Abstract
Previous research has shown that hops contain enzymes able to hydrolyze unfermentable dextrins into fermentable sugars when added during the dry-hopping process. In the presence of live yeast, these additional fermentable sugars can lead to an over-attenuation of the beer; a phenomenon known as “hop creep”. This study attempts to analyze the effect of different Saccharomyces yeast species and strains on hop creep, with the intent to find an ability to mitigate the effects of dry-hop creep by using a specific yeast. Thirty different yeast species and strains were chosen from commercial and academic collections and propagated for pilot fermentations. Brews were performed at the Anheuser-Busch Research Brewery (1.8 hL, 10 °P, 20 IBU) at UC Davis and split to 40 L cylindroconical fermenters, with one fermenter in each yeast pair receiving 10 g/L Centennial hop pellets towards the end of fermentation. Standard analytical measurements were performed over the course of fermentation, with real degrees of fermentation (RDF) and extract measured on an Anton Paar alcolyzer. In order to preemptively determine the amount of hop creep to be experienced with each unknown fermentation, bench-top fermentations with 20 g/L dry-hops were performed concurrently and compared to the pilot scale fermentations. RDF was significantly higher (p < 0.01) on dry-hopped than non-dry-hopped fermentations beginning two days post dry-hopping to the end of fermentation, with the exceptions of SafAle™ BE-134, a S. cerevisiae var. diastaticus, and UCDFST 11-510, a S. mikatae. No apparent correlation between flocculation and increased RDF was shown in dry-hopped treatments. pH was significantly different between the dry-hopped and non-hopped fermentations (p < 0.05 one day post dry-hop, p < 0.01 for all subsequent days); this may have impacted on additional attenuation. No yeasts in this study indicated their use for mitigation of dry-hop creep, but this is a first look at beer fermentation for some of the chosen yeasts. The results also present a new perspective on how hop creep varies in fermentation.
1. Introduction
Traditionally in the production of beer, hops (Humulus lupulus) are added in the brewhouse in relatively small quantities, adding bitterness to balance the sugary wort, flavor and aroma to the finished beer, foam and microbiological stability, and clarity in the brewhouse [1,2,3]. Hops (cones or pellets) are also added to beer when fermentation is active or finished, a process called dry-hopping, historically to provide packaging and transport stability [1,4] and more recently to add intense hop aroma and flavor [5,6]. In craft breweries, dry-hopping has become the standard procedure for adding hop flavor and aroma without the resultant bitterness of the α-acid content to many styles, but most frequently to the India Pale Ale (IPA) style of beer [6,7]. The Brewers’ Association, trade group for craft brewers in the USA, has reported IPA as the most purchased beer style from their members for more than a decade, and as more of these small breweries are operating than ever before in history, dry-hopping is more increasingly frequent [8,9]. Dry-hopping has become so ubiquitous that even global brands like Budweiser [10] and Guinness [11] are producing and advertising dry-hopped beers, as craft beer grows around the world [12].
Typical wort produced with malted barley has a carbohydrate content composed of 60% maltose, 15–20% maltotriose, 5–10% glucose, and less than 5% each of sucrose and fructose. The 5–10% remaining is composed of longer chain oligosaccharides, or “dextrins”, that are typically unfermentable by Saccharomyces yeasts [13,14,15]. Standard brewing yeasts assimilate the glucose, fructose, and sucrose within the first twenty-four hours, before moving to the fermentation of maltose, and finally maltotriose after the other sugars have been used, leaving behind the unfermentable dextrins [14]. These residual sugars generally remain stable through to the consumer, providing body and mouthfeel to create a balanced beer. Yeast also produces alcohol, carbon dioxide, and other desirable fermentation byproducts that contribute to the overall flavor of beer [16], and can create even more unique flavors in the presence of dry-hops [17]. There is evidence that hops contain enzymes that enzymatically alter the composition of beer during dry-hopping, contributing to the hydrolysis of the aforementioned unfermentable dextrins into fermentable sugars [4,18,19,20,21]. In the presence of live yeast, the newly present fermentable sugars can lead to an over-attenuation of the beer, which is commonly referred to by brewers as “hop creep.”
This over-attenuation can result in higher alcohol and lower residual sugar contents in the packaged product if not mitigated with pasteurization or filtration, which can come with consequences from the regulatory bodies in the United States and elsewhere [22]. If beers are dry-hopped after fermentation is complete, hop creep can cause yeast to leave dormancy, yielding higher amounts of yeast-related off-flavors such as diacetyl and acetaldehyde [23,24,25]. These off-flavors create a variation in consistency and quality for breweries and their consumers. Perhaps the most frightening effect of hop creep is when unfiltered beer is packaged with residual yeast, as is common at most craft breweries. With the residual dextrins being hydrolyzed by hop enzymes and the resultant over-attenuation, over carbonation in the container can also occur, causing safety concerns for the consumers of bottles or cans of this beer. Industry standard bottles (ISBs) suggest no greater than three volumes of CO2 (6 g/L). With a typical carbonation level in beer being 5 g/L, only 0.1 °P of additional fermentable sugar is adequate to encroach on the pressure limit of an ISB when calculating CO2 produced with the ideal gas equation [14].
