LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.3. Extraction of Phenolic Compounds
2.4. Phenolic Compound Estimation and Antioxidant Assays
2.4.1. Total Phenolic Content (TPC) Assay
2.4.2. Total Flavonoid Content (TFC) Assay
2.4.3. Total Tannins Content (TTC) Assay
2.4.4. DPPH Radical Scavenging Assay
2.4.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.4.6. ABTS Radical Scavenging Assay
2.4.7. Reducing Power Assay (RPA)
2.4.8. Hydroxyl Radical Scavenging Activity (•OH-RSA)
2.4.9. Ferrous Ion Chelating Activity (FICA)
2.4.10. Total Antioxidant Content (TAC) Assay
2.5. LC-ESI-QTOF-MS/MS Analysis
2.6. HPLC-PDA Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phenolic Compound Estimation (TFC, TPC and TTC)
3.2. Antioxidant Activities (DPPH, FRAP, ABTS, RPA, •OH-RSA, FICA and TAC)
3.3. Phenolic Content and Antioxidant Activity Correlation Analysis
3.4. LC-MS/MS Analysis
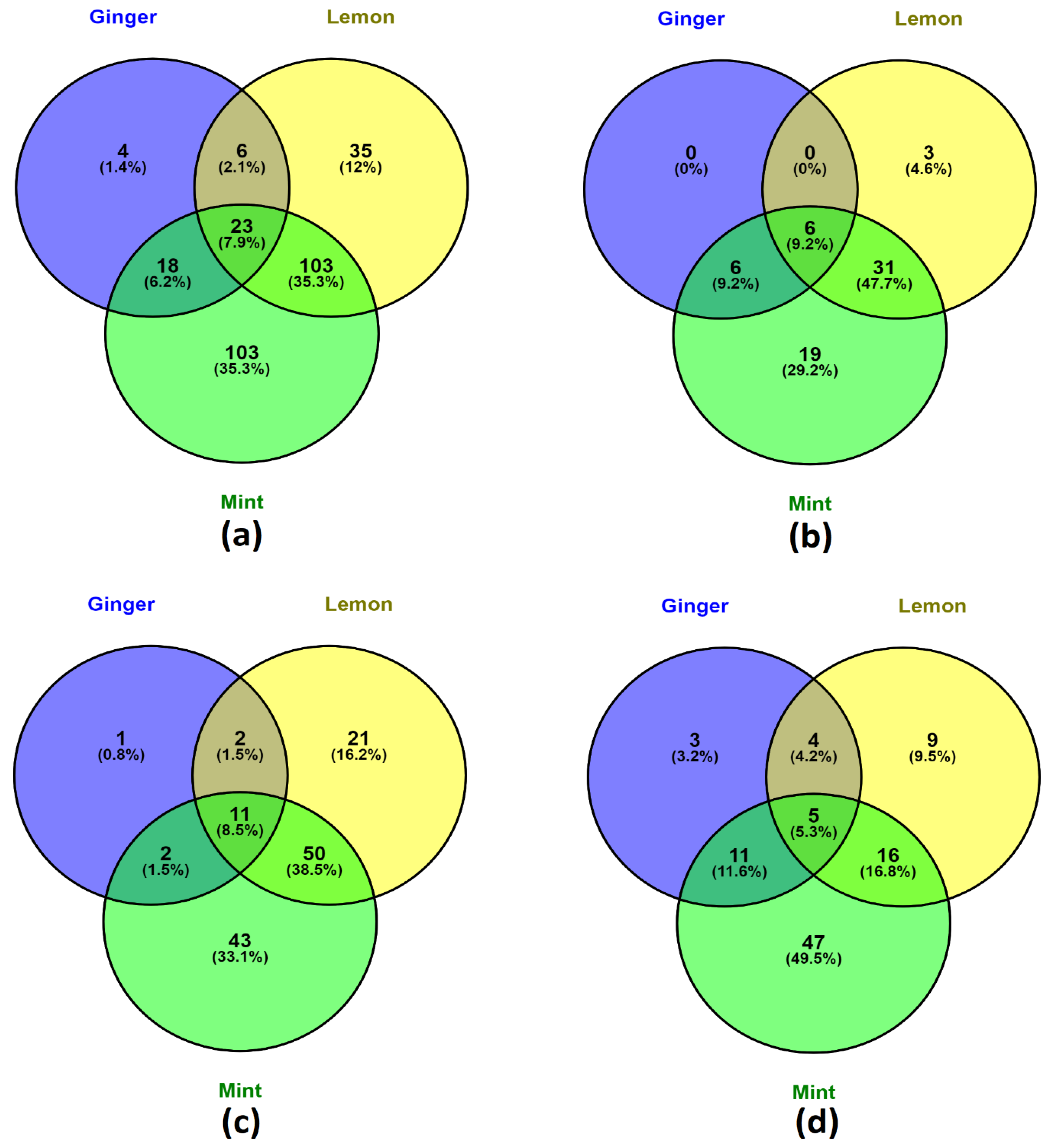
3.4.1. Phenolic Distribution—Venn Diagram Analysis
3.4.2. LC-ESI-QTOF-MS/MS Characterization
Phenolic Acids
Flavonoids
Other Phenolic Compounds
Lignans and Stilbenes
3.5. HPLC Quantification Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buyukbalci, A.; El, S.N. Determination of in vitro antidiabetic effects, antioxidant activities and phenol contents of some herbal teas. Plant. Foods Hum. Nutr. 2008, 63, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Chawla, P.; Tripathi, M.; Shukla, A.K.; Pandey, A.J.I.J.O.P.; Sciences, P. Synergistic antioxidant activity of tea with ginger, black pepper and tulsi. Int. J. Pharm. Pharm. Sci. 2014, 6, 477–479. [Google Scholar]
- Malongane, F.; McGaw, L.J.; Mudau, F.N. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: A review. J. Sci. Food Agric. 2017, 97, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Bekkouch, O.; Harnafi, M.; Touiss, I.; Khatib, S.; Harnafi, H.; Alem, C.; Amrani, S. In vitro antioxidant and in vivo lipid-lowering properties of zingiber officinale crude aqueous extract and methanolic fraction: A follow-up study. Evid. Based Complement Altern. Med. 2019, 2019, 9734390. [Google Scholar] [CrossRef]
- Nair, K.P. Turmeric (Curcuma Longa l.) and Ginger (Zingiber Officinale Rosc.)-World’s Invaluable Medicinal Spices: The Agronomy and Economy of Turmeric and Ginger; Springer Nature: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Yeh, H.-Y.; Chuang, C.-H.; Chen, H.-C.; Wan, C.-J.; Chen, T.-L.; Lin, L.-Y. Bioactive components analysis of two various gingers (zingiber officinale roscoe) and antioxidant effect of ginger extracts. Lwt Food Sci. Technol. 2014, 55, 329–334. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive compounds and bioactivities of ginger (zingiber officinale roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Jitoe, A.; Masuda, T.; Tengah, I.G.P.; Suprapta, D.N.; Gara, I.W.; Nakatani, N. Antioxidant activity of tropical ginger extracts and analysis of the contained curcuminoids. J. Agric. Food Chem. 1992, 40, 1337–1340. [Google Scholar] [CrossRef]
- Curk, F.; Ollitrault, F.; Garcia-Lor, A.; Luro, F.; Navarro, L.; Ollitrault, P. Phylogenetic origin of limes and lemons revealed by cytoplasmic and nuclear markers. Ann. Bot. 2016, 117, 565–583. [Google Scholar] [CrossRef]
- Shimizu, C.; Wakita, Y.; Inoue, T.; Hiramitsu, M.; Okada, M.; Mitani, Y.; Segawa, S.; Tsuchiya, Y.; Nabeshima, T. Effects of lifelong intake of lemon polyphenols on aging and intestinal microbiome in the senescence-accelerated mouse prone 1 (samp1). Sci. Rep. 2019, 9, 3671. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Shimoi, K.; Kumazawa, S.; Yamamoto, K.; Kinae, N.; Osawa, T.J. Identification and antioxidant activity of flavonoid metabolites in plasma and urine of eriocitrin-treated rats. J. Agric. Food Chem. 2000, 48, 3217–3224. [Google Scholar] [CrossRef] [PubMed]
- Makni, M.; Jemai, R.; Kriaa, W.; Chtourou, Y.; Fetoui, H. Citrus limon from tunisia: Phytochemical and physicochemical properties and biological activities. Biomed. Res. Int. 2018, 2018, 6251546. [Google Scholar] [CrossRef] [PubMed]
- Moosavy, M.; Hassanzadeh, P.; Mohammadzadeh, E.; Mahmoudi, R.; Khatibi, S.; Mardani, K.J.J.o.f.q. Antioxidant and antimicrobial activities of essential oil of lemon (citrus limon) peel in vitro and in a food model. J. Food Qual. Hazards Control 2017, 4, 42–48. [Google Scholar]
