Application of Hanseniaspora vineae Yeast in the Production of Rosé Wines from a Blend of Tempranillo and Albillo Grapes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains Used in Fermentation of Rosé Wines
2.2. Must and Fermentation Conditions
2.3. General Oenological Parameters Analyses
2.4. Colour Parameters Analyses
2.5. Anthocyanins Analysis
2.6. Polysaccharide Analysis
2.7. Fermentation Volatile Compounds Analysis
2.8. GC-MS Analysis
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
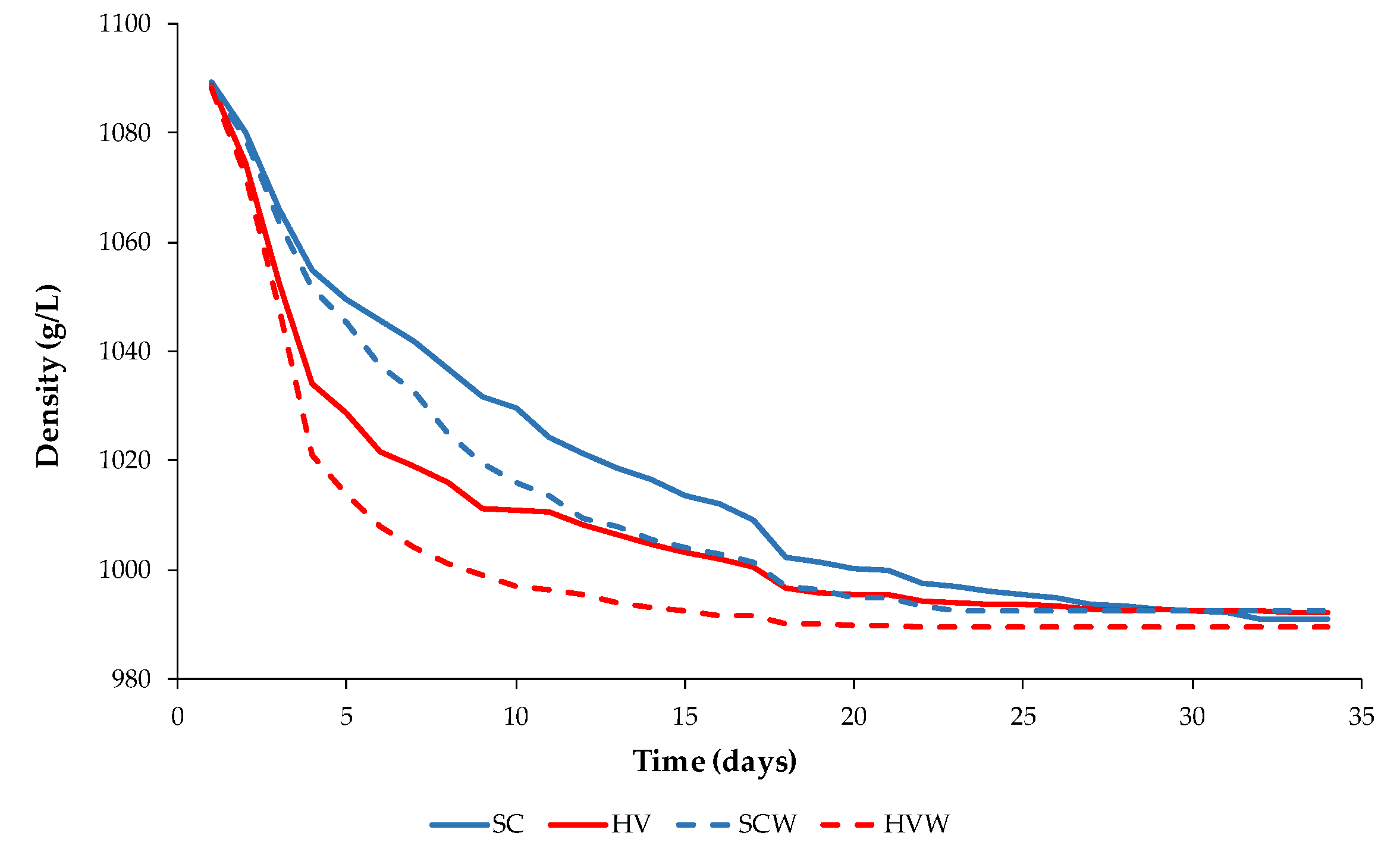
3.1. General Oenological Parameters Obtained after the Alcoholic Fermentation
3.2. Colour Parameters
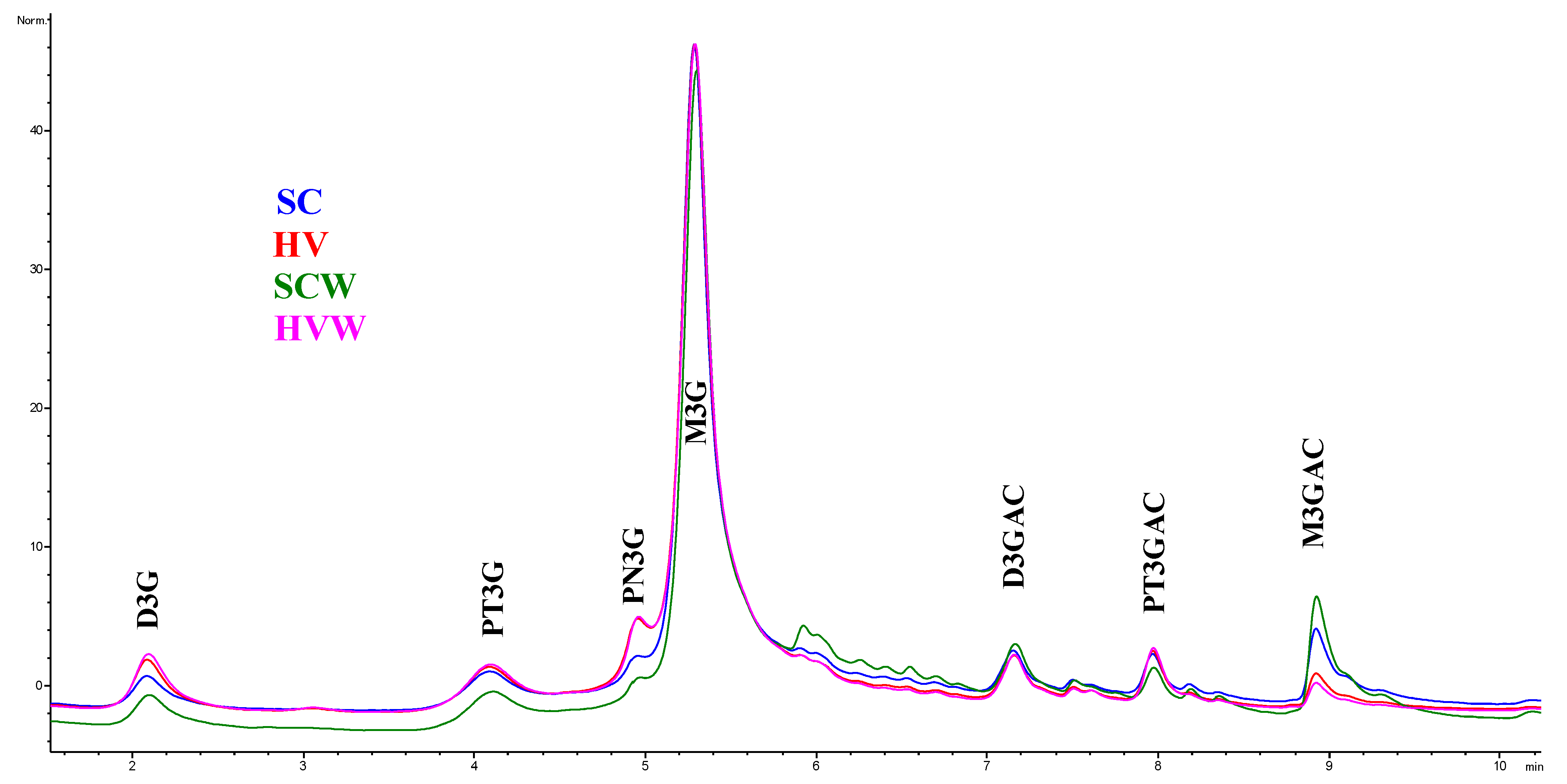
3.3. Anthocyanins
3.4. Polysaccharides Released during the Fermentation Process
3.5. Volatile Compounds Produced by Fermentation
3.6. Volatile Compounds Measured by GC-MS
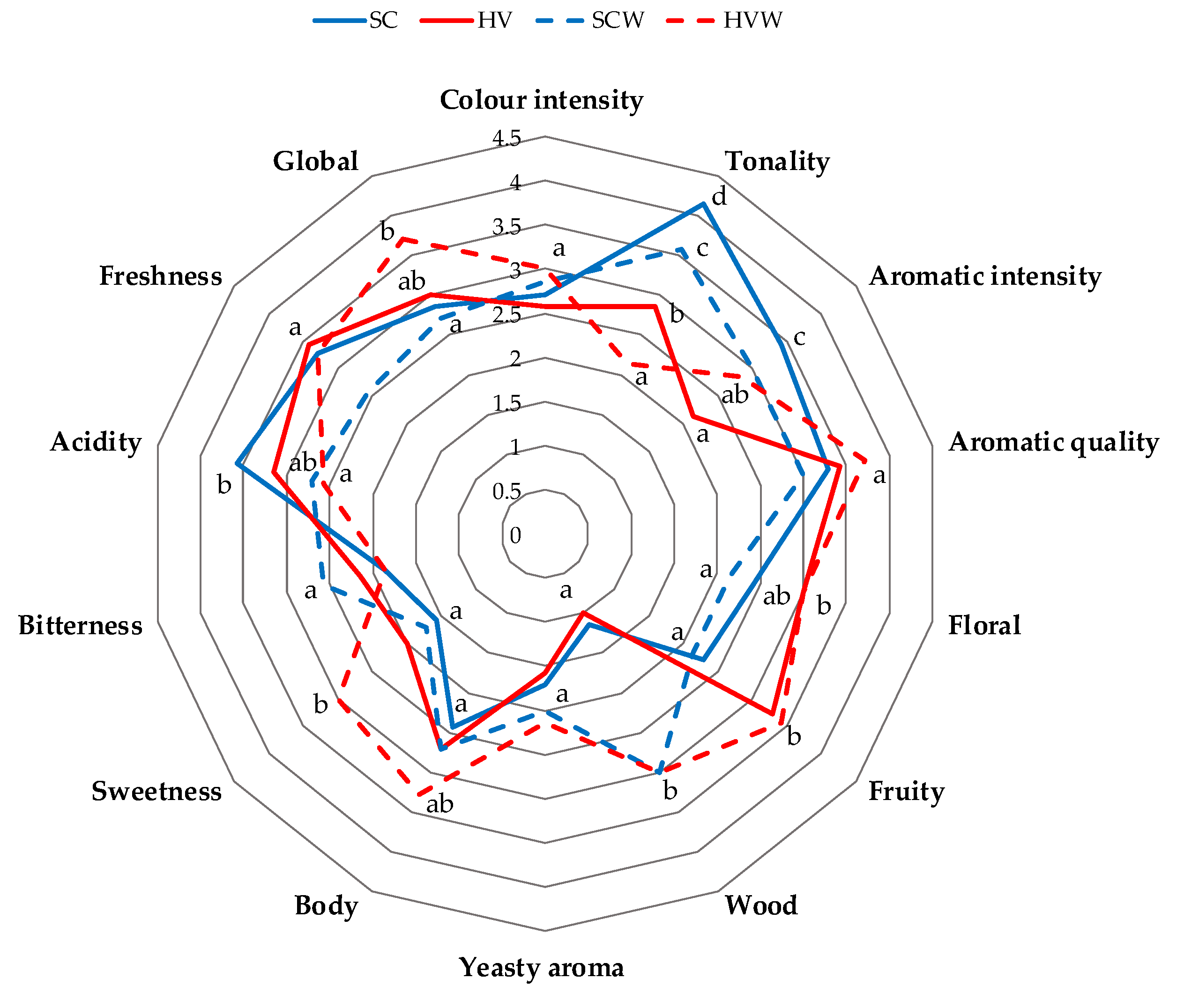
3.7. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Salinas, M.R.; Garijo, J.; Pardo, F.; Zalacain, A.; Alonso, G.L. Influence of prefermentative maceration temperature on the colour and the phenolic and volatile composition of rosé wines. J. Sci. Food Agric. 2005, 85, 1527–1536. [Google Scholar] [CrossRef]
