C.; Cho, J.-H.; Lee, O.-H. Enhancement of Immune Activities of Mixtures with Sasa quelpaertensis Nakai and Ficus erecta var. sieboldii. Foods 2020, 9, 868. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.S.; Kao, C.H.; Xu, Y.; Glucina, M.P.; Paterson, R.; Ferguson, L.R. From 2000years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry 2015, 114, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Hapuarachchi, K.K.; Wen, T.C.; Deng, C.Y.; Kang, J.C.; Hyde, K.D. Mycosphere Essays 1: Taxonomic Confusion in the Ganoderma lucidum Species Complex. Mycosphere 2015, 6, 542–559. [Google Scholar] [CrossRef]
- Wang, C.; Shi, S.; Chen, Q.; Lin, S.; Wang, R.; Wang, S.; Chen, C. Antitumor and Immunomodulatory Activities of Ganoderma lucidum Polysaccharides in Glioma-Bearing Rats. Integr. Cancer Ther. 2018, 17, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.; Xu, X.; Liu, S.; Huang, L.; Gu, J. Ganoderma: A Cancer Immunotherapy Review. Front. Pharmacol. 2018, 9, 1217. [Google Scholar] [CrossRef] [Green Version]
- Wasser, S.P.; Weis, A.L. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: A modern perspective. Crit. Rev. Immunol. 1999, 19, 65–96. [Google Scholar] [CrossRef] [PubMed]
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochemistry 2015, 114, 66–101. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Yuen, J.; Buswell, J.A.; Benzie, I.F. Ganoderma lucidum (Lingzhi or Reishi). In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; CRC Press/Taylor & Francis: Cleveland, OH, USA, 2011; pp. 53–197. [Google Scholar]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Bicker, W.; Wu, J.; Xie, M.; Lindner, W. Simultaneous Determination of 16 Nucleosides and Nucleobases by Hydrophilic Interaction Chromatography and Its Application to the Quality Evaluation of Ganoderma. J. Agric. Food Chem. 2012, 60, 4243–4252. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Min, K.M.; Cho, J.Y.; Hong, E.K. Study of Macrophage Activation and Structural Characteristics of Purified Polysaccharides from the Fruiting Body of Hericium erinaceus. J. Microbiol. Biotechnol. 2009, 19, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.P.; Kang, M.Y.; Choi, Y.H.; Kim, J.H.; Nam, S.H.; Friedman, M. Mechanism of Hericium erinaceus (Yamabushitake) mushroom-induced apoptosis of U937 human monocytic leukemia cells. Food Funct. 2011, 2, 348–356. [Google Scholar] [CrossRef]
- Mizuno, T.; Wasa, T.; Ito, H.; Suzuki, C.; Ukai, N. Antitumor-active Polysaccharides Isolated from the Fruiting Body of Hericium erinaceum, an Edible and Medicinal Mushroom Called yamabushitake or houtou. Biosci. Biotechnol. Biochem. 1992, 56, 347–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, N.; Lee, N. New Phenylpropanoids from Sasa quelpaertensis Nakai with Tyrosinase Inhibition Activities. Bull. Korean Chem. Soc. 2009, 30, 1729–1732. [Google Scholar] [CrossRef] [Green Version]
- Dhur, A.; Galan, P.; Preziosi, P.; Hercberg, S. Lymphocyte Subpopulations in the Thymus, Lymph Nodes and Spleen of Iron-Deficient and Rehabilitated Mice. J. Nutr. 1991, 121, 1418–1424. [Google Scholar] [CrossRef]
- Kang, N.S.; Park, S.Y.; Lee, K.R.; Lee, S.M.; Lee, B.G.; Shin, D.H.; Pyo, S. Modulation of macrophage function activity by ethanolic extract of larvae of Holotrichia diomphalia. J. Ethnopharmacol. 2002, 79, 89–94. [Google Scholar] [CrossRef]
- Malik, F.; Singh, J.; Khajuria, A.; Suri, K.A.; Satti, N.K.; Singh, S.; Kaul, M.K.; Kumar, A.; Bhatia, A.; Qazi, G.N. A standardized root extract of Withania somnifera and its major constituent withanolide-A elicit humoral and cell-mediated immune responses by up regulation of Th1-dominant polarization in BALB/c mice. Life Sci. 2007, 80, 1525–1538. [Google Scholar] [CrossRef]
- Bogdan, C. Nitric oxide and the immune response. Nat. Immunol. 2001, 2, 907–916. [Google Scholar] [CrossRef]
- Avni, S.; Ezove, N.; Hanani, H.; Yadid, I.; Karpovsky, M.; Hayby, H.; Gover, O.; Hadar, Y.; Schwartz, B.; Danay, O. Olive Mill Waste Enhances α-Glucan Content in the Edible Mushroom Pleurotus eryngii. Int. J. Mol. Sci. 2017, 18, 1564. [Google Scholar] [CrossRef]