- Bunsawat, J.; Elliott, N.E.; Hertweck, K.L.; Sproles, E.; Alice, L.A.J.S.B. Phylogenetics of mentha (lamiaceae): Evidence from chloroplast DNA sequences. Syst. Bot. 2004, 29, 959–964. [Google Scholar] [CrossRef]
- Riachi, L.G.; De Maria, C.A. Peppermint antioxidants revisited. Food Chem. 2015, 176, 72–81. [Google Scholar] [CrossRef]
- Singh, R.; Shushni, M.A.M.; Belkheir, A. Antibacterial and antioxidant activities of mentha piperita L. Arab. J. Chem. 2015, 8, 322–328. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Martorell Guerola, P.; Tortajada, M.; Cao, Z.; Ji, P. Chemical composition and antioxidant properties of essential oils from peppermint, native spearmint and scotch spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Profiling of Australian Mango Peel By-Product Polyphenols and Their Potential Antioxidant Activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Imran, M.; Butt, M.S.; Akhtar, S.; Riaz, M.; Iqbal, M.J.; Suleria, H.A.R. Mangiferin from Mango Peel. J. Food Process. Preserv. 2016, 40, 760–769. [Google Scholar] [CrossRef]
- Halliwell, B.; Murcia, M.A.; Chirico, S.; Aruoma, O.I. Free radicals and antioxidants in food and in vivo: What they do and how they work. Crit. Rev. Food Sci. Nutr. 1995, 35, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef]
- Briskin, D.P.J.P.p. Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol. 2000, 124, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Awan, K.A.; Butt, M.S.; Haq, I.U.; Ashfaq, F.; Suleria, H.A.R. Storage Stability of Garlic Fortified Chicken Bites. J. Food Chem. Nanotechnol. 2017, 3, 80–85. [Google Scholar] [CrossRef]
- Feng, Y.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS characterization of bioactive compounds from black spices and their potential antioxidant activities. J. Food Sci. Technol. 2020, 57, 4671–4687. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef]
- Shotorbani, N.Y.; Jamei, R.; Heidari, R.J.A.J.o.P. Antioxidant activities of two sweet pepper capsicum annuum l. Varieties phenolic extracts and the effects of thermal treatment. Avicenna J. Phytomed. 2013, 3, 25. [Google Scholar]
- Benzie, I.F.; Strain, J.J.J.A.b. The ferric reducing ability of plasma (frap) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C.J.F. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Kadam, D.; Palamthodi, S.; Lele, S.S. Lc-esi-q-tof-ms/ms profiling and antioxidant activity of phenolics from l. Sativum seedcake. J. Food Sci. Technol. 2018, 55, 1154–1163. [Google Scholar] [CrossRef]
- Pekal, A.; Drozdz, P.; Biesaga, M.; Pyrzynska, K. Screening of the antioxidant properties and polyphenol composition of aromatised green tea infusions. J. Sci. Food Agric. 2012, 92, 2244–2249. [Google Scholar] [CrossRef]
- Seo, C.-S.; Lee, M.-Y. Hplc–pda and lc–ms/ms analysis for the simultaneous quantification of the 14 marker components in sojadodamgangki-tang. Appl. Sci. 2020, 10, 2804. [Google Scholar] [CrossRef]
- Yang, D.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of australian herb and spices (garlic, ginger, and onion) and potential antioxidant activity. J. Food Process. Preserv. 2020, 44, e14497. [Google Scholar] [CrossRef]
- Tang, J.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 2019, 9, 7. [Google Scholar] [CrossRef]
- Subbiah, V.; Zhong, B.; Nawaz, M.A.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A. Screening of phenolic compounds in australian grown berries by lc-esi-qtof-ms/ms and determination of their antioxidant potential. Antioxidants 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. A comparative investigation on phenolic composition, characterization and antioxidant potentials of five different australian grown pear varieties. Antioxidants 2021, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Kılıç, C.; Can, Z.; Yılmaz, A.; Yıldız, S.; Turna, H. Antioxidant properties of some herbal teas (green tea, senna, corn silk, rosemary) brewed at different temperatures. Int. J. Second. Metab. 2017, 148–154. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of ‘tommy atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. Spj Off. Publ. Saudi Pharm. Soc. 2013, 21, 143–152. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M.J.A.b. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Suleria, H.A.; Barrow, C.J.; Dunshea, F.R.J.F. Screening and characterization of phenolic compounds and their antioxidant capacity in different fruit peels. Foods 2020, 9, 1206. [Google Scholar] [CrossRef]
