Impact of Commercial Yeasts on Phenolic Profile of Plavac Mali Wines from Croatia
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
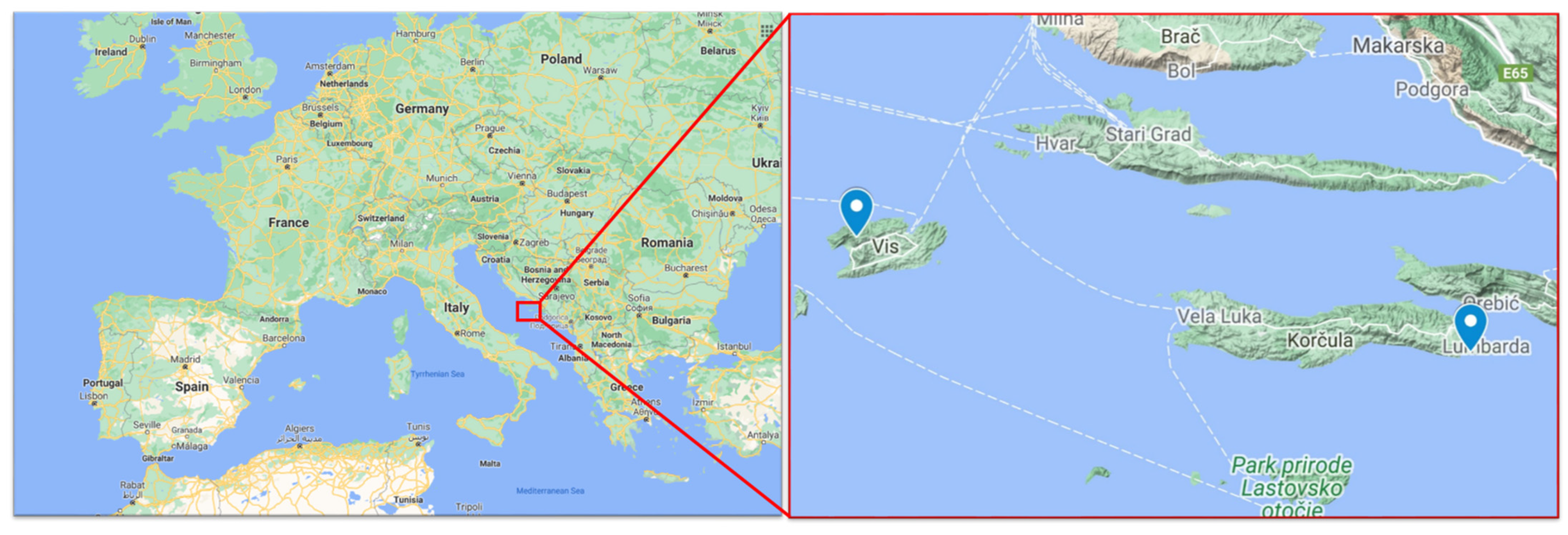
2.2. Vineyard Locations
2.3. Fermentation Trials
2.4. Physicochemical Analysis
2.5. Organic Acids Analysis
2.6. Polyphenol Compounds Determination
2.7. Color Parameters
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Composition
3.2. Phenolic Profile of Plavac Mali Wines
3.2.1. Anthocyanins
3.2.2. Flavonols
3.2.3. Phenolic Acids
3.2.4. Flavan-3-ols
3.2.5. Stilbenes
3.2.6. Chromatic Parameters
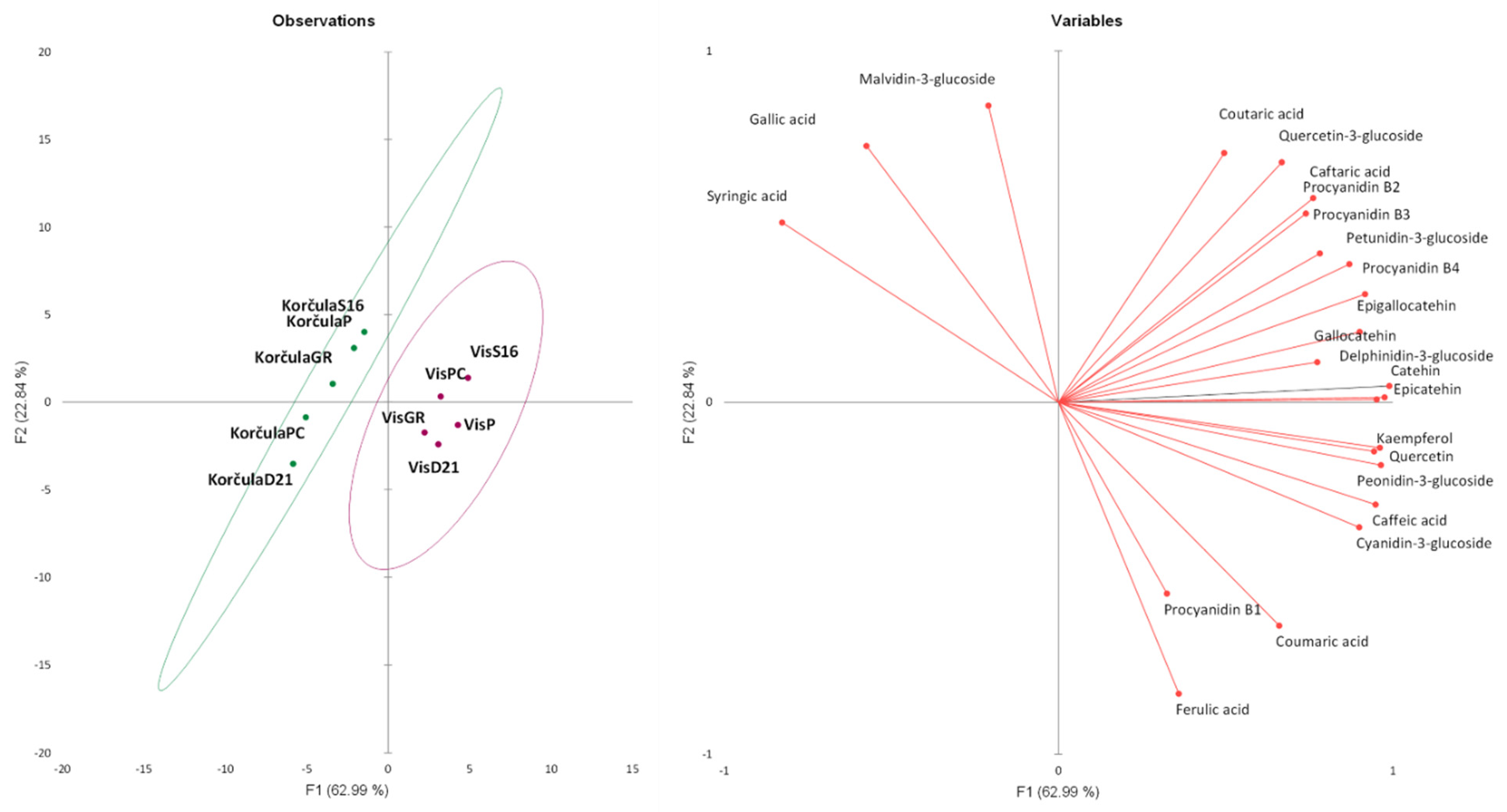
3.2.7. Multivariate Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cynkar, W.; Dambergs, R.; Smith, P.; Cozzolino, D. Classification of Tempranillo wines according to geographic origin: Combination of mass spectrometry based electronic nose and chemometrics. Anal. Chim. Acta 2010, 660, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Goldner, C.M.; Zamora, C.M. Sensory Characterization of Vitis Vinifera cv. Malbec Wines from Seven Viticulture Regions of Argentina. J. Sens. Stud. 2007, 22, 520–532. [Google Scholar] [CrossRef]
- Robinson, A.L.; Adams, D.O.; Boss, P.K.; Heymann, H.; Solomon, P.S.; Trengove, R.D. Influence of Geographic Origin on the Sensory Characteristics and Wine Composition of Vitis vinifera cv. Cabernet Sauvignon Wines from Australia. Am. J. Enol. Vitic. 2012, 63, 467–476. [Google Scholar] [CrossRef]
- Roullier-Gall, C.; Boutegrabet, L.; Gougeon, R.D.; Schmitt-Kopplin, P. A grape and wine chemodiversity comparison of different appellations in Burgundy: Vintage vs. terroir effects. Food Chem. 2014, 152, 100–107. [Google Scholar] [CrossRef]
