Abstract
In Taiwan, adult hyperuricemia affects as many as 1 in 4 males and 1 in 6 females, who are predominantly young adults aged 19–45. In this study, lactic acid bacteria (LAB) with acid tolerance, bile salt tolerance and high affinity to intestinal cells were extracted from the side products of alcohol fermentation (distillers’ grains). These bacteria were evaluated for their ability to lower uric acid levels. Qualitative identification and quantitative analysis were performed using high-performance liquid chromatography (HPLC) on the purine-degrading enzymes to select purine-decomposing LAB for animal testing. When the final concentration of purine compounds reached 0.1% and 1%, seven strains of LAB showed potential in degrading purine compounds. HPLC was used to analyze their purine-degrading abilities, and the three best performing LAB strains, (107) 8–16, (107) tau 1–3, and (107) 6–10 were screened for further animal testing with Wistar rats. By the third week, the results showed that strain (107) 6–10 could prevent formation and reduce the levels of blood urea nitrogen (BUN) in yeast extract/potassium oxonate-induced hyperuricemia. The multi-strain lactic acid bacteria (MLAB) performed best for uric acid reduction in the serum and down regulated BUN. Yeast extract/potassium oxonate-induced hyperuricemia had no impact on serum creatinine, while LAB did not affect the creatinine concentration. In summary, MLAB not only protects kidney function but is also effective in regulating uric acid concentration in the body. Hence, MLAB can be used as a functional food supplement that prevents or aids the treatment of hyperuricemia in a rodent model.
1. Introduction
Uric acid (UA) is a metabolite of purine and is mainly excreted by the kidneys, with only a small portion removed through the intestinal tract []. The in vivo uric acid concentration is determined by the balance between food content, in vivo synthesis and excretion []. When renal function is normal, uric acid can be filtered by the glomeruli and excreted in the urine. Blood uric acid concentration rises when synthesis increases too much or excretion becomes insufficient []. Blood urea nitrogen (BUN) is filtered into the urine by the glomerulus. Impaired renal function raises the blood urea nitrogen concentration, which can be used as an indicator of renal function []. Creatinine (CRE) is a product of phosphocreatine metabolism in the muscles. The CRE level reflects the blood CRE content and glomerular filtration rate. CRE concentration increases under abnormal renal function [].
Hyperuricemia, caused by abnormal uric acid levels in the blood, forms solid uric acid crystals in the joints, thus engendering pain []. Hyperuricemia is also considered a risk factor for atherosclerosis and may lead to metabolic syndrome, hypertension, chronic diabetes and kidney disease []. The potential causative factors for hyperuricemia include insulin resistance, hypertension, renal insufficiency, obesity, diet and alcohol consumption []. Hyperuricemia occurs when there is an excessively high level of uric acid in the blood. The cause of the disease may be ingesting purine-rich foods or foods that promote purine synthesis, increased in vivo synthesis and inhibition of renal metabolism []. In human purine metabolism, inosine and guanosine are the primary purine compounds that form uric acid. Current uric acid-lowering drugs may sometimes present gastrointestinal side effects, such as nausea and diarrhea, as well as mild skin rashes and, rarely, fatalities [,].
Probiotics are acid-resistant and salt-resistant, can survive in the host’s digestive tract and adhere to intestinal epithelial cells to alter the gut microbiota. Probiotics can also maintain their viability under prolonged storage []. The intestinal epithelial cells form a natural barrier against foreign objects. Upon entering the host intestinal tract, a probiotic strain may adhere to epithelial cells to prevent expulsion by peristalsis, help maintain microbiota balance, reduce the invasion and adhesion of pathogens and promote host immune function. Therefore, adsorption can be used as a criterion for screening beneficial probiotics [].
LAB are common probiotics in the host gut. In addition to promoting gut health, LAB also have immunoregulation and anti-allergy effects and may regulate blood pressure, blood lipids and blood glucose []. Recent literature has shown that LAB have the potential to lower uric acid []. Therefore, probiotics capable of effectively degrading purine compounds could represent a potential therapy to prevent hyperuricemia []. Studies have proven that a Lactobacillus plantarum strain, DM9218-A, reduces the serum uric acid concentration in hyperuricemic mice. This strain could thus serve as a supportive treatment for hyperuricemia patients and help to prevent hyperuricemia in healthy people []. In a clinical trial, the L. gasseri strain PA-3 was used to treat 25 hyperuricemia/gout patients. The results showed that the PA-3-containing yogurt improved serum uric acid levels []. Another study showed that L. paracasei obtained from screening 18 types of traditional pickles degraded uric acid in vitro and in vivo and demonstrated the potential of LAB to reduce renal damage in a rat model of chronic hyperuricemia []. The L. brevis strain DM9218 was shown to reduce serum uric acid levels and liver xanthine oxidative activity in rats and may have the potential to ameliorate fructose-induced hyperuricemia [].