- Fraile, P.; Garrido, J.; Ancín, C. Influence of a Saccharomyces cerevisiae selected strain in the volatile composition of rose wines. Evolution during fermentation. J. Agric. Food Chem. 2000, 48, 1789–1798. [Google Scholar] [CrossRef]
- Morata, A.; Escott, C.; Bañuelos, M.A.; Loira, I.; Del Fresno, J.M.; González, C.; Suárez-lepe, J.A. Contribution of non-Saccharomyces yeasts to wine freshness. A review. Biomolecules 2020, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Prakitchaiwattana, C.J.; Fleet, G.H.; Heard, G.M. Application and evaluation of denaturing gradient gel electrophoresis to analyse the yeast ecology of wine grapes. FEMS Yeast Res. 2004, 4, 865–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez, M.J.V.; Valera, M.J.; Medina, K.; Boido, E.; Carrau, F. Oenological Impact of the Hanseniaspora/Kloeckera Yeast Genus on Wines—A Review. Fermentation 2018, 4, 76. [Google Scholar] [CrossRef] [Green Version]
- Heard, G.M.; Fleet, G.H. The effects of temperature and pH on the growth of yeast species during the fermentation of grape juice. J. Appl. Bacteriol. 1988, 65, 23–28. [Google Scholar] [CrossRef]
- Medina, K.; Boido, E.; Fariña, L.; Gioia, O.; Gomez, M.; Barquet, M.; Gaggero, C.; Dellacassa, E.; Carrau, F. Increased flavour diversity of Chardonnay wines by spontaneous fermentation and co-fermentation with Hanseniaspora vineae. Food Chem. 2013, 141, 2513–2521. [Google Scholar] [CrossRef]
- Viana, F.; Belloch, C.; Vallés, S.; Manzanares, P. Monitoring a mixed starter of Hanseniaspora vineae–Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.; Henschke, P.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Cordente, A.G.; Solomon, M.; Schulkin, A.; Francis, I.L.; Barker, A.; Borneman, A.R.; Curtin, C.D. Novel wine yeast with ARO4 and TYR1 mutations that overproduce ‘floral’ aroma compounds 2-phenylethanol and 2-phenylethyl acetate. Appl. Microbiol. Biotechnol. 2018, 102, 5977–5988. [Google Scholar] [CrossRef]
- Lleixà, J.; Martin, V.; Portillo, M.C.; Carrau, F.; Beltran, G.; Mas, A. Comparison of Fermentation and Wines Produced by Inoculation of Hanseniaspora vineae and Saccharomyces cerevisiae. Front. Microbiol. 2016, 7, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Fresno, J.M.; Escott, C.; Loira, I.; Herbert-Pucheta, J.E.; Schneider, R.; Carrau, F.; Cuerda, R.; Morata, A. Impact of Hanseniaspora vineae in alcoholic fermentation and ageing on lees of high-quality whitewine. Fermentation 2020, 6, 66. [Google Scholar] [CrossRef]
- Del Fresno, J.; Escott, C.; Loira, I.; Carrau, F.; Cuerda, R.; Schneider, R.; Bañuelos, M.; González, C.; Suárez-Lepe, J.; Morata, A. The Impact of Hanseniaspora vineae Fermentation and Ageing on Lees on the Terpenic Aromatic Profile of White Wines of the Albillo Variety. Int. J. Mol. Sci. 2021, 22, 2195. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.C.; Jones, G.V.; Yuste, J. Spatial and temporal variability of cv. Tempranillo phenology and grape quality within the Ribera del Duero DO (Spain) and relationships with climate. Int. J. Biometeorol. 2015, 59, 1849–1860. [Google Scholar] [CrossRef]
- Variedades de uva|Ribera del Duero. Available online: https://www.riberadelduero.es/la-do-ribera-del-duero/variedades-de-uva (accessed on 18 June 2021).
