Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Beers
2.2. Malt
2.3. Hops
2.4. Yeasts
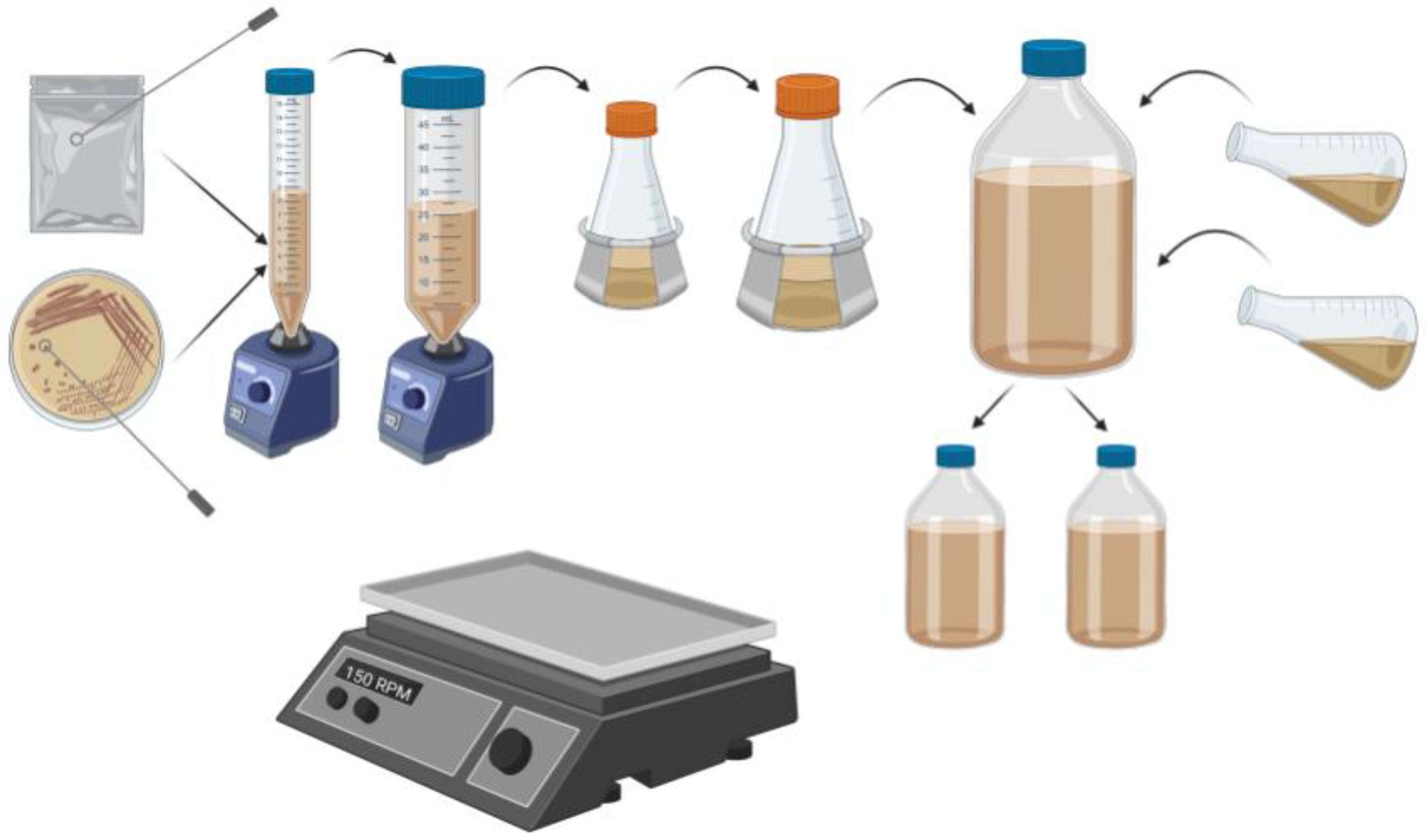
2.5. Yeast Propagation
2.6. Sample Collection and Preparation
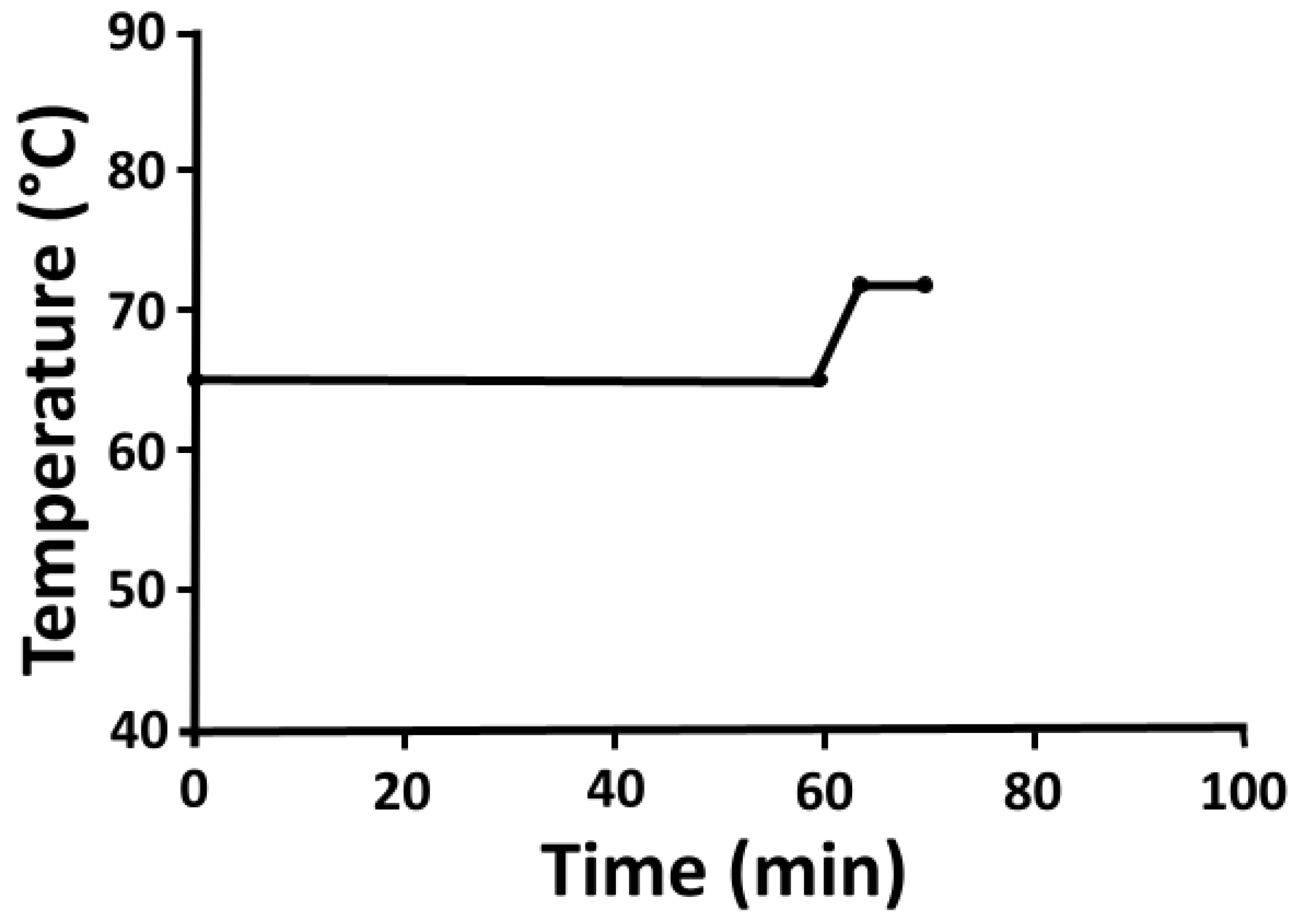
2.7. Pilot Fermentations
2.8. Bench-Top Fermentations
2.9. Analytical Measurements
2.10. Statistical Analysis
3. Results and Discussion
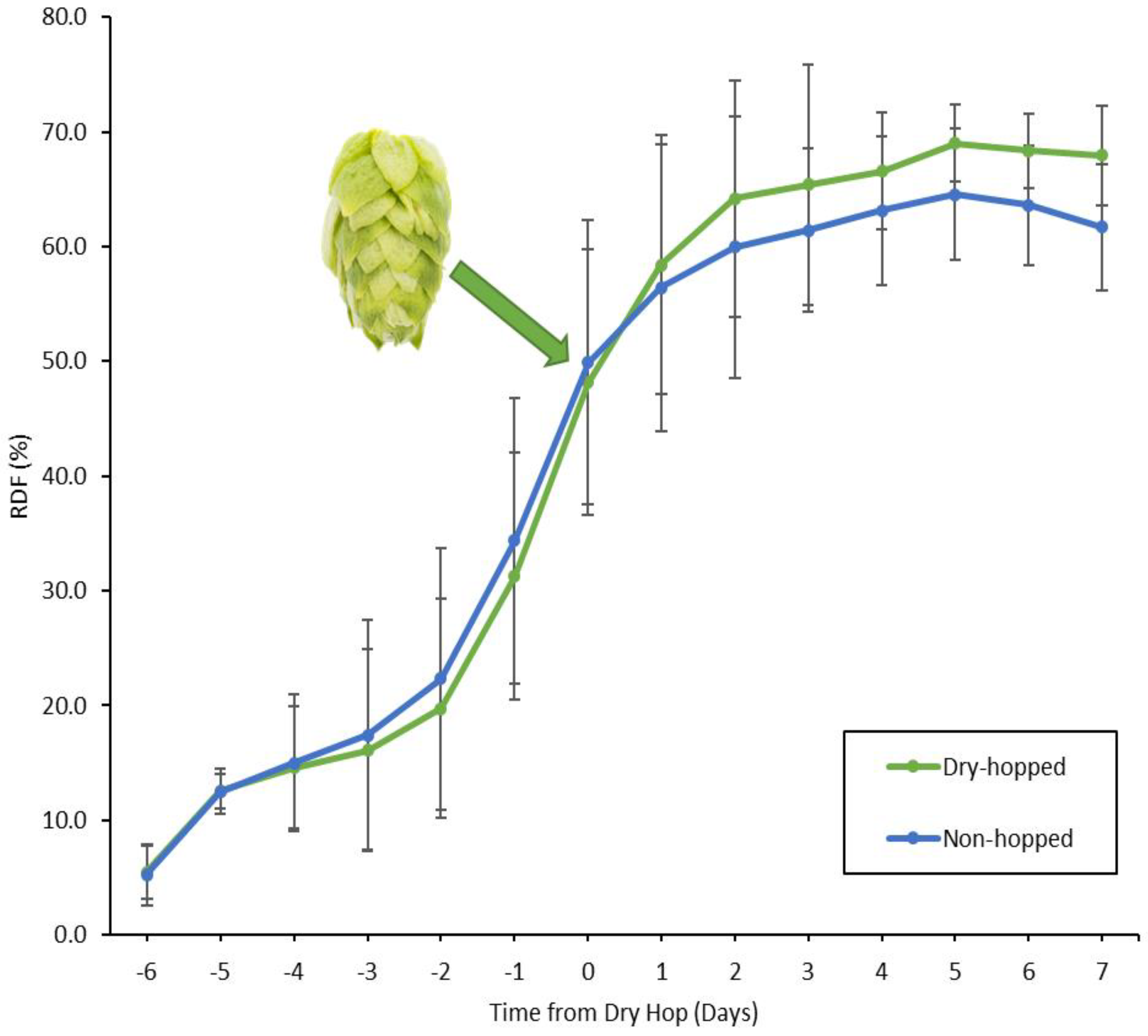
3.1. Pilot Fermentations
3.2. Bench-Top Fermentations
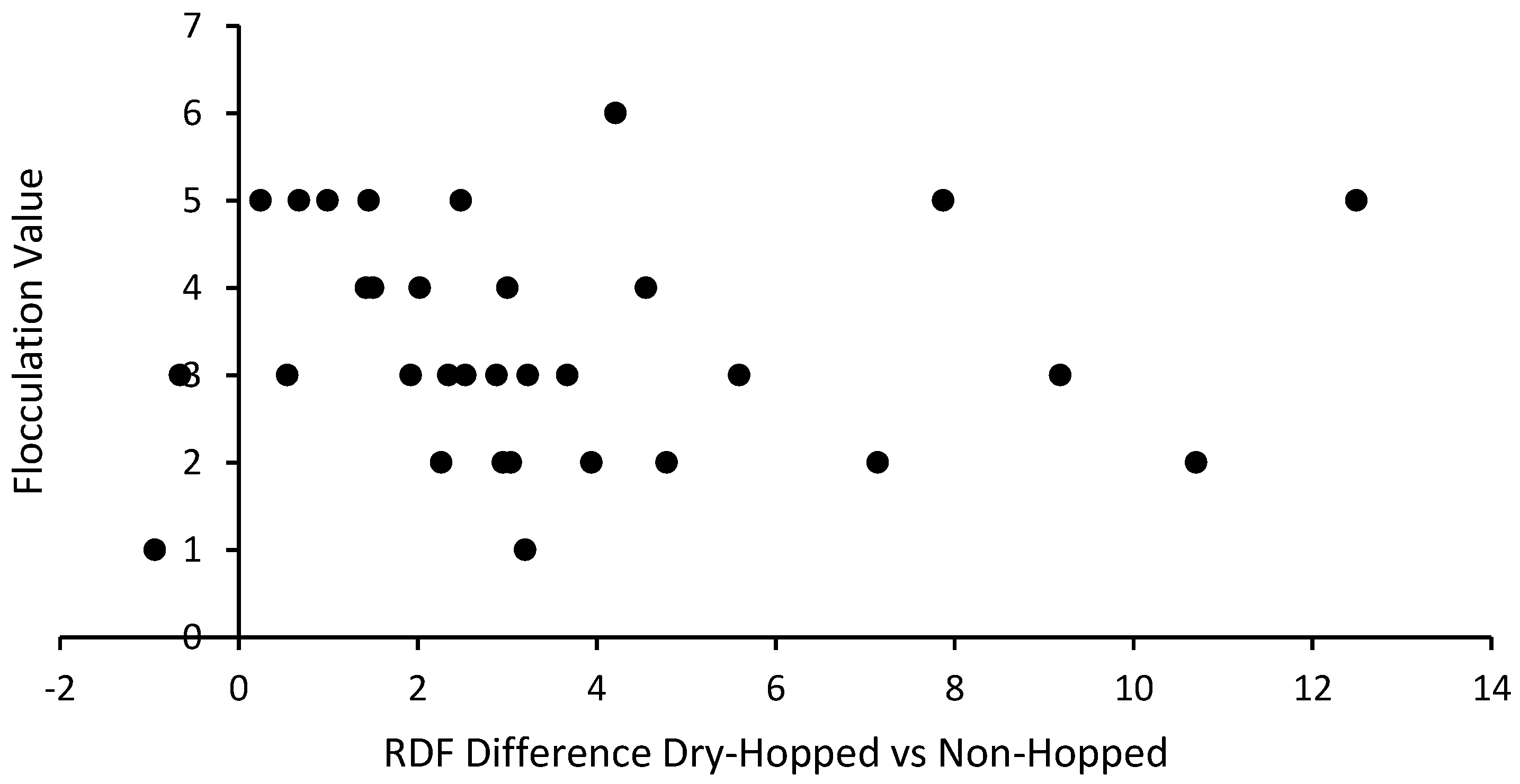
3.3. Flocculation and Attenuation
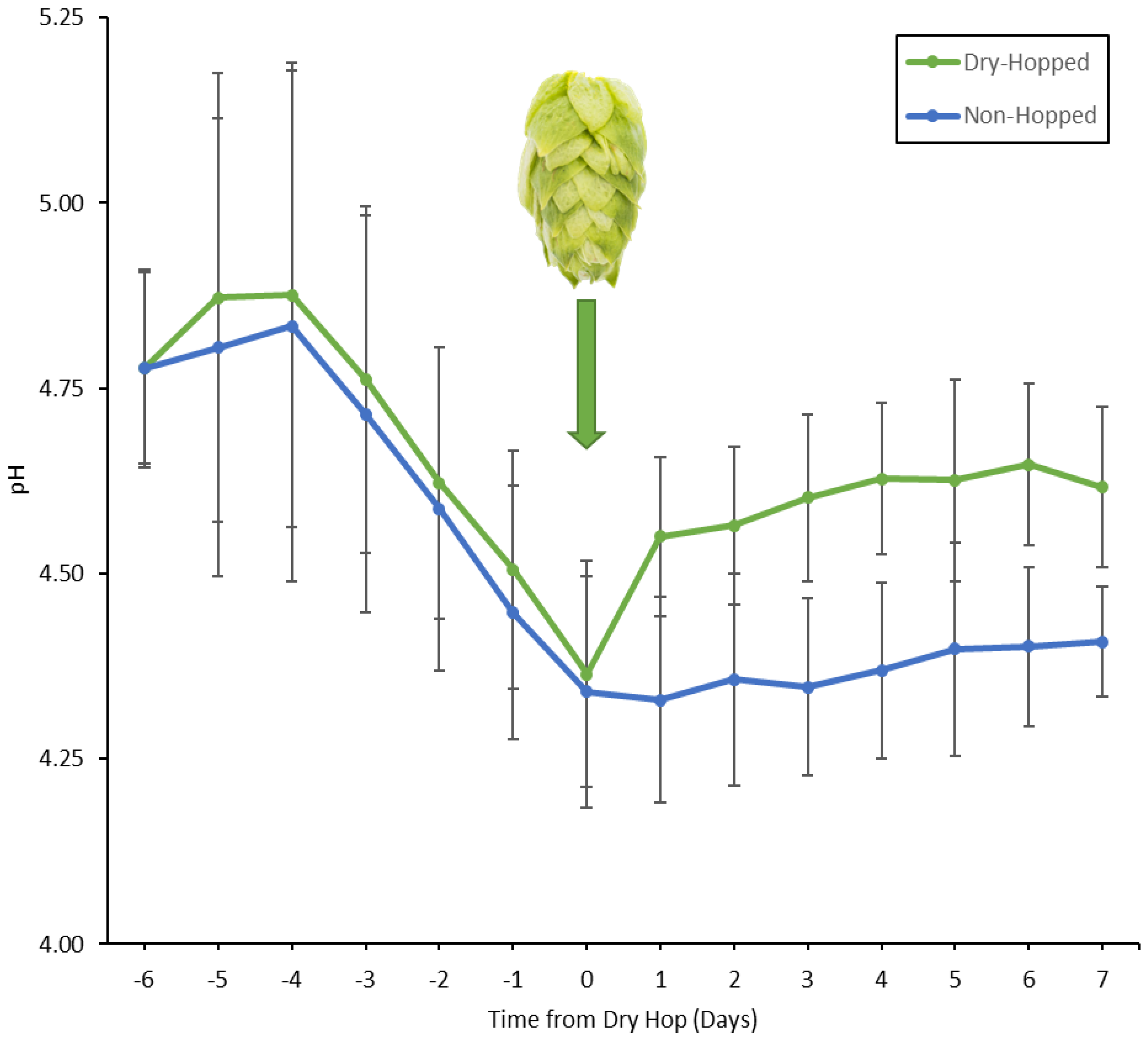
3.4. pH and Dry-Hopping
3.5. Biological Replicates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moritz, E.R.; Morris, G.H. A Text-Book of the Science of Brewing; Spon: London, UK, 1891. [Google Scholar]