- Vinci, G.; Eramo, S.; Nicoletti, I.; Restuccia, D. Influence of Environmental and Technological Parameters on Phenolic composition in red wine. J. Commode Sci. Technol. Qual. 2008, 1, 245–266. [Google Scholar]
- Merkyte, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; Suárez Lepe, J.A. Influence of Yeasts in Wine Colour; IntechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Caridi, A. Improved screening method for the selection of wine yeasts based on their pigment adsorption activity. Food Technol. Biotechnol. 2013, 51, 137–144. [Google Scholar]
- Caridi, A.; De Bruno, A.; De Salvo, E.; Piscopo, A.; Poiana, M.; Sidari, R. Selected yeasts to enhance phenolic content and quality in red wine from low pigmented grapes. Eur. Food Res. Technol. 2017, 243, 367–378. [Google Scholar] [CrossRef]
- Caridi, A.; Cufari, A.; Lovino, R.; Palumbo, R.; Tedesco, I. Influence of Yeast on Polyphenol Composition of Wine. Food Technol. Biotechnol. 2004, 42, 37–40. [Google Scholar]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Yeast interactions with anthocyanins during red wine fermentation. Am. J. Enol. Vitic. 2005, 56, 104–109. [Google Scholar]
- Morata, A.; Loira, I.; Heras, J.M.; Callejo, M.J.; Tesfaye, W.; González, C.; Suárez-Lepe, J.A. Yeast influence on the formation of stable pigments in red winemaking. Food Chem. 2016, 197, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Echeverrigaray, S.; Menegotto, M.; Delamare, A.P.L. A simple and reliable method for the quantitative evaluation of anthocyanin adsorption by wine yeasts. J. Microbiol. Methods 2019, 157, 88–92. [Google Scholar] [CrossRef]