- Zhong, B.; Robinson, N.A.; Warner, R.D.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof-ms/ms characterization of seaweed phenolics and their antioxidant potential. Mar. Drugs 2020, 18, 331. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Mohamed Ahmed, I.A. Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger (zingiber officinale) rhizome as affected by drying methods. Lwt 2020, 126, 109354. [Google Scholar] [CrossRef]
- Farnad, N.; Heidari, R.; Aslanipour, B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of peppermint (mentha piperita). J. Food Meas. Charact. 2014, 8, 113–121. [Google Scholar] [CrossRef]
- Benabdallah, A.; Rahmoune, C.; Boumendjel, M.; Aissi, O.; Messaoud, C. Total phenolic content and antioxidant activity of six wild mentha species (lamiaceae) from northeast of algeria. Asian Pac. J. Trop. Biomed. 2016, 6, 760–766. [Google Scholar] [CrossRef]
- Sarooshi, R.; Broadbent, P.J.A.J.o.E.A. Evaluation of rootstocks for eureka and lisbon lemons in replant ground in new south wales. Aust. J. Exp. Agric. 1992, 32, 205–209. [Google Scholar] [CrossRef]
- Rahmati, M.; Vercambre, G.; Davarynejad, G.; Bannayan, M.; Azizi, M.; Genard, M. Water scarcity conditions affect peach fruit size and polyphenol contents more severely than other fruit quality traits. J. Sci. Food Agric. 2015, 95, 1055–1065. [Google Scholar] [CrossRef]
- Velioglu, Y.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Contreras-López, E.; Castañeda-Ovando, A.; Jaimez-Ordaz, J.; Cruz-Cansino, N.d.S.; González-Olivares, L.G.; Rodríguez-Martínez, J.S.; Ramírez-Godínez, J.J.A.S. Release of antioxidant compounds of zingiber officinale by ultrasound-assisted aqueous extraction and evaluation of their in vitro bioaccessibility. Appl. Sci. 2020, 10, 4987. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of dpph method of antioxidant assay. J. Food Sci. Technol 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Chrpova, D.; Kouřimská, L.; Gordon, M.H.; Heřmanová, V.; Roubíčková, I.; Panek, J.J.C.J.o.F.S. Antioxidant activity of selected phenols and herbs used in diets for medical conditions. Czech J. Food Sci. 2010, 28, 317–325. [Google Scholar] [CrossRef]
- Sun, Y.; Qiao, L.; Shen, Y.; Jiang, P.; Chen, J.; Ye, X. Phytochemical profile and antioxidant activity of physiological drop of citrus fruits. J. Food Sci. 2013, 78, C37–C42. [Google Scholar] [CrossRef]
- Gu, C.; Howell, K.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms characterisation of phenolic acids and flavonoids in polyphenol-rich fruits and vegetables and their potential antioxidant activities. Antioxidants 2019, 8, 405. [Google Scholar] [CrossRef]
- Mittal, N. Estimation of antioxidant levels in pomegranate, banana, orange, lemon, sweet lime. Stud. Ethno-Med. 2019, 13. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Chong, K.L.; Tan, J.B.L.; Wong, S.K. Antioxidant properties of tropical and temperate herbal teas. J. Food Compos. Anal. 2010, 23, 185–189. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. Abts/pp decolorization assay of antioxidant capacity reaction pathways. Int J. Mol Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Assefa, A.D.; Keum, Y.-S.; Saini, R.K. A comprehensive study of polyphenols contents and antioxidant potential of 39 widely used spices and food condiments. J. Food Meas. Charact. 2018, 12, 1548–1555. [Google Scholar] [CrossRef]
- Leong, L.; Shui, G.J.F.c. An investigation of antioxidant capacity of fruits in singapore markets. Food Chem. 2002, 76, 69–75. [Google Scholar] [CrossRef]
- Bhalodia, N.R.; Nariya, P.B.; Acharya, R.N.; Shukla, V.J. In vitro antioxidant activity of hydro alcoholic extract from the fruit pulp of cassia fistula linn. Ayu 2013, 34, 209–214. [Google Scholar] [CrossRef]
- Prakash, J.J.J.o.M.P.R. Chemical composition and antioxidant properties of ginger root (zingiber officinale). J. Med. Plants Res. 2010, 4, 2674–2679. [Google Scholar]
- Kanatt, S.R.; Chander, R.; Sharma, A.J.F.C. Antioxidant potential of mint (mentha spicata L.) in radiation-processed lamb meat. Food Chem. 2007, 100, 451–458. [Google Scholar] [CrossRef]
- Stoilova, I.; Krastanov, A.; Stoyanova, A.; Denev, P.; Gargova, S. Antioxidant activity of a ginger extract (zingiber officinale). Food Chem. 2007, 102, 764–770. [Google Scholar] [CrossRef]