The fermentation of beer is typically carried out using either one of two species of Saccharomyces yeast. S. pastorianus is a bottom-fermenting lager yeast and is the most commonly utilized fermentative in the world of beer. S. cerevisiae is a top-fermenting ale yeast, which is more common in craft beer and traditionally used for IPAs [16]. The ability of these yeasts to ferment the wort sugars into alcohol is commonly referred to attenuation or degree of fermentation, expressing the relative remaining extract in the beer with considerations for the comparative destiny of ethanol produced and any other solids in suspension contributing to density. It can be measured a multitude of ways, but the most effective method given the resources is using near-infrared (NIR) detection on a density meter equipped with these capabilities [26]. Using this instrument, a researcher can accurately determine the real degree of fermentation (RDF), a direct correlation of the attenuation of a certain yeast taking into account variability in the gravity of the starting wort [27,28].
Previous research has attempted to quantify the enzymatic power of multiple hop cultivars (varieties) and relate this to their hop creep potential using only one S. cerevisiae yeast strain [29]. Thus, to shift focus from hops to how yeasts deal with potential hop creep, the aims of this research are to analyze the effect of different Saccharomyces yeast species and strains on the hop creep phenomenon as well as relate yeast flocculation and hop creep, while holding all other variables, including the hop variety, constant. Yeast strain or species potential for dry-hop creep has not yet been investigated, and research into this area has been at the top of mind for craft brewers, as participants of an industry forum requested a study such as the one performed here be initiated [30]. Due to the lack of previous beer brewing potential of a number of the yeasts used in this study, a previously developed method will be used as a way to anticipate the amount of hop creep to be experienced with each pilot fermentation [31,32]. In addition, some brewers hold the belief that hop creep yeast variability is tied to a specific strain’s flocculation, or the tendency of yeast cells to aggregate together; this research will investigate a correlation. The outcomes of this research hopes to identify specific Saccharomyces species and strains that may have an ability to mitigate the effects of dry-hop creep, as this would be of incredible value to the commercial brewing industry.
2. Materials and Methods
2.1. Experimental Beers
Sixteen pilot scale brews were performed on a 1.8 hL brewhouse in the Anheuser-Busch Research Pilot Brewery at University of California, Davis. The experimental beer attempted to emulate an American Pale Ale or Session IPA, with a target of 10.0 °P original gravity. Mash water consisted of deionized water was adjusted using CaCl2 and CaSO4 salts to 85 ppm calcium, 95 ppm sulfate, and 80.0 ppm chloride and a target mash pH of 5.3. This was added to the grist at a liquor-to-grist ratio of 3:1 (L:kg) and held at 65 ° C for 60 min., then heated to 72 °C for 5 min. for mash out (Figure 1). The grain bed was sparged with fresh deionized water using a lauter tun and the wort was extracted at an average of 4.0 L per minute until a kettle full volume was achieved. Wort was boiled for seventy-five minutes with an evaporation rate of 10% per hour on a kettle with steam-powered internal calandria. Pelletized hops were added at 0.8 g/L with sixty minutes remaining in the boil to target 20 IBUs, and 0.08 g/L of yeast nutrient (Kerry Yeastex® 82; Beloit, WI, USA) added at the end of boil. Wort was then whirlpooled and allowed ten minutes for trub to settle before being knocked out on a dual stage plate and frame heat exchanger to a target fermentation temperature of 20 °C. Each brew was split evenly by volume into four, 40 L glycol-cooled cylindroconical fermenters (JV Northwest; Canby, OR, USA).
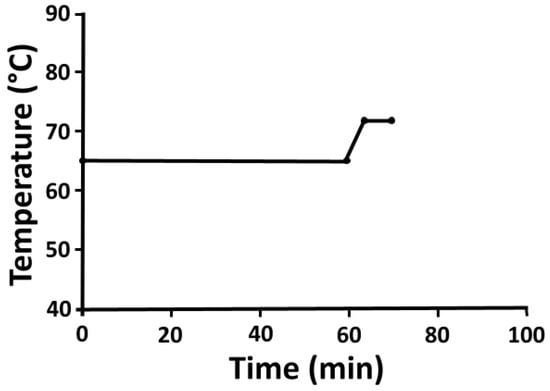
Figure 1.
Target mash profile for the experimental beers performed. Malt and pH-adjusted water was held at 65 °C for 60 min., then heated to 72 °C and held for 5 min. to mimic the typical single-step infusion mash used by many American craft brewers.