- Schönberger, C.; Kostelecky, T. 125th Anniversary Review: The Role of Hops in Brewing. J. Inst. Brew. 2011, 117, 259–267. [Google Scholar] [CrossRef]
- Kunze, W. Technology Brewing & Malting; 6th Revised English; Hendel, O., Ed.; Versuchs- und Lehranstalt für Brauerei in Berlin (VLB): Berlin, Germany, 2019. [Google Scholar]
- Brown, H.; Morris, G. On Certain Functions of Hops Used in the Dry-Hopping of Beers. Trans. Inst. Brew 1893, 6, 94–106. [Google Scholar]
- How Hoppy Beer Production Has Redefined Hop Quality and a Discussion of Agricultural and Processing Strategies to Promote It. Tech. Q. 2019. [CrossRef]
- Lafontaine, S.R.; Shellhammer, T.H. Investigating the Factors Impacting Aroma, Flavor, and Stability in Dry-Hopped Beers. MBAA Tech. Q. 2019, 56, 13–23. [Google Scholar] [CrossRef]
- Hauser, D.G.; Van Simaeys, K.R.; Lafontaine, S.R.; Shellhammer, T.H. A Comparison of Single-Stage and Two-Stage Dry-Hopping Regimes. J. Am. Soc. Brew. Chem. 2019, 77, 251–260. [Google Scholar] [CrossRef]