- Dorman, H.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R.J.J. Antioxidant properties and composition of aqueous extracts from mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef]
- Muthiah, P.; Umamaheswari, M.; Asokkumar, K.J.I.J.o.P. In vitro antioxidant activities of leaves, fruits and peel extracts of citrus. Int. J. Phytopharm. 2012, 2, 13–20. [Google Scholar]
- Ramkissoon, J.S.; Mahomoodally, M.F.; Ahmed, N.; Subratty, A.H. Therapeutic potential of common culinary herbs and spices of mauritius. In Chemistry: The Key to Our Sustainable Future; Springer: Berlin, Germany, 2014; pp. 147–162. [Google Scholar]
- Agarwal, M.; Kumar, A.; Gupta, R.; Upadhyaya, S.J.O.J.o.C. Extraction of polyphenol, flavonoid from emblica officinalis, citrus limon, cucumis sativus and evaluation of their antioxidant activity. Orient. J. Chem. 2012, 28, 993. [Google Scholar] [CrossRef][Green Version]
- Brown, N.; John, J.A.; Shahidi, F. Polyphenol composition and antioxidant potential of mint leaves. Food Prod. Process. Nutr. 2019, 1, 1–14. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of malaysia young ginger (zingiber officinale roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Sir Elkhatim, K.A.; Elagib, R.A.A.; Hassan, A.B. Content of phenolic compounds and vitamin c and antioxidant activity in wasted parts of sudanese citrus fruits. Food Sci. Nutr. 2018, 6, 1214–1219. [Google Scholar] [CrossRef]
- Vogeser, M.; Parhofer, K.G. Liquid chromatography tandem-mass spectrometry (lc-ms/ms)—technique and applications in endocrinology. Exp. Clin. Endocrinol. Diabetes 2007, 115, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through LC-ESI-QTOF-MS2 and Their Antioxidant Potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. (Amst) 2019, 24, e00370. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus limon (lemon) phenomenon-a review of the chemistry, pharmacological properties, applications in the modern pharmaceutical, food, and cosmetics industries, and biotechnological studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Tahira, R.; Naeemullah, M.; Akbar, F.; Masood, M.S.J.P.J.B. Major phenolic acids of local and exotic mint germplasm grown in islamabad. Pak. J. Bot. 2011, 43, 151–154. [Google Scholar]
- Tohma, H.; Gülçin, İ.; Bursal, E.; Gören, A.C.; Alwasel, S.H.; Köksal, E. Antioxidant activity and phenolic compounds of ginger (zingiber officinale rosc.) determined by hplc-ms/ms. J. Food Meas. Charact. 2016, 11, 556–566. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural cinnamic acids, synthetic derivatives and hybrids with antimicrobial activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Geromichalos, G. Novel cinnamic acid derivatives as antioxidant and anticancer agents: Design, synthesis and modeling studies. Molecules 2014, 19, 9655–9674. [Google Scholar] [CrossRef]
- Ben Said, R.; Hamed, A.I.; Mahalel, U.A.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Oleszek, W.; Stochmal, A. Tentative characterization of polyphenolic compounds in the male flowers of phoenix dactylifera by liquid chromatography coupled with mass spectrometry and dft. Int. J. Mol. Sci. 2017, 18, 512. [Google Scholar] [CrossRef]
- Gimenez-Bastida, J.A.; Zielinski, H.; Piskula, M.; Zielinska, D.; Szawara-Nowak, D. Buckwheat bioactive compounds, their derived phenolic metabolites and their health benefits. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Raneva, V.; Shimasaki, H.; Ishida, Y.; Ueta, N.; Niki, E.J.L. Antioxidative activity of 3, 4-dihydroxyphenylacetic acid and caffeic acid in rat plasma. Lipids 2001, 36, 1111. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Nakashima, S.; Saiki, S.; Myoi, Y.; Abe, N.; Kuwazuru, S.; Zhu, B.; Ashida, H.; Murata, Y.; Nakamura, Y. 3,4-dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. Uflc-q-tof-ms/ms-based screening and identification of flavonoids and derived metabolites in human urine after oral administration of exocarpium citri grandis extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef]
- Yu, J.; Wang, L.; Walzem, R.L.; Miller, E.G.; Pike, L.M.; Patil, B.S. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J. Agric. Food Chem. 2005, 53, 2009–2014. [Google Scholar] [CrossRef]
- Man, M.Q.; Yang, B.; Elias, P.M. Benefits of hesperidin for cutaneous functions. Evid. Based Complement. Altern. Med. 2019, 2019, 2676307. [Google Scholar] [CrossRef]