The present study aims to screen for strains with essential probiotic characteristics from fermented cereals, investigate the uric acid-lowering potential of those strains and compare those strains with common gut health supplements on the market. Thirty-three LAB strains were cultured in a differential medium supplemented with purines to screen for LAB strains that could degrade purines. Subsequently, a qualitative purine compound degradation assay and a quantitative HPLC analysis on the degraded purine compounds were used to identify strains capable of degrading and utilizing purines. These LAB strains were used in animal tests to evaluate the effectiveness of preventing hyperuricemia.
2. Materials and Methods
2.1. Strains, Culture Medium and Basic Growth Conditions
The 33 LAB strains used in this study were screened and isolated from winemaking byproducts obtained from the Taiwan Tobacco and Liquor Corporation (Taipei, Taiwan) and exhibited essential probiotic characteristics. The strains were suspended in an MRS broth containing 15% glycerol and stored in a −80 °C freezer. The strains were activated twice before the experiments using a Lactobacilli MRS broth (Difco Laboratories, Detroit, MI, USA) containing 0.05% L-cysteine. The culture condition was 37 °C for 24 h.
2.2. Screening for Lab Strains with Purine Degradation Abilities
The strains were activated once using Lactobacilli MRS broth (Difco Laboratories, Detroit, MI, USA) containing 0.05% L-cysteine at 37 °C for 16–24 h. For the second activation, 100 μL of a culture broth from the first activation was inoculated into 4 mL MRS broth without purines and cultured at 37 °C for 16–18 h. The culture was then serially diluted, and the bacterial counts were enumerated by plating. The result represented the number of normal bacteria. For the purine test, inosine and guanosine were added to 4 mL MRS broth to a final concentration of 0.1% and 1%, respectively. Next, 100 μL culture from the first activation was inoculated into the MRS broth and cultured at 37 °C for 16–18 h. The broth was then serially diluted, and bacterial counts were enumerated by plating. The solid medium used in the above experiments was Lactobacilli MRS agar (Difco Laboratories, Detroit, MI, USA) containing 0.05% L-cysteine. The culture condition was 37 °C for 24–48 h.
2.3. HPLC Analysis of Degraded Purine by Lab Strains
Strains obtained by screening were activated twice. After activation, the bacterial cells were collected via centrifuge at 8000× g for 10 min. The cells were then washed twice with PBS, and the supernatant was discarded. Next, 1 mL inosine (1.25 mM) and guanosine (1.25 mM) were added to the washed cells and mixed evenly before incubating at 37 °C/140× g. During the experiment, co-cultures were sampled at 0, 1, and 2 h and centrifuged at 4 °C/6000× g/7 min. In total, 720 µL of the supernatant was collected and mixed with 80 µL HClO4 (0.1 M) to stop the reaction. The collected supernatant was passed through a 0.22 μm filter and stored at −80 °C until HPLC analysis. The formula for calculating the degradation rate of inosine and guanosine by different probiotic strains was as follows: degradation rate (%) = (1.25 (mM) − A (mM))/1.25 (mM) × 100% (A: concentration of inosine and guanosine after reaction).
2.4. Acid Resistance and Bile Salt Resistance Tests
Phosphate buffer with pepsin (pH 2.0) was added to 1000 μL of LAB suspension (108 CFU/ mL), and the bacteria were cultured at 37 °C for 0, 1.5 and 3 h, respectively. LAB suspension mixed with phosphate buffer (pH 7.0) and cultured under the same conditions was used as the control. After culturing, the counts of viable LAB were enumerated using the pour plate method on MRS agar plates []. One mL of LAB suspension that underwent 3 h of acid treatment was then added to 9 mL of phosphate buffer with (or without) 0.3% oxgall bile (Sigma-Aldrich Corp., St. Louis, MO, USA) and 0.1% pancreatin [] and cultured for 0, 1.5 and 3 h. LAB counts were enumerated afterward using the pour plate method on MRS agar plates. Strains with acid resistance of >107 CFU /mL and bile salt resistance of >106 CFU /mL were selected.
2.5. Caco-2 Cell Adhesion Test
One milliliter of Caco-2 cell suspension (5 × 104 cell /mL) in DMEM (Dulbecco’s Modified Eagle Medium) was added per well in a 24-well plate and cultured at 37 °C for 40–45 h in a 5% CO2 incubator. One milliliter of LAB suspension was added to an Eppendorf tube and centrifuged at 8000× g for 10 min. The supernatant was then discarded, and 1 mL 1 × PBS was added and mixed evenly before another centrifugation. Following that, the pellet was resuspended in 1 mL DMEM.
The old culture medium was aspirated from the 24-well plates containing Caco-2 cells, and the plates were washed twice with 1 × PBS. Nine-hundred microliters of fresh culture medium and 100 μL PBS washed LAB suspension were then added to the plates. The plates were co-cultured at 37 °C in an incubator with 5% CO2 for 2 h. To fix the bacteria and cells, the culture medium was aspirated, and the plates were washed twice with 1 × PBS, followed by adding 200 μL 6–10% formalin to each well and letting the plates stand for 30 min. The formalin was then aspirated, and the plates were washed twice with 1 × PBS. Following that, 200 μL crystal violet solution was added and allowed to react for 5 min. The number of bacteria that adhered to the cells was counted using an inverted microscope []. The bacteria were considered to have an adhesion ability if 15 or more bacteria adhered to each cell [].