- Park, H.; Ka, K.-H.; Ryu, S.-R. Enhancement of β-Glucan Content in the Cultivation of Cauliflower Mushroom (Sparassis latifolia) by Elicitation. Mycobiology 2014, 42, 41–45. [Google Scholar] [CrossRef] [Green Version]
- Kerékgyártó, C.; Virág, L.; Tankó, L.; Chihara, G.; Fachet, J. Strain differences in the cytotoxic activity and TNF production of murine macrophages stimulated by lentinan. Int. J. Immunopharmacol. 1996, 18, 347–353. [Google Scholar] [CrossRef]
- Chan, W.K.; Law, H.K.-W.; Lin, Z.-B.; Lau, Y.L.; Chan, G.C.F. Response of human dendritic cells to different immunomodulatory polysaccharides derived from mushroom and barley. Int. Immunol. 2007, 19, 891–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Dong, S.; Liu, C.; Wang, W.; Sun, S.; Gu, J.; Wang, Y.; Boraschi, D.; Qu, D. β-Glucan Oligosaccharide EnhancesCD8+T Cells Immune Response Induced by a DNA Vaccine Encoding Hepatitis B Virus Core Antigen. J. Biomed. Biotechnol. 2010, 2010, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mc Cormack, E.; Skavland, J.; Mujić, M.; Bruserud, Ø.; Gjertsen, B.T. Lentinan: Hematopoietic, Immunological, and Efficacy Studies in a Syngeneic Model of Acute Myeloid Leukemia. Nutr. Cancer 2010, 62, 574–583. [Google Scholar] [CrossRef]
- Zhou, L.-D.; Zhang, Q.-H.; Zhang, Y.; Liu, J.; Cao, Y.-M. The shiitake mushroom-derived immuno-stimulant lentinan protects against murine malaria blood-stage infection by evoking adaptive immune-responses. Int. Immunopharmacol. 2009, 9, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, S.; Tabata, T.; Hazama, S.; Iizuka, N.; Yamamoto, K.; Hirayama, M.; Tangoku, A.; Oka, M. Immunoregulatory effects of the antitumor polysaccharide lentinan on Th1/Th2 balance in patients with digestive cancers. Anticancer. Res. 2001, 20, 4707–4711. [Google Scholar]
- Akhapkina, I.G.; Antropova, A.B.; Akhmatov, E.A.; Zheltikova, T.M. Effects of the Linear Fragments of Beta-(1→3)-Glucans on Cytokine Production in vitro. Biochemistry 2018, 83, 1002–1006. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, Y.; Liu, F. IL-10 producing B cells regulated 1,3-β-glucan induced Th responses in coordinated with Treg. Immunol. Lett. 2021, 235, 15–21. [Google Scholar] [CrossRef]
- Ohno, N.; Egawa, Y.; Hashimoto, T.; Adachi, Y.; Yadomae, T. Effect of BETA-Glucans on the Nitric Oxide Synthesis by Peritoneal Macrophage in Mice. Biol. Pharm. Bull. 1996, 19, 608–612. [Google Scholar] [CrossRef] [Green Version]



| Total Glucan | α-Glucan | β-Glucan | |
|---|---|---|---|
| S. quelpaertensis extract (SQ) | 3.73 ± 0.50 bc1) | 0.49 ± 0.02 d | 3.24 ± 0.49 bc |
| S. quelpaertensis. Ganoderma lucidum extract (SQGL) | 7.40 ± 0.89 a | 1.83 ± 0.06 a | 5.57 ± 0.86 a |
| S. quelpaertensis Hericium erinaceum extract (SQHE) | 4.49 ± 0.89 b | 0.67 ± 0.02 b | 3.82 ± 0.89 b |
| S. quelpaertensis Phellinus linteus extract (SQPL) | 4.43 ± 0.57 b | 0.63 ± 0.02 b c | 3.80 ± 0.58 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.-H.; Kim, J.-H.; Kim, S.; Son, H.-S.; Hwang, K. Immunomodulatory Effect of Sasa quelpaertensis Leaves Fermentation Products in Mice. Fermentation 2021, 7, 142. https://doi.org/10.3390/fermentation7030142
Cho J-H, Kim J-H, Kim S, Son H-S, Hwang K. Immunomodulatory Effect of Sasa quelpaertensis Leaves Fermentation Products in Mice. Fermentation. 2021; 7(3):142. https://doi.org/10.3390/fermentation7030142
Chicago/Turabian StyleCho, Ju-Hyun, Jung-Hyon Kim, Sunoh Kim, Hong-Seok Son, and Kwontack Hwang. 2021. "Immunomodulatory Effect of Sasa quelpaertensis Leaves Fermentation Products in Mice" Fermentation 7, no. 3: 142. https://doi.org/10.3390/fermentation7030142
APA StyleCho, J.-H., Kim, J.-H., Kim, S., Son, H.-S., & Hwang, K. (2021). Immunomodulatory Effect of Sasa quelpaertensis Leaves Fermentation Products in Mice. Fermentation, 7(3), 142. https://doi.org/10.3390/fermentation7030142