- Mcharek, N.; Hanchi, B.J.J.A.B.F.Q. Maturational effects on phenolic constituents, antioxidant activities and lc-ms/ms profiles of lemon (citrus limon) peels. J. Appl. Bot. Food Qual. 2017, 90, 1–9. [Google Scholar]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, E. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Lin, Y.; Fang, S.; Liu, Y.; Shang, X. Phytochemical content and antioxidant activity in aqueous extracts of cyclocarya paliurus leaves collected from different populations. PeerJ 2019, 7, e6492. [Google Scholar] [CrossRef]
- Shirai, M.; Moon, J.-H.; Tsushida, T.; Terao, J.J.J.o.A.; Chemistry, F. Inhibitory effect of a quercetin metabolite, quercetin 3-o-β-d-glucuronide, on lipid peroxidation in liposomal membranes. J. Agric. Food Chem. 2001, 49, 5602–5608. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Nie, B.; Liu, T.; Yuan, F.; Feng, F.; Zhang, Y.; Zhou, W.; Xu, X.; Yao, M.; Zhang, F. Simultaneous determination of coumarin and its derivatives in tobacco products by liquid chromatography-tandem mass spectrometry. Molecules 2016, 21, 1511. [Google Scholar] [CrossRef]
- Al-Majedy, Y.; Al-Amiery, A.; Kadhum, A.A.; BakarMohamad, A. Antioxidant activity of coumarins. Syst. Rev. Pharm. 2016, 8, 24–30. [Google Scholar] [CrossRef]
- Shyamala, B.; Naidu, M.M.; Sulochanamma, G.; Srinivas, P. Studies on the antioxidant activities of natural vanilla extract and its constituent compounds through in vitro models. J. Agric. Food Chem. 2007, 55, 7738–7743. [Google Scholar] [CrossRef]
- Melliou, E.; Zweigenbaum, J.A.; Mitchell, A.E. Ultrahigh-pressure liquid chromatography triple-quadrupole tandem mass spectrometry quantitation of polyphenols and secoiridoids in california-style black ripe olives and dry salt-cured olives. J. Agric. Food Chem. 2015, 63, 2400–2405. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elias, R.; Gepdiremen, A.; Boyer, L. Antioxidant activity of lignans from fringe tree (chionanthus virginicus L.). Eur. Food Res. Technol. 2006, 223, 759–767. [Google Scholar] [CrossRef]
- Huang, X.; Song, F.; Liu, Z.; Liu, S. Studies on lignan constituents from schisandra chinensis (turcz.) baill. Fruits using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. J. Mass Spectrom 2007, 42, 1148–1161. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Yi, H.K. Schisandrin c enhances mitochondrial biogenesis and autophagy in c2c12 skeletal muscle cells: Potential involvement of anti-oxidative mechanisms. Naunyn Schmiedebergs Arch. Pharm. 2018, 391, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Tanweer, S.; Mehmood, T.; Zainab, S.; Ahmad, Z.; Shehzad, A. Comparison and hplc quantification of antioxidant profiling of ginger rhizome, leaves and flower extracts. Clin. Phytosci. 2020, 6. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Variation of the phytochemical constituents and antioxidant activities of zingiber officinale var. Rubrum theilade associated with different drying methods and polyphenol oxidase activity. Molecules 2016, 21, 780. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Astola, J.; Kumpulainen, J. Determination of flavonoids in plant material by hplc with diode-array and electro-array detections. J. Agric. Food Chem. 2000, 48, 5834–5841. [Google Scholar] [CrossRef]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M.J. Quantitation of flavonoid constituents in citrus fruits. J. Agric. Food Chem. 1999, 47, 3565–3571. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (citrus limon burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef]
- Mišan, A.; Mimica-Dukić, N.; Mandić, A.; Sakač, M.; Milovanović, I.; Sedej, I. Development of a rapid resolution hplc method for the separation and determination of 17 phenolic compounds in crude plant extracts. Open Chem. 2011, 9, 133–142. [Google Scholar] [CrossRef]
- Dolzhenko, Y.; Bertea, C.M.; Occhipinti, A.; Bossi, S.; Maffei, M.E. Uv-b modulates the interplay between terpenoids and flavonoids in peppermint (mentha x piperita L.). J. Photochem. Photobiol. B 2010, 100, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kapp, K. Polyphenolic and Essential Oil Composition of Mentha and Their Antimicrobial Effect; Helsingin Yliopisto: Helsinki, Finland, 2015. [Google Scholar]
| Assays | Ginger | Lemon | Mint | GLMT |
|---|---|---|---|---|
| TPC (mg GAE/g) | 2.93 ± 0.07 c | 0.12 ± 0.04 d | 14.35 ± 0.19 a | 5.85 ± 0.08 b |