- Clare, S.S.; Skurray, G.R.; Shalliker, R.A. Effect of yeast strain selection on the concentration of cis- and trans-resveratrol and resveratrol glucoside isomers in wine. Aust. J. Grape Wine Res. 2005, 11, 9–14. [Google Scholar] [CrossRef]
- Carew, A.L.; Smith, P.; Close, D.C.; Curtin, C.; Dambergs, R.G. Yeast Effects on Pinot noir Wine Phenolics, Color, and Tannin Composition. J. Agric. Food Chem. 2013, 61, 9892–9898. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdyło, A.; Chmielewska, J.; Nofer, J. Effect of Different Yeast Strains and Temperature of Fermentation on Basic Enological Parameters, Polyphenols and Volatile Compounds of Aurore White Wine. Foods 2019, 8, 599. [Google Scholar] [CrossRef]
- Grieco, F.; Carluccio, M.A.; Giovinazzo, G. Autochthonous Saccharomyces cerevisiae Starter Cultures Enhance Polyphenols Content, Antioxidant Activity, and Anti-Inflammatory Response of Apulian Red Wines. Foods 2019, 8, 453. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, K.; Bisson, F.L.; Adams, O.D. A Review of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Available online: https://www.apprrr.hr/registri/ (accessed on 2 April 2021).
- Lukić, I.; Radeka, S.; Budić-Leto, I.; Bubola, M.; Vrhovsek, U. Targeted UPLC-QqQ-MS/MS profiling of phenolic compounds for differentiation of monovarietal wines and corroboration of particular varietal typicity concepts. Food Chem. 2019, 300, 125251. [Google Scholar] [CrossRef]
- OIV Microbiological Analysis of Wines and Musts Method OIV-MA-AS4-01 Type IV Method. Compend. Int. Methods Anal. OIV 2010, 1–32.