- Escott, C.; Vaquero, C.; del Fresno, J.M.; Bañuelos, M.A.; Loira, I.; Han, S.-Y.; Bi, Y.; Morata, A.; Suárez-Lepe, J.A. Pulsed Light Effect in Red Grape Quality and Fermentation. Food Bioprocess Technol. 2017, 10, 1540–1547. [Google Scholar] [CrossRef]
- Loira, I.; Vejarano, R.; Morata, A.; Ricardo-Da-Silva, J.M.; Laureano, O.; González, M.; Suárez-Lepe, J. Effect of Saccharomyces strains on the quality of red wines aged on lees. Food Chem. 2013, 139, 1044–1051. [Google Scholar] [CrossRef]
- Abalos, D.; Vejarano, R.; Morata, A.; González, C.; Suárez-Lepe, J.A.; Vejarano-Mantilla, R.D. The use of furfural as a metabolic inhibitor for reducing the alcohol content of model wines. Eur. Food Res. Technol. 2011, 232, 663–669. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Zohre, D.; Erten, H. The influence of Kloeckera apiculata and Candida pulcherrima yeasts on wine fermentation. Process. Biochem. 2002, 38, 319–324. [Google Scholar] [CrossRef]
- Comitini, F.; Ciani, M. The zymocidial activity of Tetrapisispora phaffii in the control of Hanseniaspora uvarum during the early stages of winemaking. Lett. Appl. Microbiol. 2010, 50, 50–56. [Google Scholar] [CrossRef]
- Ciani, M.; Beco, L.; Comitini, F. Fermentation behaviour and metabolic interactions of multistarter wine yeast fermentations. Int. J. Food Microbiol. 2006, 108, 239–245. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Gómez-Plaza, E.; Martínez, A.; López-Roca, J. Evolution of the CIELAB and other spectrophotometric parameters during wine fermentation. Influence of some pre and postfermentative factors. Food Res. Int. 1997, 30, 699–705. [Google Scholar] [CrossRef]
- Prez-Magario, S.; Gonzalez-SanJose, M.L. Prediction of red and rosé wine CIELab parameters from simple absorbance measurements. J. Sci. Food Agric. 2002, 82, 1319–1324. [Google Scholar] [CrossRef]
- Bakker, J.; Bridle, P.; Timberlake, C.F. Tristimulus measurements (CIELAB 76) of port wine colour. Vitis 1986, 25, 67–78. [Google Scholar]
- Almela, L.; Fernández-López, J.A.; Javaloy, S.; Fernhndez, J.A.; Lopez-Rota, M. Comparison between the tristimulus measurements Yxy and L*a*b* to evaluate the color of young red wines. Food Chem. 1995, 53, 321–327. [Google Scholar] [CrossRef]
- Núñez, V.; Monagas, M.; Gomez-Cordovés, M.; Bartolomé, B. Vitis vinifera L. cv. Graciano grapes characterized by its anthocyanin profile. Postharvest Biol. Technol. 2004, 31, 69–79. [Google Scholar] [CrossRef]
- Puértolas, E.; Saldaña, G.; Álvarez, I.; Raso, J. Experimental design approach for the evaluation of anthocyanin content of rosé wines obtained by pulsed electric fields. Influence of temperature and time of maceration. Food Chem. 2011, 126, 1482–1487. [Google Scholar] [CrossRef]