| TFC (mg QE/g) | 0.98 ± 0.02 c | 0.07 ± 0.03 d | 1.29 ± 0.07 a | 1.25 ± 0.04 b |
| TTC (mg CE/g) | 0.02 ± 0.04 d | 0.04 ± 0.01 c | 2.13 ± 0.08 a | 0.45 ± 0.09 b |
| DPPH (mg AAE/g) | 1.24 ± 0.09 c | 0.09 ± 0.04 d | 3.15 ± 0.12 a | 2.24 ± 0.01 b |
| FRAP (mg AAE/g) | 0.83 ± 0.03 c | 0.08 ± 0.01 d | 7.15 ± 0.14 a | 1.91 ± 0.07 b |
| ABTS (mg AAE/g) | 0.11 ± 0.01 c | 0.07 ± 0.03 d | 24.25 ± 2.18 a | 5.48 ± 0.21 b |
| RPA (mg AAE/g) | 0.08 ± 0.01 a | 0.02 ± 0.03 c | 0.04 ± 0.02 b | 1.01 ± 0.04 b |
| •OH-RSA (mg AAE/g) | 0.53 ± 0.03 b | 0.39 ± 0.07 a | 0.27± 0.01 c | 0.77± 0.08 c |
| FICA (mg EDTA/g) | 0.04 ± 0.03 b | - | 0.09 ± 0.02 a | 0.07 ± 0.04 a |
| TAC (mg AAE/g) | 0.73 ± 0.08 c | 0.03 ± 0.04 d | 6.74 ± 0.58 a | 1.35 ± 0.41 b |
| Assays | TPC | TFC | TTC | DPPH | FRAP | ABTS | RAP | OH-RSA | FICA |
|---|---|---|---|---|---|---|---|---|---|
| TFC | 0.747 | ||||||||
| TTC | 0.971 * | 0.565 | |||||||
| DPPH | 0.942 * | 0.915 * | 0.839 | ||||||
| FRAP | 0.988 * | 0.639 | 0.994 ** | 0.879 | |||||
| ABTS | 0.976 * | 0.583 | 1.000 ** | 0.852 | 0.996 ** | ||||
| RAP | 0.004 | 0.440 | −0.150 | 0.293 | −0.126 | −0.126 | |||
| •OH-RSA | −0.357 | 0.269 | −0.537 | −0.030 | −0.492 | −0.515 | 0.886 * | ||
| FICA | 0.907 * | 0.948 * | 0.783 | 0.995 ** | 0.831 * | 0.798 | 0.353 | 0.055 | |
| TAC | 0.977 * | 0.604 | 0.994 ** | 0.850 | 0.998 ** | 0.993 ** | −0.192 | −0.546 | 0.799 |
| No. | Proposed Compounds | Molecular Formula | RT (min) | Ionisation (ESI+/ESI−) | Molecular Weight | Theoretical (m/z) | Observed (m/z) | Error (ppm) | MS2 Productions | Samples |
|---|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | ||||||||||
| Hydroxybenzoic Acids | ||||||||||
| 1 | Gallic acid 4-O-glucoside | C13H16O10 | 6.580 | [M − H]− | 332.0743 | 331.067 | 331.0684 | 4.2 | 169, 125 | M |
| 2 | 4-Hydroxybenzoic acid 4-O-glucoside | C13H16O8 | 11.898 | [M − H]− | 300.0845 | 299.0772 | 299.077 | −0.7 | 255, 137 | M |
| 3 | 2,3-Dihydroxybenzoic acid | C7H6O4 | 12.378 | [M − H]− | 154.0266 | 153.0193 | 153.0194 | 0.7 | 109 | M |
| 4 | Protocatechuic acid 4-O-glucoside | C13H16O9 | 13.256 | [M − H]− | 316.0794 | 315.0721 | 315.0723 | 0.6 | 153 | M |
| 5 | 2-Hydroxybenzoic acid | C7H6O3 | 19.932 | ** [M − H]− | 138.0317 | 137.0244 | 137.0245 | 0.7 | 93 | M |
| 6 | Paeoniflorin | C23H28O11 | 58.033 | [M − H]− | 480.1632 | 479.1559 | 479.1577 | 3.8 | 449, 357, 327 | L |
| Hydroxycinnamic Acids | ||||||||||
| 7 | p-Coumaroyl tartaric acid | C13H12O8 | 8.232 | ** [M − H]− | 296.0532 | 295.0459 | 295.0466 | 2.4 | 115 | *G, L |
| 8 | Cinnamic acid | C9H8O2 | 9.166 | [M − H]− | 148.0524 | 147.0451 | 147.0448 | −2.0 | 103 | L |
| 9 | Caffeoyl tartaric acid | C13H12O9 | 13.438 | [M − H]− | 312.0481 | 311.0408 | 311.0413 | 1.6 | 161 | M |
| 10 | Ferulic acid | C10H10O4 | 15.708 | ** [M − H]− | 194.0579 | 193.0506 | 193.0515 | 4.7 | 178, 149,134 | L, *M |
| 11 | Caffeic acid 3-O-glucuronide | C15H16O10 | 16.354 | ** [M − H]− | 356.0743 | 355.067 | 355.0685 | 4.2 | 179 | M |
| 12 | 3-p-Coumaroylquinic acid | C16H18O8 | 17.695 | ** [M − H]− | 338.1002 | 337.0929 | 337.0943 | 4.2 | 265, 173, 162 | M |
| 13 | p-Coumaric acid 4-O-glucoside | C15H18O8 | 19.137 | [M − H]− | 326.1002 | 325.0929 | 325.093 | 0.3 | 163 | M |
| 14 | m-Coumaric acid | C9H8O3 | 19.153 | [M − H]− | 164.0473 | 163.04 | 163.0395 | −3.1 | 119 | M |
| 15 | Caffeic acid 4-sulfate | C9H8O7S | 19.248 | [M + H]+ | 259.9991 | 261.0064 | 261.0057 | −2.7 | 179, 135 | M |
| 16 | 3-Caffeoylquinic acid | C16H18O9 | 19.766 | ** [M − H]− | 354.0951 | 353.0878 | 353.0878 | 0.0 | 253, 190, 144 | L, *M |
| 17 | Feruloyl tartaric acid | C14H14O9 | 22.185 | [M − H]− | 326.0638 | 325.0565 | 325.0573 | 2.5 | 193, 149 | M |
| 18 | Ferulic acid 4-O-glucoside | C16H20O9 | 25.779 | ** [M − H]− | 356.1107 | 355.1034 | 355.1019 | −4.2 | 193, 178, 149, 134 | M |
| 19 | Chicoric acid | C22H18O12 | 35.138 | [M − H]− | 474.0798 | 473.0725 | 473.0753 | 5.0 | 293, 311 | M |
| 20 | 1-Sinapoyl-2-feruloylgentiobiose | C33H40O18 | 35.768 | [M − H]− | 724.2215 | 723.2142 | 723.2184 | 4.1 | 529, 499 | M |
| 21 | 1,5-Dicaffeoylquinic acid | C25H24O12 | 48.341 | ** [M − H]− | 516.1268 | 515.1195 | 515.122 | 4.9 | 353, 335, 191, 179 | M |
| Hydroxyphenylacetic Acids | ||||||||||
| 22 | 3,4-Dihydroxyphenylacetic acid | C8H8O4 | 10.059 | ** [M − H]− | 168.0423 | 167.035 | 167.0354 | 2.4 | 149, 123 | G, *M |