2.6. Animal Tests on the Uric Acid-Lowering Effects of Lab Strains
The animal experiment protocol approval number is HK-10805 (date: 5 July 2019) from HungKuang University, Taichung, Taiwan. The experimental animals included 56 30-day-old (about four weeks) male Wistar rats, which were purchased from BioLASCO Taiwan Co. Ltd. (Taipei, Taiwan). The temperature in the animal room was controlled at 23 ± 1 °C and the relative humidity at 40–60%. An automatic controller was used to create a 12/12 h light-dark cycle (light period: 8:00 to 20:00; dark period: 20:00 to 8:00). The animals were kept in individually ventilated cages (IVC) and given ad libitum access to feed and sterile distilled water. After one week of acclimatization, S-shaped cages were used for grouping, with eight rats per group. A total of seven groups were established to test the uric acid-lowering effects of LAB strains from different sources. These groups included a blank control (C), a multi-strain LAB blank control (CM), a hyperuricemia induction group (HUA), a hyperuricemia induction + strain (107) 8–16 treatment group, a hyperuricemia induction + strain (107) 1–3 treatment group, a hyperuricemia induction + strain (107) 6–10 treatment group and a hyperuricemia induction + multi-strain LAB treatment group (MLAB). Intraperitoneal injection of potassium oxonate (0.35 mg/100 g BW/day) and gavage with yeast extract (15 g/kg BW/day) were used to induce hyperuricemia in the rats (Figure 1).
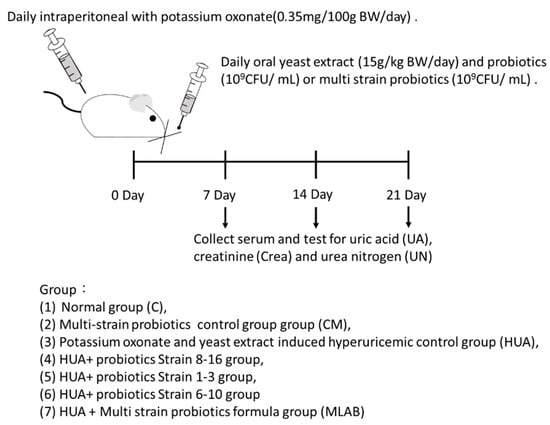
Figure 1.
Animal model experimental structure and schedule.
Strains obtained from screening were activated twice. After activation, the culture medium was centrifuged at 9000× g for 10 min to collect the bacteria. The bacterial cells were washed twice with PBS, and the supernatant was discarded. The washed bacterial cells were then resuspended in PBS, the volume of which was the same as that of the MRS used in the second culture. The suspension was stored at 4 °C for up to 3 days. The dose of MLAB used in each group was 109 CFU/mL, and the ratio of MLAB used was 1:1:1. One milliliter of LAB was fed to the rats after the daily induction procedure. Weight and food intake were recorded every week, and fasting venous blood was collected. The isolated serum was measured for uric acid, creatinine and urea nitrogen contents (Figure 1).
2.7. Statistical Analysis
The results of this study are based on statistical analyses performed using SPSS 23.00 for Windows (IBM Corporation, Armonk, NY, USA). The experimental results are expressed as the mean ± standard deviation (SD) based on a single factor one-way analysis of variance (one-way ANOVA) test. Duncan’s Multiple Range Test was used to test the difference between the averages of the experimental groups, and p < 0.05 was considered as a significant difference with a two-tailed test.
3. Results
3.1. Screening for LAB Strains with Potential Purine Degradation Abilities
When the final concentration of purines was 0.1% and 1%, the bacterial counts of most strains were not severely affected (Table 1). However, the bacterial counts for (107) 8–16, (107) tau 3–5, (107) tau 2–1, (107) 6–10 and (107) tau 2–8 were slightly increased (from 0.99 to 0.74 Log CFU/mL) compared to the original bacterial count. Moreover, the bacterial counts for (107) tau 6–2 and (107) tau 1–3 were increased by 1 log CFU/mL (Table 1). If the addition of purines causes the bacterial count to remain constant or increase, the strain may be able to utilize purines for growth. Therefore, we hypothesized that the above seven probiotic strains might be able to degrade purines. Subsequently, HPLC was used to determine the ratio of degraded purines in the above seven strains.

Table 1.
Tolerance of lactic acid bacteria to the purine compound medium.
The excessive intake of purines in the diet will increase serum uric acid levels, which is a risk factor for hyperuricemia and gout. Lifestyle guides recommend nutritional therapy, such as caloric restriction, to reduce obesity and limit purine intake, as well as to restrict alcoholic beverage intake and engage in adequate exercise to avoid raising serum uric acid levels []. The ability of probiotics to degrade purine compounds in food to prevent hyperuricemia was previously reported [].