- Stávek, J.; Papoušková, B.; Balik, J.; Bednar, P. Effect of Storage Conditions on Various Parameters of Colour and the Anthocyanin Profile of Rosé Wines. Int. J. Food Prop. 2012, 15, 1133–1147. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Properties of Wine Polysaccharides. In Pectins—Extraction, Purification, Characterization and Applications, 1st ed.; IntechOpen: London, UK, 2020; Volume 1, pp. 1–21. [Google Scholar]
- Guadalupe, Z.; Martínez-Pinilla, O.; Garrido, Á.; Carrillo, J.D.; Ayestarán, B. Quantitative determination of wine polysaccharides by gas chromatography-mass spectrometry (GC-MS) and size exclusion chromatography (SEC). Food Chem. 2012, 131, 367–374. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Loira, I.; Morata, A.; González, C.; Suárez-Lepe, J.A.; Cuerda, R. Application of ultrasound to improve lees ageing processes in red wines. Food Chem. 2018, 261, 157–163. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; Gonzalez, M.; Suárez-Lepe, J. Conventional and enzyme-assisted autolysis during ageing over lees in red wines: Influence on the release of polysaccharides from yeast cell walls and on wine monomeric anthocyanin content. Food Chem. 2007, 105, 838–846. [Google Scholar] [CrossRef]
- Rapp, A.; Versini, G. Influence of Nitrogen Compounds in Grapes on Aroma Compounds of Wines; Elsevier: Amsterdam, The Netherlands, 1995; pp. 1659–1694. [Google Scholar] [CrossRef]
- Vilanova, M.; Martínez, C. First study of determination of aromatic compounds of red wine from Vitis vinifera cv. Castañal grown in Galicia (NW Spain). Eur. Food Res. Technol. 2006, 224, 431–436. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Shen, J.Y.; Duan, C.Q.; Yan, G.L. Use of indigenous Hanseniaspora vineae and Metschnikowia pulcherrima Co-fermentation with Saccharomyces cerevisiae to improve the aroma diversity of Vidal blanc ice wine. Front. Microbiol. 2018, 9, 2303. [Google Scholar] [CrossRef] [PubMed]
- Sumby, K.; Grbin, P.; Jiranek, V. Microbial modulation of aromatic esters in wine: Current knowledge and future prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Plata, C.; Millán, C.; Mauricio, J.; Ortega, J. Formation of ethyl acetate and isoamyl acetate by various species of wine yeasts. Food Microbiol. 2003, 20, 217–224. [Google Scholar] [CrossRef]
- Martin, V.; Giorello, F.; Fariña, L.; Minteguiaga, M.; Salzman, V.; Boido, E.; Aguilar, P.S.; Gaggero, C.; Dellacassa, E.; Mas, A.; et al. De NovoSynthesis of Benzenoid Compounds by the Yeast Hanseniaspora vineae Increases the Flavor Diversity of Wines. J. Agric. Food Chem. 2016, 64, 4574–4583. [Google Scholar] [CrossRef] [Green Version]
- Kotseridis, Y.; Baumes, R.L.; Bertrand, A.; Skouroumounis, G.K. Quantitative determination of β-ionone in red wines and grapes of Bordeaux using a stable isotope dilution assay. J. Chromatogr. A 1999, 848, 317–325. [Google Scholar] [CrossRef]