| 23 | 2-Hydroxy-2-phenylacetic acid | C8H8O3 | 15.310 | ** [M − H]− | 152.0473 | 151.04 | 151.0408 | 3.8 | 136, 92 | M |
| Hydroxyphenylpropanoic Acids | ||||||||||
| 24 | Dihydroferulic acid 4-O-glucuronide | C16H20O10 | 6.978 | ** [M − H]− | 372.1056 | 371.0983 | 371.0999 | 4.3 | 195 | M |
| Flavonoids | ||||||||||
| Flavanols | ||||||||||
| 25 | 4’-O-Methylepigallocatechin | C16H16O7 | 33.560 | [M + H]+ | 320.0896 | 321.0969 | 321.0958 | −3.4 | 302 | L, *M |
| 26 | 4’-O-Methyl-(-)-epigallocatechin 7-O-glucuronide | C22H24O13 | 48.622 | [M − H]− | 496.1217 | 495.1144 | 495.115 | 1.2 | 451, 313 | M |
| 27 | (+)-Catechin 3-O-gallate | C22H18O10 | 50.445 | ** [M − H]− | 442.09 | 441.0827 | 441.0833 | 1.4 | 289, 169, 125 | M |
| 28 | (+)-Gallocatechin 3-O-gallate | C22H18O11 | 63.448 | ** [M − H]− | 458.0849 | 457.0776 | 457.0794 | 3.9 | 305, 169 | M |
| Flavones | ||||||||||
| 29 | Apigenin 6,8-di-C-glucoside | C27H30O15 | 26.791 | ** [M − H]− | 594.1585 | 593.1512 | 593.1556 | 4.7 | 503, 473 | G, *L, M-T |
| 30 | Apigenin 7-O-apiosyl-glucoside | C26H28O14 | 31.067 | [M + H]+ | 564.1479 | 565.1552 | 565.1545 | −1.2 | 296 | *G, L, M-T |
| 31 | 7,4’-Dihydroxyflavone | C15H10O4 | 37.337 | [M + H]+ | 254.0579 | 255.0652 | 255.0659 | 2.7 | 227, 199, 171 | M |
| 32 | 6-Hydroxyluteolin 7-O-rhamnoside | C21H20O11 | 39.131 | ** [M − H]− | 448.1006 | 447.0933 | 447.095 | 3.8 | 301 | L, *M |
| 33 | Rhoifolin | C27H30O14 | 43.471 | ** [M − H]− | 578.1636 | 577.1563 | 577.1582 | 3.3 | 413, 269 | L, *M |
| 34 | Cirsilineol | C18H16O7 | 45.338 | [M + H]+ | 344.0896 | 345.0969 | 345.0966 | −0.9 | 330, 312, 297, 284 | L, *M |
| 35 | Apigenin 7-O-glucuronide | C21H18O11 | 47.673 | [M + H]+ | 446.0849 | 447.0922 | 447.0901 | −4.7 | 271, 253 | M |
| 36 | Chrysoeriol 7-O-glucoside | C22H22O11 | 54.565 | [M + H]+ | 462.1162 | 463.1235 | 463.1234 | −0.2 | 445, 427, 409, 381 | L, *M |
| 37 | Diosmin | C28H32O15 | 59.17 | ** [M + H]+ | 608.1741 | 609.1814 | 609.1819 | 0.8 | 301, 286 | L, *M |
| Flavanones | ||||||||||
| 38 | Naringin 4’-O-glucoside | C33H42O19 | 25.233 | [M − H]- | 742.232 | 741.2247 | 741.2279 | 4.3 | 433, 271 | M |
| 39 | Neoeriocitrin | C27H32O15 | 36.946 | ** [M − H]− | 596.1741 | 595.1668 | 595.1658 | −1.7 | 431, 287 | *L, M |
| 40 | Hesperidin | C28H34O15 | 42.745 | ** [M + H]+ | 610.1898 | 611.1971 | 611.1956 | −2.5 | 593, 465, 449, 303 | *L, M |
| Flavonols | ||||||||||
| 41 | Quercetin 3’-O-glucuronide | C21H18O13 | 12.512 | ** [M − H]− | 478.0747 | 477.0674 | 477.067 | −0.8 | 301 | *L, M |
| 42 | Quercetin 3-O-glucosyl-xyloside | C26H28O16 | 15.395 | [M − H]− | 596.1377 | 595.1304 | 595.1299 | −0.8 | 265, 138, 116 | L |
| 43 | Kaempferol 3,7-O-diglucoside | C27H30O16 | 23.162 | ** [M − H]− | 610.1534 | 609.1461 | 609.1486 | 4.1 | 447, 285 | L, *M |
| 44 | Kaempferol 3-O-(2’’-rhamnosyl-galactoside) 7-O-rhamnoside | C33H40O19 | 34.660 | ** [M − H]− | 740.2164 | 739.2091 | 739.2114 | 3.1 | 593, 447, 285 | G, *L, M-T |
| 45 | Kaempferol 3-O-glucosyl-rhamnosyl-galactoside | C33H40O20 | 37.254 | ** [M − H]− | 756.2113 | 755.204 | 755.2037 | −0.4 | 285 | *G, L, M-T |
| 46 | Myricetin 3-O-rhamnoside | C21H20O12 | 39.479 | ** [M − H]− | 464.0955 | 463.0882 | 463.0874 | −1.7 | 317 | L, *M |
| 47 | 3-Methoxysinensetin | C21H22O8 | 61.671 | [M + H]+ | 402.1315 | 403.1388 | 403.1388 | 0.0 | 388, 373, 355, 327 | M |
| 48 | Myricetin 3-O-rutinoside | C27H30O17 | 81.239 | ** [M − H]− | 626.1483 | 625.141 | 625.1404 | −1.0 | 301 | L, *M |
| Dihydroflavonols | ||||||||||
| 49 | Dihydromyricetin 3-O-rhamnoside | C21H22O12 | 39.926 | [M − H]− | 466.1111 | 465.1038 | 465.1051 | 2.8 | 301 | M |
| Anthocyanins | ||||||||||
| 50 | Isopeonidin 3-O-arabinoside | C21H21O10 | 29.965 | [M + H]+ | 433.1135 | 434.1208 | 434.1213 | 1.2 | 271, 253, 243 | M |
| Isoflavonoids | ||||||||||
| 51 | 3’-O-Methylviolanone | C18H18O6 | 12.494 | ** [M − H]− | 330.1103 | 329.103 | 329.1041 | 3.3 | 314, 299, 284, 256 | G, *M |
| 52 | Sativanone | C17H16O5 | 14.051 | [M − H]− | 300.0998 | 299.0925 | 299.0919 | −2.0 | 284, 269, 225 | M |
| 53 | 2’-Hydroxyformononetin | C16H12O5 | 28.896 | [M + H]+ | 284.0685 | 285.0758 | 285.076 | 0.7 | 270, 229 | *L, M |
| 54 | 5,6,7,3’,4’-Pentahydroxyisoflavone | C15H10O7 | 31.563 | ** [M + H]+ | 302.0427 | 303.05 | 303.0501 | 0.3 | 285, 257 | *L, M |
| 55 | 3’-Hydroxygenistein | C15H10O6 | 39.466 | ** [M + H]+ | 286.0477 | 287.055 | 287.0543 | −2.4 | 269, 259 | *G, L, M-T |