- Dykstra, J. The Beer Connoisseur; CafeMedia: New York, NY, USA, 2020; pp. 18–29. [Google Scholar]
- National Beer Sales & Production Data|Brewers Association. Available online: https://www.brewersassociation.org/statistics-and-data/national-beer-stats/ (accessed on 11 January 2020).
- Bud Light Crisp. Available online: https://www.budlight.com/en/our-beers/crisp.html (accessed on 31 March 2021).
- Guinness® Nitro IPA|Guinness®. Available online: https://www.guinness.com/en/our-beers/guinness-nitro-ipa/ (accessed on 30 March 2021).
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Zhao, H. Non-Alcoholic and Craft Beer Production and Challenges. Processes 2020, 8, 1382. [Google Scholar] [CrossRef]
- Otter, G.E.; Taylor, L. Determination of the sugar composition of wort and beer by gas liquid chromatography. J. Inst. Brew. 1967, 73, 570–576. [Google Scholar] [CrossRef]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2001; p. 656. [Google Scholar]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Bamforth, C.W. Scientific Principles of Malting and Brewing; American Society of Brewing Chemists, Ed.; American Society of Brewing Chemists: St. Paul, MN, USA, 2006; p. 246. [Google Scholar]
- Takoi, K.; Koie, K.; Itoga, Y.; Katayama, Y.; Shimase, M.; Nakayama, Y.; Watari, J. Biotransformation of Hop-Derived Monoterpene Alcohols by Lager Yeast and Their Contribution to the Flavor of Hopped Beer. J. Agric. Food Chem. 2010, 58, 5050–5058. [Google Scholar] [CrossRef]
- Kirkpatrick, K.R.; Shellhammer, T.H. Evidence of Dextrin Hydrolyzing Enzymes in Cascade Hops (Humulus lupulus). J. Agric. Food Chem. 2018, 66, 9121–9126. [Google Scholar] [CrossRef] [PubMed]
- Olodokun, O.; Cowley, T.; James, S.; Smart, K.A. Dry-hopping: The effects of temperature and hop variety on the bittering profiles and properties of resultant beers. Brew. Sci. 2017, 70, 187–196. [Google Scholar]
- Kirkendall, J.A.; Mitchell, C.A.; Chadwick, L.R. The Freshening Power of Centennial Hops. J. Am. Soc. Brew. Chem. 2018, 76, 178–184. [Google Scholar] [CrossRef]
- Janicki, J.; Kotasthane, W.V.; Parker, A.; Walker, T.K. The DIAStatic activity of hops, together with a note on maltase in hops. J. Inst. Brew. 1941, 47, 24–36. [Google Scholar] [CrossRef]
- U.S. Department of the Treasury. The Beverage Alcohol Manual (BAM): A Practical Guide; Basic Mandatory Labeling Information for Malt Bev-Erages; US Gov., Tax and Trade Bureau: Washington, DC, USA, 2007; Chapter 1; Volume 3, Section 5; p. 7.