- Langen, J.; Wegmann-Herr, P.; Schmarr, H.G. Quantitative determination of α-ionone, β-ionone, and β-damascenone and enantio differentiation of α-ionone in wine for authenticity control using multidimensional gas chromatography with tandem mass spectrometric detection. Anal. Bioanal. Chem. 2016, 408, 6483–6496. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Arapitsas, P.; Antonopoulos, A.; Stefanou, E.; Dourtoglou, V. Artificial aging of wines using oak chips. Food Chem. 2004, 86, 563–570. [Google Scholar] [CrossRef]
- Garcia, R.; Soares, B.; Dias, C.B.; Freitas, A.M.C.; Cabrita, M.J. Phenolic and furanic compounds of Portuguese chestnut and French, American and Portuguese oak wood chips. Eur. Food Res. Technol. 2012, 235, 457–467. [Google Scholar] [CrossRef]
- Towey, J.P.; Waterhouse, A.L. The Extraction of Volatile Compounds From French and American Oak Barrels in Chardonnay During Three Successive Vintages. Am. J. Enol. Vitic 1996, 47, 163–172. [Google Scholar]
- Pérez-Prieto, L.J.; López-Roca, J.M.; Martínez-Cutillas, A.; Pardo Mínguez, F.; Gómez-Plaza, E. Maturing Wines in Oak Barrels. Effects of Origin, Volume, and Age of the Barrel on the Wine Volatile Composition. J. Agric. Food Chem. 2002, 50, 3272–3276. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Dubourdie, D.; Boidron, J.-N.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]



| Rosé Wines | Ethanol (% v/v) | pH | Glucose-Fructose (g/L) | Volatile Acidity (g/L Acetic Acid) |
|---|---|---|---|---|
| SC | 12.90 ± 0.53 a,b | 3.51 ± 0.03 c | 3.45 ± 0.45 a | 0.44 ± 0.11 a,b |
| HV | 12.70 ± 0.10 a | 3.43 ± 0.04 b | 2.95 ± 1.75 a | 0.42 ± 0.01 a,b |
| SCW | 13.27 ± 0.12 b | 3.39 ± 0.03 a,b | 2.10 ± 0.10 a | 0.52 ± 0.02 b |
| HVW | 13.20 ± 0.10 a,b | 3.36 ± 0.02 a | 3.35 ± 0.15 a | 0.40 ± 0.01 a |
| Delphinidin 3-O-Glucoside (D3G) (mg/L) | Petunidin 3-O-Glucoside (PT3G) (mg/L) | Peonidin 3-O-Glucoside (PN3G) (mg/L) | Malvidin 3-O-Glucoside (M3G) (mg/L) | Delphinidin 3-O-(6”-O-Acetyl) Glucoside (D3GAC) (mg/L) | Petunidin 3-O-(6”-O-Acetyl) Glucoside (PT3GAC) (mg/L) | Malvidin 3-O-(6”-O-Acetyl) Glucoside (M3GAC) (mg/L) | Total Anthocyanin Content (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| SC | 3.78 ± 0.20 a | 4.28 ± 0.22 b | 3.61 ± 0.10 a | 31.69 ± 1.27 b | 4.06 ± 0.07 b | 3.82 ± 0.07 b | 4.64 ± 0.02 c | 55.87 ± 1.69 b |
| HV | 4.79 ± 0.32 b | 4.96 ± 0.13 c | 4.84 ± 0.40 b | 35.98 ± 0.27 c | 4.25 ± 0.03 c | 4.24 ± 0.00 c | 3.63 ± 0.09 b | 62.70 ± 0.40 c |
| SCW | 3.63 ± 0.16 a | 3.80 ± 0.02 a | 3.33 ± 0.15 a | 23.60 ± 2.35 a | 3.78 ± 0.02 a | 3.39 ± 0.11 a | 4.76 ± 0.05 c | 46.29 ± 2.71 a |
| HVW | 5.54 ± 0.15 c | 4.98 ± 0.03 c | 5.16 ± 0.14 b | 38.56 ± 0.42 d | 4.41 ± 0.02 d | 4.46 ± 0.02 d | 3.50 ± 0.03 a | 66.61 ± 0.60 d |
| Compound | SC | HV | SCW | HVW |
|---|---|---|---|---|