| 56 | 2’,7-Dihydroxy-4’,5’-dimethoxyisoflavone | C17H14O6 | 41.246 | [M + H]+ | 314.079 | 315.0863 | 315.085 | −4.1 | 300, 282 | M |
| 57 | 6’’-O-Acetylglycitin | C24H24O11 | 45.345 | ** [M + H]+ | 488.1319 | 489.1392 | 489.1378 | −2.9 | 285, 270 | *L, M |
| 58 | 3’-Hydroxydaidzein | C15H10O5 | 46.895 | [M + H]+ | 270.0528 | 271.0601 | 271.0603 | 0.7 | 253, 241, 225 | L, *M |
| 59 | Glycitin | C22H22O10 | 70.633 | [M + H]+ | 446.1213 | 447.1286 | 447.1276 | −2.2 | 285 | M |
| Other Phenolic Compounds | ||||||||||
| Hydroxycoumarins | ||||||||||
| 60 | Coumarin | C9H6O2 | 60.230 | [M + H]+ | 146.0368 | 147.0441 | 147.0436 | −3.4 | 103, 91 | M |
| Hydroxybenzaldehydes | ||||||||||
| 61 | 4-Hydroxybenzaldehyde | C7H6O2 | 19.932 | [M − H]− | 122.0368 | 121.0295 | 121.0299 | 3.3 | 77 | M |
| Hydroxybenzoketones | ||||||||||
| 62 | 2,3-Dihydroxy-1-guaiacylpropanone | C10H12O5 | 13.157 | ** [M − H]− | 212.0685 | 211.0612 | 211.062 | 3.8 | 167, 123, 105, 93 | M |
| Phenolic Terpenes | ||||||||||
| 63 | Carnosic acid | C20H28O4 | 80.86 | [M − H]− | 332.1988 | 331.1915 | 331.1922 | 2.1 | 287, 269 | L |
| Tyrosols | ||||||||||
| 64 | Hydroxytyrosol 4-O-glucoside | C14H20O8 | 10.49 | [M − H]− | 316.1158 | 315.1085 | 315.109 | 1.6 | 153, 123 | M |
| 65 | Oleoside 11-methylester | C17H24O11 | 14.217 | [M − H]− | 404.1319 | 403.1246 | 403.1262 | 4.0 | 223, 165 | M |
| 66 | 3,4-DHPEA-AC | C10H12O4 | 33.080 | ** [M − H]− | 196.0736 | 195.0663 | 195.0671 | 4.1 | 135 | *G, L, M-T |
| Other Phenolic Compounds | ||||||||||
| 67 | Lithospermic acid | C27H22O12 | 49.119 | ** [M − H]− | 538.1111 | 537.1038 | 537.1054 | 3.0 | 493, 339, 295 | M |
| 68 | Salvianolic acid B | C36H30O16 | 76.568 | ** [M − H]− | 718.1534 | 717.1461 | 717.1491 | 4.2 | 519, 339, 321, 295 | M |
| Lignans | ||||||||||
| 69 | 7-Oxomatairesinol | C20H20O7 | 30.089 | [M + H]+ | 372.1209 | 373.1282 | 373.1297 | 4.0 | 358, 343, 328, 325 | L |
| 70 | Conidendrin | C20H20O6 | 45.653 | [M + H]+ | 356.126 | 357.1333 | 357.1325 | −2.2 | 339, 221, 206 | M |
| 71 | Pinoresinol | C20H22O6 | 50.544 | [M − H]− | 358.1416 | 357.1343 | 357.1364 | 1.3 | 342, 327, 313, 221 | M |
| 72 | Schisandrin C | C22H24O6 | 80.132 | ** [M + H]+ | 384.1573 | 385.1646 | 385.163 | −4.2 | 370, 315, 300 | G, *L |
| Stilbenes | ||||||||||
| 73 | 4-Hydroxy-3,5,4’-trimethoxystilbene | C17H18O4 | 76.456 | [M + H]+ | 286.1205 | 287.1278 | 287.1266 | −4.2 | 271, 241, 225 | G |
| No. | Compound Name | Ginger (mg/g) | Lemon (mg/g) | Mint (mg/g) | GLMT (mg/g) |
|---|---|---|---|---|---|
| 1 | Gallic acid | 3.21 ± 0.15 d | 4.42 ± 0.25 c | 7.21 ± 0.12 a | 6.85 ± 0.08 b |
| 2 | Protocatechuic acid | 2.36 ± 0.14 b | - | 4.27 ± 0.13 a | 2.16 ± 0.11 c |
| 3 | p-Hydroxybenzoic acid | 7.87 ± 0.23 a | 3.17 ± 0.023 c | 6.37 ± 0.31 b | 7.84 ± 0.36 a |
| 4 | Chlorogenic acid | 15.78 ± 1.12 d | 21.45 ± 1.72 b | 18.79 ± 1.05 c | 31.47 ± 1.86 a |
| 5 | Caffeic acid | 4.39 ± 0.18 b | 2.16 ± 0.02 d | 3.47 ± 0.05 c | 8.73 ± 0.40 a |
| 6 | Catechin | 11.95 ± 0.48 c | 4.56 ± 0.09 d | 17.87 ± 0.91 b | 21.56 ± 1.42 a |
| 7 | Epicatechin | 2.34 ± 0.03 c | - | 5.43 ± 0.33 a | 3.71 ± 0.02 b |
| 8 | Epicatechin gallate | - | 1.25 ± 0.05 b | 3.42 ± 0.14 a | 3.32 ± 0.10 a |
| 9 | Quercetin | 32.56 ± 1.00 a | 6.78 ± 0.26 d | 8.45 ± 0.40 c | 17.76 ± 0.66 b |
| 10 | Kaempferol | 14.37 ± 0.66 a | 7.98 ± 0.34 d | 11.43 ± 0.29 b | 9.74 ± 0.32 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, O.; Ali, A.; Subbiah, V.; Barrow, C.J.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential. Fermentation 2021, 7, 73. https://doi.org/10.3390/fermentation7020073
Chou O, Ali A, Subbiah V, Barrow CJ, Dunshea FR, Suleria HAR. LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential. Fermentation. 2021; 7(2):73. https://doi.org/10.3390/fermentation7020073
Chicago/Turabian StyleChou, Osbert, Akhtar Ali, Vigasini Subbiah, Colin J. Barrow, Frank R. Dunshea, and Hafiz A. R. Suleria. 2021. "LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential" Fermentation 7, no. 2: 73. https://doi.org/10.3390/fermentation7020073
APA StyleChou, O., Ali, A., Subbiah, V., Barrow, C. J., Dunshea, F. R., & Suleria, H. A. R. (2021). LC-ESI-QTOF-MS/MS Characterisation of Phenolics in Herbal Tea Infusion and Their Antioxidant Potential. Fermentation, 7(2), 73. https://doi.org/10.3390/fermentation7020073