3.2. HPLC Analysis of the Ratio of Inosine and Guanosine Degraded by Lab Strains
Microorganisms degrade nucleosides mainly through the biosynthesis of nucleoside hydrolases. The existence of nucleoside hydrolase in the strain can be proven by the degradation of nucleoside substances, such as guanosine and inosine [,]. The addition of various ratios of purines was used to screen for LAB strains capable of degrading purines. Seven strains were isolated: (107) tau 6–2, (107) tau 2–1, (107) 6–10, (107) tau 2–8, (107) 8–16, (107) tau 1–3 and (107) tau 3–5.
Figure 2 showed the inosine degradation experiment results for the LAB strains. At all time points, the inosine degradation rates of all strains were 95% or higher. The preferred strains were (107) 8–16 and (107) 6–10. Both strains maintained stable degradation rates of 99% across all time points. The next best strains were (107) tau 1–3 and (107) tau 3–5. Although (107) tau 1–3 had a degradation rate lower than that of the preferred strains, it reached 95% and above and showed an increasing trend. On the other hand, (107) tau 3–5showed a reduction to 97% at 1 h but then rose to 99% at 2 h. Therefore, both strains were retained as candidates. The analysis of guanosine degradation by LAB found that the guanosine degradation rates of all tested strains reached 95% or higher (Figure 3). The preferred strain was (107) tau 1–3, with a degradation rate reaching 99.5% and a stable increase. The second was (107) tau 2–8, with an overall stable degradation rate of 99.5%. In third place were (107) 8–16 and (107) tau 2–1. Although the rate of (107) 8–16 dropped to 99.36% at 2 h, the overall degradation rate was high. There was a stable degradation rate in (107) tau 2–1 at 0 and 1 h, which increased at 2 h (Figure 3). The guanosine degradation rates of the (107) 6–10 strains were 99.2, 98.8 and 98.9% at different time point (Figure 3). In summary, the HPLC analysis of inosine degradation tested strains (107) 8–16, (107) 6–10, (107) tau 1–3 and (107) tau 3–5, while the analysis of guanosine degradation tested strains (107) tau 1–3, (107) tau 2–8, (107) 8–16 and (107) tau 2–1.
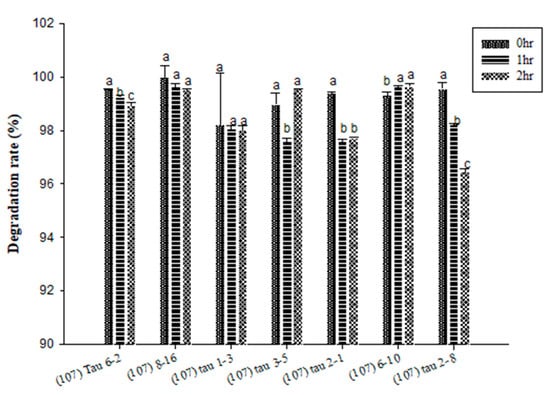
Figure 2.
The degradation effect of potential probiotics on the purine compound inosine at different time points. a,b,c values in different superscripts indicate a significant difference (p < 0.05) using Duncan’s multiple range test.
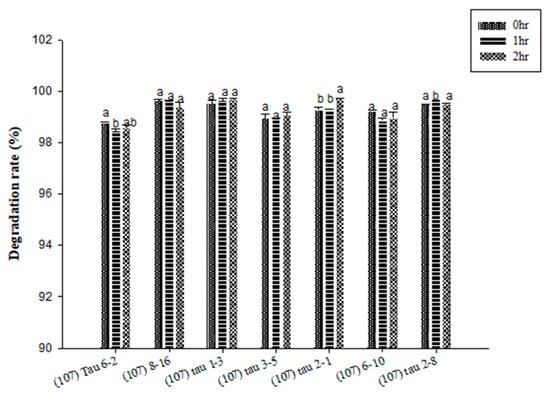
Figure 3.
The degradation effect of potential probiotics on the purine compound guanosine at different time points. a,b values in different superscripts indicate a significant difference (p < 0.05) using Duncan’s multiple range test.
3.3. Bacteria Identification
The API kit was used for strain identification during the HPLC analysis. As shown in the appendix, (107) tau 2–1, (107) tau 2–8, and 6–10 were identified as Lactobacillus paracasei, (107) 8–16 as L. plantarum, (107) tau 3–5 as L. curvatus and (107) tau 1–3 as Pediococcus acidilactici. However, the API results showed other possible bacterial species and streaking was used to isolate single strains for further identification. The current literature proves that the LAB able to reduce hyperuricemia includes L. plantarum, L. brevis, L. gasseri and L. paracasei [,,,]. In this study, we found that L. curvatus and P. acidilactici isolated from distillers’ grains might also have the ability to degrade purine and reduce uric acid.
3.4. Acid and Bile Salt Resistance and Intestinal Cell Adhesion Tests
The bacterial counts of LAB strains (107) 8–16 and (107) 6–10 at 3 h were at least 108 CFU/mL, indicating acid resistance (Table 2). The bacterial counts of LAB strains (107) 8–16 and (107) 6–10 at 3 h were at least 107 CFU/mL, indicating bile salt resistance (Table 2). After 2 h of co-culture with Caco-2 cells, the adhesion capacity of LAB strains (107) 8–16 and (107) 6–10 was 26.8 ± 3.77 CFU/cell and 27.8 ± 9.94 CFU/cell, respectively, which was higher than the 15 CFU/cell threshold, indicating that both strains could adhere to intestinal epithelial cells (Figure 4).