- Oiv Standard for International Wine and Spirituous Beverages of Viticultural Origin Competitions. Resolution OIV 332A/2009. 2009, pp. 1–19. Available online: https://www.oiv.int/public/medias/4661/oiv-concours-332a-2009-en.pdf (accessed on 2 April 2021).
- Tomaz, I.; Maslov, L. Simultaneous Determination of Phenolic Compounds in Different Matrices using Phenyl-Hexyl Stationary Phase. Food Anal. Methods 2016, 9, 401–410. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges ll, Connaissance de la Vigne et du vin. Vigne Vin 1984, 18, 253–271. [Google Scholar]
- de Klerk, J.-L. Succinic acid Production by Wine Yeasts. Master’s Thesis, University of Stellenbosch, Stellenbosch, Africa, 2010. [Google Scholar]
- Korenika, A.-M.J.; Marinov, L.; Anđelini, D.; Jeromel, A. Yeasts and wine acidity profile. J. Cent. Eur. Agric. 2020, 21, 861–869. [Google Scholar] [CrossRef]
- Chidi, B.S.; Rossouw, D.; Buica, A.S.; Bauer, F.F. Determining the Impact of Industrial Wine Yeast Strains on Organic Acid Production Under White and Red Wine-like Fermentation Conditions. S. Afr. J. Enol. Vitic. 2015, 36, 316–327. [Google Scholar] [CrossRef]
- Caridi, A. Effect of protectants on the fermentation performance of wine yeasts subjected to osmotic stress. Food Technol. Biotechnol. 2003, 41, 145–148. [Google Scholar]
- Margalit, Y. Must and Wine Composition-Concepts in Wine Chemistry; Wine Appreciation Guild Ltd.: Hong Kong, China, 1997. [Google Scholar]
- Otteneder, H.; Marx, R.; Zimmer, M. Analysis of the anthocyanin composition of Cabernet Sauvignon and Portugieser wines provides an objective assessment of the grape varieties. Aust. J. Grape Wine Res. 2008, 10, 3–7. [Google Scholar] [CrossRef]
- Pérez-TrujilloJ, P.; Hernández, Z.; López-Bellido, F.J.; Hermosín-Gutiérrez, I. Characteristic Phenolic Composition of Single-Cultivar Red Wines of the Canary Islands (Spain). J. Agric. Food Chem. 2011, 59, 6150–6164. [Google Scholar] [CrossRef]
- Urvieta, R.; Buscema, F.; Bottini, R.; Coste, B.; Fontana, A. Phenolic and sensory profiles discriminate geographical indications for Malbec wines from different regions of Mendoza, Argentina. Food Chem. 2018, 265, 120–127. [Google Scholar] [CrossRef]
- Makris, D.P.; Kallithraka, S.; Mamalos, A. Differentiation of young red wines based on cultivar and geographical origin with application of chemometrics of principal polyphenolic constituents. Talanta 2006, 70, 1143–1152. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Colomo, B.; Suárez, J.A. Cell wall anthocyanin adsorption by different Saccharomyces strains during the fermentation of Vitis vinifera L. cv Graciano grapes. Eur. Food Res. Technol. 2005, 220, 341–346. [Google Scholar] [CrossRef]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404. [Google Scholar] [CrossRef]