- Otter, G.E.; Taylor, L. Estimation and occurrence of acetaldehyde in beer. J. Inst. Brew. 1971, 77, 467–472. [Google Scholar] [CrossRef]
- Wainwright, T. diacetyl-a review: Part i-analytical and biochemical considerations: Part ii-brewing experience. J. Inst. Brew. 1973, 79, 451–470. [Google Scholar] [CrossRef]
- Tian, J. Determination of several flavours in beer with headspace sampling–gas chromatography. Food Chem. 2010, 123, 1318–1321. [Google Scholar] [CrossRef]
- Technical Committee. Alcohol. In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Cutaia, A.J.; Munroe, J.H. A Method for the Consistent Estimation of Real Degree of Fermentation. J. Am. Soc. Brew. Chem. 1979, 37, 188–189. [Google Scholar] [CrossRef]
- Huerta-Zurita, R.; Horsley, R.D.; Schwarz, P.B. Is the Apparent Degree of Fermentation a Reliable Estimator of Fermentability? J. Am. Soc. Brew. Chem. 2019, 77, 1–9. [Google Scholar] [CrossRef]
- Kirkpatrick, K.R.; Shellhammer, T.H. A Cultivar-Based Screening of Hops for Dextrin Degrading Enzymatic Potential. J. Am. Soc. Brew. Chem. 2018, 76, 247–256. [Google Scholar] [CrossRef]
- Shellhammer, T.H.; Beauchamp, A.; Kravitz, M.; Vaughn, C.; Cilurzo, V. Hop Creep: What It Is and Approaches to Man-Aging It; Brewers’ Association: Boulder, CO, USA, 2020. [Google Scholar]
- Bruner, J.; Williams, J.; Fox, G. Further Exploration of Hop Creep Variability with Humulus lupulus Cultivars and Proposed Method for Determination of Secondary Fermentation. Tech. Q. 2020, 57, 57. [Google Scholar] [CrossRef]
- Stokholm, A.; Lindsey, N.R.; Shellhammer, T.H. Evaluating a benchtop fermentation method for estimating dextrin degra-dation by hop’ ‘ diastatic enzymes during dry-hopping. Brew. Sci. 2020, 73, 140–148. [Google Scholar]
- Gallagher, L.W.; Silberstein, R.; Prato, L.; Vogt, H. ‘Butta 12’, a two-rowed malting barley adapted to the California Central Valley with proven floor-malting success and craft brewer acceptance. J. Plant Regist. 2020, 14, 250–265. [Google Scholar] [CrossRef]
- Bruner, J.; Fox, G. Novel Non-Cerevisiae Saccharomyces Yeast Species Used in Beer and Alcoholic Beverage Fermentations. Fermentation 2020, 6, 116. [Google Scholar] [CrossRef]
- Schisler, D.O. Comparison of Revised Yeast Counting Methods. J. Am. Soc. Brew. Chem. 1986, 44, 81–85. [Google Scholar] [CrossRef]
- Lafontaine, S.R.; Shellhammer, T.H. Impact of static dry-hopping rate on the sensory and analytical profiles of beer. J. Inst. Brew. 2018, 124, 434–442. [Google Scholar] [CrossRef]
- Magalhães, F.; Vidgren, V.; Ruohonen, L.; Gibson, B. Maltose and maltotriose utilisation by group I strains of the hybrid lager yeastSaccharomyces pastorianus. FEMS Yeast Res. 2016, 16. [Google Scholar] [CrossRef]
- Gibson, B.R.; Storgårds, E.; Krogerus, K.; Vidgren, V. Comparative physiology and fermentation performance of Saaz and Frohberg lager yeast strains and the parental speciesSaccharomyces eubayanus. Yeast 2013, 30, 255–266. [Google Scholar] [CrossRef]
- Yamashita, I.; Suzuki, K.; Fukui, S. Nucleotide sequence of the extracellular glucoamylase gene STA1 in the yeast Saccharomyces diastaticus. J. Bacteriol. 1985, 161, 567–573. [Google Scholar] [CrossRef]
- Sakai, K.; Fukui, S.; Yabuuchi, S.; Aoyagi, S.; Tsumura, Y. Expression of theSaccharomyces Diastaticus STA1Gene in Brewing Yeasts. J. Am. Soc. Brew. Chem. 1989, 47, 87–91. [Google Scholar] [CrossRef]
- Bellon, J.R.; Schmid, F.; Capone, D.L.; Dunn, B.L.; Chambers, P.J. Introducing a New Breed of Wine Yeast: Interspecific Hybridisation between a Commercial Saccharomyces cerevisiae Wine Yeast and Saccharomyces mikatae. PLoS ONE 2013, 8, e62053. [Google Scholar] [CrossRef]