Table 2.
Acid and bile salt tolerance of LAB strains.
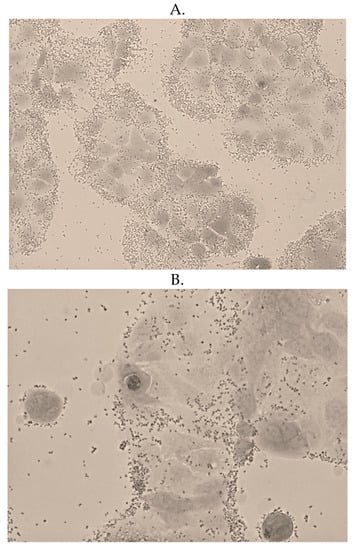
Figure 4.
Adhesion of LAB (A) strain (107) 6–10 and (B) strain (107) 8–16 to the Caco-2 intestinal epithelium cells.
The biological characteristics of tolerance to acid and bile salt and cell adhesion abilities were important to determine whether the tested LAB strains could survive in and successfully colonize the gastrointestinal tract []. Current studies demonstrate that the factors facilitating Lactobacillus adhesion on epithelial cells include hydrophobic effects, lipoteichoic acids (LTA), lectins, proteins and exopolysaccharides (EPA) [,,,]. EPA often attaches to cell surfaces or is secreted into an extracellular medium. One study noted that the removal of EPA from the cell surface of L. plantarum significantly decreased adhesion []. The same study also showed that adhesion was affected by trypsin treatment []. Moreover, probiotics mainly exert their effects in the posterior segment of the gut. Therefore, probiotics must be able to resist gastric acid and bile acid before reaching the site of effect. Studies show that gastric acid pH changes with the time of gastric juice secretion and type of food, usually fluctuating between pH 1.5 and 4.58 over 4 h. The survival of LAB is easily affected by an extremely low pH. The main constituents of bile salts are mixtures of glycine or taurine and bile acids, as well as chenodeoxycholic acid, deoxycholic acid, lithocholic acids and their derivatives. The presence of bile salts changes the permeability of the outer membrane of the intestinal bacteria, and bile acids in bile salts also inhibit the growth of intestinal bacteria [,].
3.5. Animal Tests to Determine the Uric-Acid-Lowering Effects of the Lab Strains
The HPLC analysis of inosine degradation tested strains (107) 8–16, (107) 6–10, (107) tau 1–3, and (107) tau 3–5, while the analysis of guanosine degradation tested strains (107) tau 1–3, (107) tau 2–8, (107) 8–16 and (107) tau 2–1. The three probiotic strains used for animal experiments were (107) 8–16, (107) tau 1–3 and (107) 6–10. The criterium for selecting these three strains was their high inosine and guanosine degradation rates in the in vitro experiments. Therefore, these three probiotic strains were expected to show positive effects in the animal tests.
The uric acid results in the experimental animals are shown in Figure 5. There were no significant differences among the different groups at week 0 (p > 0.05). At week 2, the uric acid concentration in the HUA group was 17.86% higher than that in group C (p < 0.05). After induction and feeding with LAB strains from different sources, the serum uric acid concentrations in the treatment groups, including in strain (107) 8–16, (107) tau 1–3, (107) 6–10, and MLAB, were significantly lower than those in the HUA group (p < 0.05) by 16.43%, 20% and 32.14%, respectively. Among these groups, uric acid reduction was the greatest in the MLAB group, while there was no significant difference between the strain (107) tau 1–3 treatment group and the HUA group (p > 0.05). There was no significant difference between the CM and C groups (p > 0.05). At week 3, the uric acid concentration in the HUA group was 23% higher than that in the C group (p < 0.05). After induction and feeding with LAB strains from different sources, the serum uric acid concentrations in the treatment groups, including strains (107) 8–16, (107) tau 1–3, (107) 6–10, and MLAB, were significantly lower than those in the HUA group (p < 0.05) by 14.5%, 12.25%, and 28%, respectively. Among these groups, the uric acid reduction was the greatest in the MLAB group, while there was no significant difference between the CM and C groups (p > 0.05). The results showed that mixed probiotics could prevent and alleviate the uric acid issue in hyperuricemia induced by potassium oxonate and yeast extract.
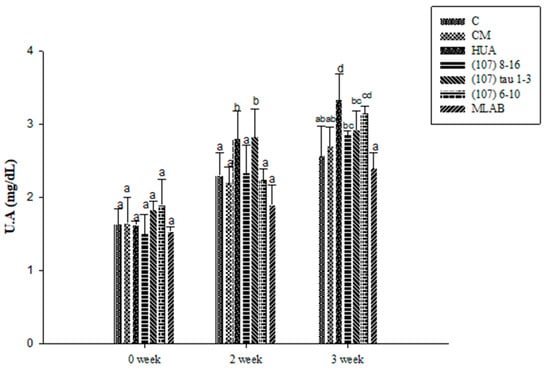
Figure 5.