- Rizzo, M.; Ventrice, D.; Varone, M.A.; Sidari, R.; Caridi, A. HPLC determination of phenolics adsorbed on yeasts. J. Pharm. Biomed. Anal. 2006, 42, 46–55. [Google Scholar] [CrossRef]
- Šikuten, I.; Štambuk, P.; Andabaka, Ž.; Tomaz, I.; Marković, Z.; Stupić, D.; Maletić, E.; Kontić, J.K.; Preiner, D. Grapevine as a Rich Source of Polyphenolic Compounds. Molecules 2020, 25, 5604. [Google Scholar] [CrossRef]
- Žurga, P.; Vahčić, N.; Pasković, I.; Banović, M.; Staver, M.M. Croatian Wines from Native Grape Varieties Have Higher Distinct Phenolic (Nutraceutic) Profiles than Wines from Non-Native Varieties with the Same Geographic Origin. Chem. Biodivers. 2019, 16, e1900218. [Google Scholar] [CrossRef]
- Smit, A.; Otero, R.R.C.; Lambrechts, M.G.; Pretorius, I.S.; Van Rensburg, P. Enhancing Volatile Phenol Concentrations in Wine by Expressing Various Phenolic Acid Decarboxylase Genes inSaccharomyces cerevisiae. J. Agric. Food Chem. 2003, 51, 4909–4915. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, V.A.P.; Glories, Y.; Monique, A. Developmental changes of procyanidins in grapes of red Vitis vinifera varieties and their composition in respective wines. Am. J. Enol. Vitic. 2000, 51, 397–403. [Google Scholar]
- Rastija, V.; Srečnik, G.; Medić-Šarićc, M. Polyphenolic composition of Croatian wines with different geographical origins. Food Chem. 2009, 115, 54–60. [Google Scholar] [CrossRef]
- Cantos, E.; García-Viguera, C.; De Pascual-Teresa, S.; Tomás-Barberán, F.A. Effect of postharvest ultraviolet irradiation on resveratrol and other phenolics of cv. Napoleon table grapes. J. Agric. Food Chem. 2000, 48, 4606–4612. [Google Scholar] [CrossRef] [PubMed]
- Celotti, E.; Ferrarini, R.; Zironi, R.; Conte, L.S. Resveratrol content of some wines obtained from dried Valpolicella grapes: Recioto and Amarone. J. Chromatogr. A 1996, 730, 47–52. [Google Scholar] [CrossRef]
- Jeandet, P.; Bessis, R.; Maume, B.F.; Meunier, P.; Peyron, D.; Trollat, P. Effect of Enological Practices on the Resveratrol Isomer Content of Wine. J. Agric. Food Chem. 1995, 43, 316–319. [Google Scholar] [CrossRef]
- Vacca, V.; Leccis, L.; Fenu, P.; Pretti, L.; Farris, G.A. Wine yeasts and resveratrol content. Biotechnol. Lett. 1997, 19, 497–498. [Google Scholar] [CrossRef]
- Rojas, I.B.; Smith, P.A.; Bartowsky, E.J. Influence of choice of yeasts on volatile fermentation-derived compounds, colour and phenolics composition in Cabernet Sauvignon wine. World J. Microbiol. Biotechnol. 2012, 28, 3311–3321. [Google Scholar] [CrossRef] [PubMed]