| Acetaldehyde | 63.25 ± 4.63 a | 70.23 ± 2.76 a,b | 74.75 ± 0.65 b,c | 79.08 ± 6.57 c |
| Methanol | 26.64 ± 0.84 b | 27.21 ± 2.07 b | 22.84 ± 2.86 a | 25.31 ± 0.68 a,b |
| Diacetyl | 1.55 ± 0.07 b | 1.60 ± 0.12 b | 1.56 ± 0.01 b | 0.49 ± 0.84 a |
| Acetoin | 5.50 ± 0.05 a | 6.46 ± 1.32 a | 5.74 ± 1.66 a | 5.70 ± 0.40 a |
| 2,3-butanediol | 536.22 ± 80.81 b | 381.94 ± 41.65 a | 360.03 ± 13.82 a | 394.49 ± 89.51 a |
| Isobutanol | 21.05 ± 0.85 b | 17.73 ± 0.40 a | 26.24 ± 1.04 c | 18.21 ± 0.40 a |
| 1-propanol | 24.23 ± 0.63 b | 20.23 ± 1.59 a | 24.41 ± 0.49 b | 20.22 ± 1.67 a |
| 2-methyl-1-butanol | 24.17 ± 0.57 b,c | 19.44 ± 1.07 a | 25.46 ± 1.47 c | 22.58 ± 0.91 b |
| 3-methyl-1-butanol | 120.25 ± 0.72 c | 93.13 ± 4.75 a | 125.40 ± 2.97 c | 108.64 ± 1.34 b |
| Hexanol | 4.46 ± 0.18 b | 4.17 ± 0.22 a,b | 4.10 ± 0.29 a,b | 3.86 ± 0.03 a |
| 2-phenyl-ethanol | 16.46 ± 0.96 a | 15.21 ± 0.27 a | 17.08 ± 1.63 a | 16.16 ± 1.47 a |
| ∑ Higher alcohols | 210.61 ± 1.29 c | 169.91 ± 7.56 a | 222.69 ± 4.97 d | 189.67 ± 4.80 b |
| Ethyl lactate | 15.25 ± 1.60 b | 14.50 ± 3.75 a,b | 10.11 ± 0.83 a | 15.08 ± 3.19 b |
| Ethyl acetate | 70.96 ± 4.09 b | 57.55 ± 4.62 a | 85.08 ± 2.99 c | 63.30 ± 3.20 a |
| Isoamyl acetate | 4.63 ± 0.11 a,b | 4.52 ± 0.37 a | 5.08 ± 0.22 b | 4.89 ± 0.39 a,b |
| 2-phenylethyl acetate | 9.28 ± 1.63 b | 15.38 ± 1.22 c | 6.66 ± 0.60 a | 7.35 ± 0.40 a,b |
| ∑ Esters | 100.12 ± 6.34 a,b | 93.45 ± 5.37 a | 106.93 ± 3.16 b | 90.62 ± 5.76 a |
| ∑ Total volatiles | 943.90 ± 78.80 b | 750.80 ± 28.39 a | 794.54 ± 22.04 a | 785.37 ± 100.17 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fresno, J.M.D.; Loira, I.; Escott, C.; Carrau, F.; González, C.; Cuerda, R.; Morata, A. Application of Hanseniaspora vineae Yeast in the Production of Rosé Wines from a Blend of Tempranillo and Albillo Grapes. Fermentation 2021, 7, 141. https://doi.org/10.3390/fermentation7030141
Fresno JMD, Loira I, Escott C, Carrau F, González C, Cuerda R, Morata A. Application of Hanseniaspora vineae Yeast in the Production of Rosé Wines from a Blend of Tempranillo and Albillo Grapes. Fermentation. 2021; 7(3):141. https://doi.org/10.3390/fermentation7030141
Chicago/Turabian StyleFresno, Juan Manuel Del, Iris Loira, Carlos Escott, Francisco Carrau, Carmen González, Rafael Cuerda, and Antonio Morata. 2021. "Application of Hanseniaspora vineae Yeast in the Production of Rosé Wines from a Blend of Tempranillo and Albillo Grapes" Fermentation 7, no. 3: 141. https://doi.org/10.3390/fermentation7030141
APA StyleFresno, J. M. D., Loira, I., Escott, C., Carrau, F., González, C., Cuerda, R., & Morata, A. (2021). Application of Hanseniaspora vineae Yeast in the Production of Rosé Wines from a Blend of Tempranillo and Albillo Grapes. Fermentation, 7(3), 141. https://doi.org/10.3390/fermentation7030141