Effects of serum uric acid (UA) in Wistar rats with hyperuricemia induced by potassium oxalate and yeast extract and simultaneously fed with probiotics. a,b,c,d values in different superscripts indicate significant differences (p < 0.05) using a Duncan’s multiple range test.
The BUN results in the experimental animals are shown in Figure 6. There were no significant differences among the different groups at week 0 (p > 0.05). At week 2, the BUN concentration in the HUA group was 13.64% higher than that in the C group (p < 0.05). After induction and feeding with LAB strains from different sources, the serum BUN concentrations in the treatment groups, including strains (107) 8–16, (107) tau 1–3, (107) 6–10, and MLAB, were significantly lower than those in the HUA group (p < 0.05) by 22.42%, 19.07%, 27.27% and 23.12%, respectively. Among these groups, BUN reduction was the greatest in the 6–10 group, while there was no significant difference observed between the CM and C groups (p > 0.05). At week 3, the BUN concentration in the HUA group was 13.19% higher than that in the C group (p < 0.05). After induction and feeding with LAB strains from different sources, the serum BUN concentrations in the treatment groups including strains (107) 8–16, (107) tau 1–3, (107) 6–10, and MLAB, were significantly lower than those in the HUA group (p < 0.05) by 11.1%, 17.02%, 19.63%, and 16.11%, respectively. Among these groups, BUN reduction was the greatest in the 6–10 group, while there was no significant difference observed between the CM and C groups (p > 0.05). The results showed that strains 6–10 could prevent and decrease BUN in hyperuricemia induced by potassium oxonate and yeast extract.
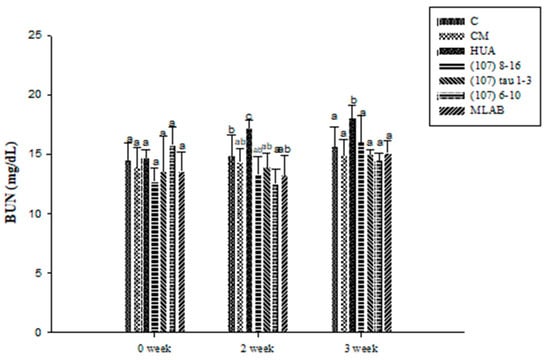
Figure 6.
Effects of blood urea nitrogen (BUN) in Wistar rats with hyperuricemia induced by potassium oxalate and yeast extract and simultaneously fed with probiotics. a, b, c values in different superscripts indicate significant differences (p < 0.05) using a Duncan’s multiple range test.
Figure 7 shows the blood creatinine results for the experimental animals. There were no significant differences observed between the groups at week 0 (p > 0.05). At weeks 2 and 3, there were no significant differences found between groups after induction and feeding with LAB strains from different sources (p > 0.05). The results showed that hyperuricemia induced by potassium oxonate and yeast extract did not affect serum creatinine and that probiotics from different sources did not affect the creatinine concentration.
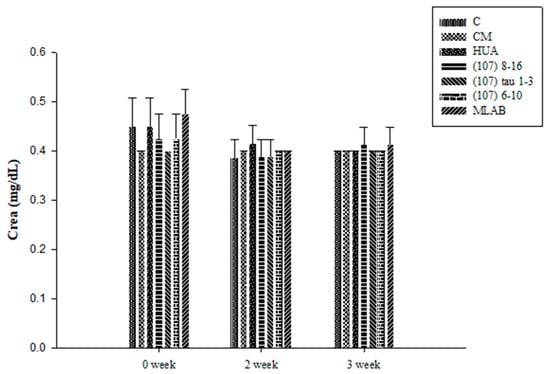
Figure 7.
Effects of creatinine (Crea) in Wistar rats with hyperuricemia induced by potassium oxalate and yeast extract and simultaneously fed with probiotics. Values showed no significant differences (p > 0.05) based on a Duncan’s multiple range test.
The literature review found that at least two to three weeks is required for a uric acid-lowering test in animals with probiotics alone []. Rodents such as rats and mice are used in most animal experiments. These animals possess uric acid (oxidation) enzymes, such as uricase (urate oxidase), that convert uric acid into more water-soluble allantoin for excretion. Therefore, rodents are less susceptible to hyperuricemia [,]. There are three main methods for inducing hyperuricemia in experimental animals. The first is to increase the uric acid level in vivo (i.e., inducing the body to generate a large amount of uric acid, leading to hyperuricemia) such as by using yeast extract, a high-purine diet or uric acid precursors []. The second method is to inhibit the uric acid excretion pathways, thus impairing renal uric acid metabolism and causing hyperuricemia through treatment with adenine, niacin, or ethambutol. The final method is uricase inhibition, which involves using uric acid analogs, such as potassium oxonate, to competitively inhibit uricase and induce hyperuricemia []. However, inducing hyperuricemia by inhibiting uric acid excretion or uricase activity may lead to renal impairment in the lab animals and does not match the mechanism of hyperuricemia in humans. On the other hand, it is difficult to maintain long term hyperuricemia using yeast extracts or high-purine diets alone, which may prolong the research period. Therefore, in the present study, we combined potassium oxonate and yeast extract to induce hyperuricemia in rats. This method not only inhibited uricase activity but also increased in vivo purine levels, thereby causing hyperuricemia [].