| Lalvin ICV D21 | Fermol Power | Fermol Grand Rouge | Fermol Premier Cru | Fermol Super 16 | |
|---|---|---|---|---|---|
| Yeast | S. cerevisiae | S. cerevisiae | S. cerevisiae | S. cerevisiae | S. cerevisiae |
| Temperature range | 16–30 °C | low temperatures | up to 30 °C | 18–34 °C | up to 34 °C |
| Fermentation speed | moderate | normal | normal | normal | normal |
| SO2 production | low | low | low | low | low |
| Alcohol tolerance | high (16 vol%) | high (14 vol%) | high (15 vol%) | high | high (18 vol%) |
| Nitrogen needs | medium | low | medium | medium | medium |
| Compounds | Vis | Korčula | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S16 | GR | P | PC | D21 | S16 | GR | P | PC | D21 | |
| Alcohol (%, v/v) | 13.9 | 14.1 | 14.4 | 14.2 | 14.1 | 13.8 | 13.8 | 13.6 | 13.9 | 13.5 |
| Dry extract (g/L) | 29.8 b | 30.5 ab | 31.1 a | 29.2 b | 30.4 ab | 26.5 b | 27.7 a | 26.4 b | 25.6 b | 26.9 b |
| Reducing sugars (g/L) | 3.5 | 3.2 | 3.2 | 3.8 | 3.9 | 3.1 | 3.8 | 3.5 | 3.3 | 3.1 |
| Total acidity * (g/L) | 6.5 b | 6.9 ab | 7.0 a | 6.4 b | 7.0 a | 5.2 b | 5.8 a | 5.3 b | 5.4 b | 5.7 a |
| Volatile acidity ** (g/L) | 0.40 | 0.45 | 0.44 | 0.48 | 0.37 | 0.34 | 0.36 | 0.38 | 0.32 | 0.30 |
| pH | 3.52 | 3.48 | 3.52 | 3.51 | 3.51 | 3.72 | 3.57 | 3.63 | 3.59 | 3.65 |
| Malic acid (g/L) | 1.01 b | 1.13 ab | 1.13 a | 0.93 ab | 1.31 a | 0.64 b | 0.69 b | 0.66 b | 0.75 b | 0.95 a |
| Succinic acid (g/L) | 0.85 b | 0.86 b | 0.92 a | 0.82 b | 0.99 a | 0.64 c | 0.74 b | 0.85 a | 0.75 b | 0.88 a |
| Location | Alcohol (%, v/v) | Dry Extract (g/L) | Reducing Sugars (g/L) | Total Acidity * (g/L) | Volatile Acidity ** (g/L) | pH | Malic Acid (g/L) | Succinic Acid (g/L) |
|---|---|---|---|---|---|---|---|---|
| VIS | 14.14 a | 30.20 a | 3.52 a | 6.76 a | 0.43 a | 3.51 b | 1.10 a | 0.89 a |
| KORČULA | 13.72 b | 26.62 b | 3.36 a | 5.48 b | 0.32 b | 3.63 a | 0.74 b | 0.77 b |
| Compounds (mg/L) | Vis | Korčula | Vis | Korčula | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S16 | GR | P | PC | D21 | S16 | GR | P | PC | D21 | |||
| Delphinidin-3-O-glucoside | 32.63 a | 24.64 b | 30.55 a | 25.47 b | 25.84 b | 18.95 a | 11.41 c | 14.15 b | 7.75 d | 6.78 e | 27.82 a | 11.81 b |
| Cyanidin-3-O-glucoside | 1.46 bc | 1.35 c | 1.84 a | 0.85 d | 1.53 b | 0.22 a | 0.11 b | 0.13 b | 0.24 a | 0.14 b | 1.41 a | 0.17 b |
| Petunidin-3-O-glucoside | 17.90 a | 13.52 d | 16.67 b | 16.26 b | 14.85 c | 17.01 a | 10.42 c | 12.38 b | 7.82 d | 7.76 d | 15.84 a | 11.08 b |
| Peonidin-3-O-glucoside | 9.64 b | 8.61 c | 11.18 a | 7.45 d | 9.98 b | 4.45 a | 3.01 b | 4.17 a | 2.30 c | 2.12 c | 9.37 a | 3.21 b |