- Bellon, J.; Schmidt, S.; Solomon, M. Case study: Development of Saccharomyces cerevisiae × Saccharomyces mikatae wine yeast hybrids and their potential to deliver alternative wine styles. AWRI Tech. Rev. 2019, 241, 6–11. [Google Scholar]
- Technical Committee. Yeast Fermentable Extract. In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Technical Committee. End fermentation (yeast fermentable extract). In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Nikulin, J.; Vidgren, V.; Krogerus, K.; Magalhães, F.; Valkeemäki, S.; Kangas-Heiska, T.; Gibson, B. Brewing potential of the wild yeast species Saccharomyces paradoxus. Eur. Food Res. Technol. 2020, 246, 2283–2297. [Google Scholar] [CrossRef]
- D’Hautcourt, O.; Smart, K.A. Measurement of Brewing Yeast Flocculation. J. Am. Soc. Brew. Chem. 1999, 57, 123–128. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Verachtert, H.; Delvaux, F.R. Yeast flocculation: What brewers should know. Appl. Microbiol. Biotechnol. 2003, 61, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.G. Yeast Flocculation—Sedimentation and Flotation. Ferment 2018, 4, 28. [Google Scholar] [CrossRef]
- Bendiak, D.S. Quantification of the Helm’s Flocculation Test. J. Am. Soc. Brew. Chem. 1994, 52, 120–122. [Google Scholar] [CrossRef]
- Humulinone Formation in Hops and Hop Pellets and Its Implications for Dry Hopped Beers. Tech. Q. 2016, 53, 23–27. [CrossRef]
- Maye, J.P.; Smith, R.; Leker, J. Dry Hopping and Its Effect on Beer Bitterness, the IBU Test, and pH. BrauW. Int. 2018, 2018, 25–29. [Google Scholar]
- Vanbeneden, N.; Van Roey, T.; Willems, F.; Delvaux, F.; Delvaux, F.R. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008, 111, 83–91. [Google Scholar] [CrossRef]
- García, A.I.; García, L.A.; Díaz, M. Prediction of ester production in industrial beer fermentation. Enzym. Microb. Technol. 1994, 16, 66–71. [Google Scholar] [CrossRef]
- García, A.I.; García, L.A.; Díaz, M. Fusel Alcohols Production in Beer Fermentation Processes. Process. Biochem. 1994, 29, 303–309. [Google Scholar] [CrossRef]
| Yeast Name | Scientific Name | Comparable Strains | Origin | Flocculation | Attenuation |
|---|---|---|---|---|---|
| BY881 | S. cerevisiae | (R&D) ** | Oakland, CA, USA | Medium | 75–85% |
| SafAle™ BE-134 | S. cerevisiae var. diastaticus | WLP566 | Belgium | Low | 89–93% |
| SafAle™ BE-256 | S. cerevisiae | WLP530; WY3787 | Belgium | High | 82–86% |
| SafAle™ K-97 | S. cerevisiae | WLP029 | Koln, Germany | Medium High | 80–84% |
| SafAle™ S-33 | S. cerevisiae | WLP005 | England | Medium Low | 68–72% |
| SafAle™ T-58 | S. cerevisiae | WLP565 | Belgium | Medium Low | 72–78% |
| SafAle™ US-05 * | S. cerevisiae | WLP001; UCDFST 96-12 | USA | Medium | 78–82% |
| SafLager™ W 34/70 | S. pastorianus | WLP830 | Germany | High | 80–84% |
| SafŒno™ BC S103 | S. bayanus | ** | France | High | High |
| SafŒno™ CK S102 | S. cerevisiae | ** | France | High | High |
| SafŒno™ HD T18 | S. cerevisiae x S. bayanus | (R&D) ** | Marcq-en-Baroeul, France | Medium | High |
| SafSpirit™ USW-6 | S. cerevisiae | ** | USA | Medium Low | High |
| UCDFST 01-135 | S. bayanus | CBS 380; DVBPG 6171 | Turbid Beer—Italy | Medium | Moderate |
| UCDFST 01-157 | S. pastorianus | CBS 1538; DBVPG 6047 | Carlsberg—Denmark | Medium High | 72–78% |
| UCDFST 01-161 | S. paradoxus | CBS 432; DBVPG 6411 | Northeast Europe | Medium | Moderate |
| UCDFST 11-510 | S. mikatae | CBS 8839; NCYC 2888 | Soil—Japan | Medium | Moderate Low |
| UCDFST 11-512 | S. uvarum | CBS 395; DBVPG 6179 | Fruit—Scandinavia | High | Moderate |
| UCDFST 11-515 | S. kudriavzevii | CBS 8840, NCYC 2889 | Western Europe | Medium High | Moderate |