4. Conclusions
We screened LAB from distillers’ grains and indicated their ability to degrade purine in vitro and reduce UA in vivo. Based on the above results, when probiotics from different sources were fed to Wistar rats with hyperuricemia induced by potassium oxonate and yeast extract, the MLAB group showed the best serum uric acid-lowering effect. MLAB also reduced BUN without affecting creatinine. Although strain (107) 6–10 showed the best BUN-lowering effects, it did not control uric acid to the same extent as MLAB did. Therefore, employing a fixed ratio of mixed probiotics not only presented some nephroprotective effects but also well-regulated in vivo uric acid. In the future, we will study the effectiveness of probiotics through clinical research.
Author Contributions
H.-Y.C. and C.-C.T. designed the study and acquired funding; M.-W.H. performed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Taiwan Tobacco and Liquor Corporation, Taipei, Taiwan under grant contract case number 107-0620-1-001.
Institutional Review Board Statement
The animal experiment protocol approval number is HK-10805 (date: 2019/07/05) from HungKuang University, Taichung, Taiwan.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
This study was funded by the contract case number 107-0620-1-001 project from Taiwan Tobacco and Liquor Corporation, Taipei, Taiwan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cicero, A.F.G.; Fogacci, F.; Kuwabara, M.; Borghi, C. Therapeutic strategies for the treatment of chronic hyperuricemia: An evidence-based update. Medicina 2021, 57, 58–76. [Google Scholar] [CrossRef] [PubMed]
- Lima, W.G.; Martins-Santos, M.E.; Chaves, V.E. Uric acid as a modulator of glucose and lipid metabolism. Biochimie 2015, 116, 17–23. [Google Scholar] [CrossRef]
- Giordano, C.; Karasik, O.; King-Morris, K.; Asmar, A. Uric acid as a marker of kidney disease: Review of the current literature. Dis. Markers 2015, 2015, 382918. [Google Scholar] [CrossRef]
- Akkasilpa, S.; Avihingsanon, Y.; Hanvivadhanakul, P.; Wonchinsri, J. Clinical manifestations of patients with hyperuricemia. J. Med. Assoc. Thai. 2004, 87, 41–44. [Google Scholar]
- Li, M.; Yang, D.; Mei, L.; Yuan, L.; Xie, A.; Yuan, J. Screening and characterization of purine nucleoside degrading lactic acid bacteria isolated from Chinese sauerkraut and evaluation of the serum uric acid lowering effect in hyperuricemic rats. PLoS ONE 2014, 9, e105577. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Cheng, J.; Huangfu, N.; Zhao, R.; Xu, Z.; Zhang, F.; Zheng, W.; Zhang, D. Hyperuricemia and cardiovascular disease. Curr. Pharm. Des. 2019, 25, 700–709. [Google Scholar] [CrossRef]
- Li, C.; Hsieh, M.C.; Chang, S.J. Metabolic syndrome, diabetes, and hyperuricemia. Curr. Opin. Rheumatol. 2013, 25, 210–216. [Google Scholar] [CrossRef]
- Chalès, G. How should we manage asymptomatic hyperuricemia? Jt. Bone Spine 2019, 86, 437–443. [Google Scholar] [CrossRef]
- García-Arroyo, F.E.; Gonzaga, G.; Muñoz-Jiménez, I.; Blas-Marron, M.G.; Silverio, O.; Tapia, E.; Soto, V.; Ranganathan, N.; Ranganathan, P.; Vyas, U.; et al. Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PLoS ONE 2018, 13, e0202901. [Google Scholar] [CrossRef]
- Strilchuk, L.; Fogacci, F.; Cicero, A.F. Safety and tolerability of available urate-lowering drugs: A critical review. Expert. Opin. Drug Saf. 2019, 18, 261–271. [Google Scholar] [CrossRef]
- Vinderola, G.; Binetti, A.; Burns, P.; Reinheimer, J. Cell viability and functionality of probiotic bacteria in dairy products. Front. Microbiol. 2011, 2, 70. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Zoumpopoulou, G.; Foligné, B.; Alexandraki, V.; Kazou, M.; Pot, B.; Tsakalidou, E. Discovering probiotic microorganisms: In vitro, in vivo, genetic and omics approaches. Front. Microbiol. 2015, 6, 58. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Sedaghat, A.; Karimi Torshizi, M.A. Immune responses, intestinal microbiota, performance and blood characteristics of Japanese quail fed on diets containing camphor. Animal 2017, 11, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, H.; Taniguchi, A.; Tsuboi, H.; Kano, H.; Asami, Y. Hypouricaemic effects of yoghurt containing Lactobacillus gasseri PA-3 in patients with hyperuricaemia and/or gout: A randomised, double-blind, placebo-controlled study. Mod. Rheumatol. 2019, 29, 146–150. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, C.; Zeng, X.; Yuan, Z. Microecological treatment of hyperuricemia using Lactobacillus from pickles. BMC Microbiol. 2020, 20, 195–204. [Google Scholar] [CrossRef]