| Malvidin-3-O-glucoside | 225.96 a | 196.05 c | 223.06 a | 226.07 a | 214.08 b | 349.16 a | 239.70 c | 298.64 b | 210.74 d | 215.70 d | 217.04 b | 262.79 a |
| Ʃ Anthocyanins | 287.58 a | 244.16 d | 283.29 a | 276.10 b | 266.27 c | 389.78 a | 264.63 c | 329.46 b | 228.83 d | 232.48 d | 271.48 a | 289.04 a |
| Quercetin-3-O-glucoside | 7.86 a | 5.44 d | 7.14 b | 7.02 b | 6.12 c | 7.16 b | 5.44 c | 7.95 a | 4.08 d | 3.27 e | 6.72 a | 5.58 b |
| Quercetin | 3.07 a | 1.77 d | 2.43 b | 2.57 b | 2.23 c | 0.96 a | 0.67 a | 0.79 a | 0.69 a | 0.75 a | 2.41a | 0.77 b |
| Kaempferol | 0.33 a | 0.28 a | 0.32 a | 0.28 a | 0.30 a | 0.24 a | 0.18 b | 0.23 a | 0.19 b | 0.20 ab | 0.30 a | 0.21 b |
| Ʃ Flavonols | 11.25 a | 7.49 d | 9.89 b | 9.87 b | 8.64 c | 8.34 b | 6.29 c | 8.97 a | 4.95 d | 4.22 e | 9.42 a | 6.55 b |
| trans-caftaric acid | 33.59 a | 30.24 c | 31.98 b | 33.75 a | 31.13 bc | 31.94 ab | 28.66 bc | 32.49 a | 25.17 c | 18.11 d | 32.14 a | 27.27 b |
| Caffeic acid | 3.80 ab | 3.28 c | 4.23 a | 3.44 bc | 3.91 ab | 1.63 b | 1.56 b | 1.85 a | 1.54 b | 1.55 b | 3.73 a | 1.63 b |
| trans-coutaric acid | 5.12 b | 4.42 c | 5.11 b | 5.57 a | 5.09 b | 5.36 b | 4.65 c | 6.38 a | 4.01 d | 2.86 e | 5.06 a | 4.65 b |
| trans-coumaric acid | 0.89 c | 1.82 a | 1.75 a | 1.10 b | 1.84 a | 0.32 c | 0.75 a | 0.53 b | 0.43 bc | 0.87 a | 1.48 a | 0.58 b |
| Ferulic acid | 0.32 c | 0.54 a | 0.48 ab | 0.44 b | 0.57 a | 0.19 b | 0.29 b | 0.21 b | 0.20 b | 0.57 a | 0.47 a | 0.29 b |
| Gallic acid | 21.59 a | 17.89 b | 17.67 b | 16.34 b | 16.00 b | 29.03 a | 27.21 ab | 25.32 b | 21.96 c | 20.92 c | 17.90 b | 24.89 a |
| Syringic acid | 2.44 a | 2.12 ab | 1.86 b | 2.17 ab | 1.98 b | 3.87 a | 3.34 bc | 3.55 b | 3.20 c | 3.41 bc | 2.11 b | 3.47 a |
| Ʃ Phenolic acids | 67.74 a | 60.32 b | 63.08 b | 62.79 b | 60.50 b | 72.32 a | 66.45 b | 70.32 a | 56.50 c | 48.27 d | 62.88 a | 62.77 a |
| (+)-Gallocatechin | 1.65 a | 1.49 b | 1.54 ab | 1.44 b | 1.57 ab | 1.16 c | 1.17 c | 1.57 a | 1.34 b | 0.96 d | 1.53 a | 1.24 b |
| Procyanidin B1 | 70.04 c | 59.14 e | 101.70 b | 66.96 d | 128.73 a | 74.55 b | 57.87 c | 49.75 d | 57.79 c | 92.75 a | 71.31 a | 66.54 b |
| (-)-Epigallocatechin | 20.67 a | 17.53 c | 17.38 c | 19.17 b | 13.60 d | 14.67 a | 11.90 bc | 12.58 b | 10.85 cd | 9.42 d | 17.67 a | 11.88 b |
| Procyanidin B3 | 2.16 a | 2.06 a | 1.94 a | 2.08 a | 2.17 a | 2.22 a | 1.87 b | 1.84 b | 1.44 c | 1.39 c | 2.08 a | 1.75 b |
| (+)-Catechin | 29.97 a | 23.71 c | 24.45 c | 26.76 b | 22.43 c | 16.08 a | 13.72 b | 13.87 b | 10.40 c | 9.45 c | 25.46 a | 12.70 b |