| UCDFST 21-101 | S. cerevisiae var. chevalieri | CBS 400; DBVPG 6174 | Ivory Coast | Medium Low | 15% |
| UCDFST 77-65 | Saccharomyces cerevisiae | WLP076 | Santa Rosa, CA, USA | Medium | 70–74% |
| UCDFST 96-12 | S. cerevisiae | WLP001; US-05 | Chico, CA, USA | Medium Low | 73–78% |
| WLP001 | S. cerevisiae | US-05; UCDFST 96-12 | Chico, CA, USA | Medium | 73–80% |
| WLP002 | S. cerevisiae | UCD 96-17; WY1968 | London, UK | Very High | 63–70% |
| WLP013 | S. cerevisiae | OYL003; UCDFST 96-11 | London, UK | Medium | 67–75% |
| WLP030 | S. cerevisiae | WY1275 | Trent, UK | High | 72–78% |
| WLP066 | S. cerevisiae | A38; OYL011 | London, UK | Medium Low | 75–82% |
| WLP090 | S. cerevisiae | OYL043 | San Diego, CA, USA | Medium High | 76–83% |
| WLP095 | S. cerevisiae | OYL052; GY054 | Burlington, VT, USA | Medium High | 75–80% |
| WLP351 | S. bayanus | UCDFST 02-124; OYL025 | Weiss—Germany | Low | 75–82% |
| WLP518 | S. cerevisiae | NCYC 4285 | Kveik—Norway | High | 70–80% |
| Yeast Name | Non-Hopped | Dry-Hopped | Difference | RDF (%) | |
|---|---|---|---|---|---|
| BY881 | 2.26 | ||||
| SafAle™ BE-134 | −0.94 | ||||
| SafAle™ BE-256 | 1.45 | ||||
| SafAle™ K-97 | 4.55 | ||||
| SafAle™ S-33 | 3.04 | ||||
| SafAle™ T-58 | 7.14 | 75% | |||
| SafAle™ US-05 * | 2.56 | ||||
| SafLager™ W-34/70 | 2.48 | ||||
| SafŒno™ BC S103 | 12.49 | ||||
| SafŒno™ CK S102 | 0.67 | ||||
| SafŒno™ HD T18 | 2.88 | ||||
| SafSpirit™ USW-6 | 2.95 | ||||
| UCDFST 01-135 | 3.67 | ||||
| UCDFST 01-157 | 2.02 | ||||
| UCDFST 01-161 | 0.54 | ||||
| UCDFST 11-510 | −0.66 | 65% | |||
| UCDFST 11-512 | 0.24 | ||||
| UCDFST 11-515 | 1.42 | ||||
| UCDFST 21-101 | 10.70 | ||||
| UCDFST 77-65 | 9.18 | ||||
| UCDFST 96-12 | 4.78 | ||||
| WLP001 | 2.34 | ||||
| WLP002 | 4.21 | ||||
| WLP013 | 5.59 | ||||
| WLP030 | 0.99 | ||||
| WLP066 | 3.94 | 55% | |||
| WLP090 | 1.50 | ||||
| WLP095 | 3.00 | ||||
| WLP351 | 3.20 | ||||
| WLP518 | 7.87 |
| Yeast Name | Non-Hopped | Dry-Hopped | Difference | RDF (%) | |
|---|---|---|---|---|---|
| BY881 | 20.68 | ||||
| SafAle™ BE-134 | −3.07 | ||||
| SafAle™ BE-256 | 3.25 | ||||
| SafAle™ K-97 | 20.71 | ||||
| SafAle™ S-33 | 5.40 | ||||
| SafAle™ T-58 | 5.16 | 75% | |||
| SafAle™ US-05 * | 3.50 | ||||
| SafLager™ W-34/70 | 2.90 | ||||
| SafŒno™ BC S103 | 10.41 | ||||
| SafŒno™ CK S102 | 4.94 | ||||
| SafŒno™ HD T18 | 1.83 | ||||
| SafSpirit™ USW-6 | 3.13 | ||||
| UCDFST 01-135 | 4.33 | ||||
| UCDFST 01-157 | −42.97 | ||||
| UCDFST 01-161 | −0.37 | ||||
| UCDFST 11-510 | 1.68 | 65% | |||
| UCDFST 11-512 | 31.85 | ||||
| UCDFST 11-515 | 38.13 | ||||
| UCDFST 21-101 | 1.49 | ||||
| UCDFST 77-65 | 11.23 | ||||
| UCDFST 96-12 | 43.33 | ||||
| WLP001 | 3.39 | ||||
| WLP002 | 2.69 | ||||
| WLP013 | 2.43 | ||||
| WLP030 | 1.78 | ||||
| WLP066 | 1.97 | 55% | |||
| WLP090 | 4.15 | ||||
| WLP095 | 0.37 | ||||
| WLP351 | 1.92 | ||||
| WLP518 | 9.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruner, J.; Marcus, A.; Fox, G. Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains. Fermentation 2021, 7, 66. https://doi.org/10.3390/fermentation7020066
Bruner J, Marcus A, Fox G. Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains. Fermentation. 2021; 7(2):66. https://doi.org/10.3390/fermentation7020066
Chicago/Turabian StyleBruner, James, Andrew Marcus, and Glen Fox. 2021. "Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains" Fermentation 7, no. 2: 66. https://doi.org/10.3390/fermentation7020066
APA StyleBruner, J., Marcus, A., & Fox, G. (2021). Dry-Hop Creep Potential of Various Saccharomyces Yeast Species and Strains. Fermentation, 7(2), 66. https://doi.org/10.3390/fermentation7020066