- Wang, H.; Mei, L.; Deng, Y.; Liu, Y.; Wei, X.; Liu, M.; Zhou, J.; Ma, H.; Zheng, P.; Yuan, J.; et al. Lactobacillus brevis DM9218 ameliorates fructose-induced hyperuricemia through inosine degradation and manipulation of intestinal dysbiosis. Nutrition 2019, 62, 63–73. [Google Scholar] [CrossRef]
- Gómez Zavaglia, A.; Kociubinski, G.; Pérez, P.; De Antoni, G. Isolation and characterization of Bifidobacterium strains for probiotic formulation. J. Food Prot. 1998, 61, 865–873. [Google Scholar] [CrossRef]
- Gilliland, S.E.; Walker, D.K. Factors to consider when selecting a culture of Lactobacillus acidophilus as a dietary adjunct to produce a hypocholesterolemic effect in humans. J. Dairy Sci. 1990, 73, 905–911. [Google Scholar] [CrossRef]
- Banerjee, P.; Merkel, G.J.; Bhunia, A.K. Lactobacillus delbrueckii ssp. bulgaricus B-30892 can inhibit cytotoxic effects and adhesion of pathogenic Clostridium difficile to Caco-2 cells. Gut Pathog. 2009, 1, 8. [Google Scholar] [CrossRef][Green Version]
- Pedersen, K.; Tannock, G.W. Colonization of the porcine gastrointestinal tract by lactobacilli. Appl. Environ. Microbiol. 1989, 55, 279–283. [Google Scholar] [CrossRef]
- Kaneko, K.; Takayanagi, F.; Fukuuchi, T.; Yamaoka, N.; Yasuda, M.; Mawatari, K.I.; Fujimori, S. Determination of total purine and purine base content of 80 food products to aid nutritional therapy for gout and hyperuricemia. Nucleosides Nucleotides Nucleic Acids 2020, 39, 1449–1457. [Google Scholar] [CrossRef]
- Guimaraes, A.P.; Oliveir, A.A.; Ramalho, T.C. Analysis of bacillus anthracis nucleoside hydrolase via in silico docking with inhibitors and molecular dynamics simulation. J. Mol. Model. 2011, 17, 2939–2951. [Google Scholar] [CrossRef] [PubMed]
- Babot, J.D.; Argañaraz-Martínez, E.; Saavedra, L.; Apella, M.C.; Chaia, A.P. Compatibility and safety of five lectin-binding putative probiotic strains for the development of a multi-strain protective culture for poultry. Benef. Microbes 2018, 9, 927–935. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Zhao, J.; Xia, Y.; Lai, P.F.; Ai, L. A surface protein from Lactobacillus plantarum increases the adhesion of Lactobacillus strains to human epithelial cells. Front. Microbiol. 2018, 9, 2858. [Google Scholar] [CrossRef]
- Castro-Bravo, N.; Wells, J.M.; Margolles, A.; Ruas-Madiedo, P. Interactions of surface exopolysaccharides from Bifidobacterium and Lactobacillus within the intestinal environment. Front. Microbiol. 2018, 9, 2426. [Google Scholar] [CrossRef] [PubMed]
- Deepika, G.; Charalampopoulos, D. Surface and adhesion properties of lactobacilli. Adv. Appl. Microbiol. 2010, 70, 127–152. [Google Scholar]
- Sun, J.; Le, G.W.; Shi, Y.H.; Su, G.W. Factors involved in binding of Lactobacillus plantarum Lp6 to rat small intestinal mucus. Lett. Appl. Microbiol. 2007, 44, 79–85. [Google Scholar] [CrossRef]
- Gilliland, S.E. Acidophilus milk products: A review of potential benefits to consumers. J. Dairy Sci. 1989, 72, 2483–2494. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Tapia, E.; Soto, V.; Avila-Casado, C.; Franco, M.; Zhao, L.; Johnson, R.J. Treatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemia. Nephrol. Dial. Transplant. 2008, 23, 1179–1185. [Google Scholar] [CrossRef]
- Sánchez-Lozada, L.G.; Soto, V.; Tapia, E.; Avila-Casado, C.; Sautin, Y.Y.; Nakagawa, T.; Franco, M.; Rodríguez-Iturbe, B.; Johnson, R.J. Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am. J. Physiol. Renal. Physiol. 2008, 295, 1134–1141. [Google Scholar] [CrossRef]
- Haryono, A.; Nugrahaningsih, D.A.A.; Sari, D.C.R.; Romi, M.M.; Arfian, N. Reduction of serum uric acid associated with attenuation of renal injury, inflammation and macrophages M1/M2 ratio in hyperuricemic mice model. Kobe J. Med. Sci. 2018, 64, 107–114. [Google Scholar]
- Wu, P.; Li, J.; Zhang, X.; Zeng, F.; Liu, Y.; Sun, W. Study of the treatment effects of compound tufuling granules in hyperuricemic rats using serum metabolomics. Evid. Based Complement Alternat. Med. 2018, 2018, 3458185. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).