| Procyanidin B4 | 4.12 a | 3.71 b | 3.74 b | 4.14 a | 3.60 b | 3.68 a | 3.08 b | 3.14 b | 2.59 c | 2.40 c | 3.86 a | 2.98 b |
| Procyanidin B2 | 7.94 a | 6.14 c | 6.65 b | 6.55 b | 5.58 d | 6.67 a | 6.31 b | 5.80 c | 4.68 d | 4.38 e | 6.57 a | 5.57 b |
| (-)-Epicatechin | 18.70 a | 14.63 c | 16.58 b | 16.10 b | 13.57 d | 10.95 a | 10.80 a | 7.90 b | 7.01 c | 6.83 c | 15.92 a | 8.69 b |
| Ʃ Flavan-3-ols | 155.24 c | 128.39 e | 173.95 b | 143.19 d | 191.24 a | 129.96 a | 106.70 b | 96.45 c | 96.09 c | 127.56 a | 158.40 a | 111.35 b |
| trans-piceid | 11.06 ab | 9.51 c | 12.29 a | 9.90 bc | 10.57 bc | 3.94 b | 3.61 c | 4.49 a | 2.74 d | 2.12 e | 10.67 a | 3.38 b |
| Wine | A420 | A520 | A620 | I.C. | T | Chromatic Structure | ||
|---|---|---|---|---|---|---|---|---|
| % Yellow Pigments | % Red Pigments | % Blue Pigments | ||||||
| Vis D21 | 2.21 | 3.33 | 0.55 | 6.09 b | 0.66 a | 36.28 a | 54.67 c | 9.03 b |
| Vis P | 2.39 | 3.68 | 0.55 | 6.62 a | 0.64 a | 36.10 a | 55.58 b | 8.30 c |
| Vis PC | 2.45 | 3.75 | 0.58 | 6.78 a | 0.65 a | 36.13 a | 55.30 bc | 8.55 c |
| Vis S16 | 2.43 | 3.99 | 0.58 | 7.00 a | 0.60 b | 34.71 c | 57.00 a | 8.28 c |
| Vis GR | 2.30 | 3.50 | 0.61 | 6.41 b | 0.65 a | 35.88 b | 54.60 c | 9.51 a |
| Korčula D21 | 1.84 | 2.57 | 0.45 | 4.87 d | 0.71 a | 37.80 a | 52.87 c | 9.33 b |
| Korčula P | 2.85 | 4.56 | 0.84 | 8.25 b | 0.62 b | 34.54 c | 55.27 a | 10.18 a |
| Korčula PC | 1.89 | 2.88 | 0.51 | 5.29 d | 0.65 b | 35.72 b | 54.44 b | 9.64 b |
| Korčula S16 | 3.18 | 5.12 | 0.89 | 9.20 a | 0.62 b | 34.56 c | 55.65 a | 9.67 a |
| Korčula GR | 2.33 | 3.51 | 0.60 | 6.44 c | 0.64 b | 36.18 b | 54.50 b | 9.31 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagatić Korenika, A.-M.; Tomaz, I.; Preiner, D.; Plichta, V.; Jeromel, A. Impact of Commercial Yeasts on Phenolic Profile of Plavac Mali Wines from Croatia. Fermentation 2021, 7, 92. https://doi.org/10.3390/fermentation7020092
Jagatić Korenika A-M, Tomaz I, Preiner D, Plichta V, Jeromel A. Impact of Commercial Yeasts on Phenolic Profile of Plavac Mali Wines from Croatia. Fermentation. 2021; 7(2):92. https://doi.org/10.3390/fermentation7020092
Chicago/Turabian StyleJagatić Korenika, Ana-Marija, Ivana Tomaz, Darko Preiner, Vedran Plichta, and Ana Jeromel. 2021. "Impact of Commercial Yeasts on Phenolic Profile of Plavac Mali Wines from Croatia" Fermentation 7, no. 2: 92. https://doi.org/10.3390/fermentation7020092
APA StyleJagatić Korenika, A.-M., Tomaz, I., Preiner, D., Plichta, V., & Jeromel, A. (2021). Impact of Commercial Yeasts on Phenolic Profile of Plavac Mali Wines from Croatia. Fermentation, 7(2), 92. https://doi.org/10.3390/fermentation7020092