2.2. Malt
All malt was supplied by Admiral Maltings (Alameda, CA, USA; admiralmaltings.com, accessed on 26 April 2021) and milled fresh for each brew at the pilot brewhouse on a Seeger two-roller dry mill, type ZSM-0 mini (Schmidt-Seeger AG; Beilngries, Germany) to standard crush: 70% retained above the 75 mm sieve, with 25% above and 5% below the 45 mm sieve. The grist consisted of 25 kg of pilsner malt (84.2%, equal blend of the two batches) made with Butta 12 barley [33] grown in Esparto, CA, USA, 3.1 kg (10.4%) of chit malt made with organic Copeland barley grown in Tulelake, CA, USA, and 1.6 kg (5.4%) of kilned caramel malt made with UC Davis Experimental barley grown in Esparto, CA. Malt specifications available below with values reported from supplier (Table S1).
2.3. Hops
Pelletized T-90 hops of the Centennial cultivar were provided by Hopsteiner (S.S. Steiner; New York, NY, USA). They were used both in the boil and for dry-hop due to their use as a dual-purpose hop, common in American craft brewing as both aroma and bittering uses. This hop was also used extensively in previous research regarding the hop creep phenomenon [20,29,31]. The hops were of 2020 crop year and reported to contain 8.3% α-acid, 3.7% β-acid, 8.3% moisture, a hop storage index (HSI) of 0.502, and delivered in 5 kg packages sealed with nitrogen cover gas in mylar packages. Upon receiving, hops were sorted into separate vacuum-sealed packages for each bittering and dry-hop addition for all brews, then stored in a refrigerated room at 1 °C until use.
2.4. Yeasts
Yeasts (Table 1) were chosen based on either their widespread commercial use in dry-hopped beer, historical significance, or unique characteristics. Yeast from Berkeley Yeast (Oakland, CA, USA; berkeleyyeast.com, accessed on 26 April 2021) was provided on a yeast peptone dextrose agar (YPD) plate and stored at room temperature until propagation. Yeasts from White Labs (San Diego, CA, USA; www.whitelabs.com, accessed on 26 April 2021) were provided in 35 mL PurePitch™ packages and stored at 4 °C until propagation. Yeasts from Fermentis (Marcq-en-Baroeul, France; fermentis.com/en/, accessed on 26 April 2021) were provided as active dry yeast in mylar sachets with an emulsifier (E491, sorbitan monostearate) and stored at 4 °C until use. Novel yeasts were supplied by the UC Davis Phaff Yeast Culture Collection (Davis, CA, USA; phaffcollection.ucdavis.edu, accessed on 26 April 2021), and were revived from cryogenic storage and streaked onto potato dextrose agar (PDA) plates, then incubated for two days at 30 °C before being moved to room temperature storage. Due to time constraints with research brewing, only one yeast was chosen on which to perform three biological replicates to ferment from three separate brews: SafAle™ US-05.

Table 1.
Thirty Saccharomyces yeast species used in the fermentations of the experimental beer, reported in alphabetical order. Yeasts were sourced from either the Phaff Yeast Culture Collection at University of California, Davis (UCD), White Labs of San Diego, CA, USA (WLP), Berkeley Yeast of Oakland, CA, USA (BY), or from Fermentis LeSaffre of Marcq-en-Baroeul, France (Saf), signified in the “Yeast Name” column. “Comparable Strains” determined as best estimate by the researcher, or genetic sequence for UCDFST yeasts. “Origin” as defined by original source or colloquial name. “Attenuation” and “Flocculation” is defined in yeast supplier literature or research as previously known values, designated as percent values when possible [34].
2.5. Yeast Propagation
Propagation wort consisted of 10% w/v (10 °P, 1.040 Specific Gravity) dried pilsner malt extract (Briess CBW® Pilsen Light; Chilton, WI, USA) in deionized water with 20 ppm CaCl2 salts and 0.1% w/v yeast nutrient, targeting 5.2 pH. Wort was boiled for ten minutes then sterilized via autoclave before being sterile filtered to remove protein and trub particulate. All transfers of yeast and wort were done in a laminar flow hood or positive pressure room. Yeast cell counts and viability were performed on all propagations and fermentations according to standard methods [35].
Yeast colonies were transferred from plate or package via sterile inoculation loop to 10 mL of propagation wort in a 15 mL conical tube and placed on an orbital shaker table (Innova™ 2000, New Brunswick Scientific; Edison, NJ, USA) set to 150 rpm for 24 h. at room temperature. The contents of that tube were then vortexed and transferred to a 50 mL conical tube containing 20 mL of fresh sterile propagation wort and placed on the orbital shaker table as above for an additional twenty-four hours at room temperature. The tube was then vortexed and transferred to a 125 mL Erlenmeyer flask containing 50 mL of fresh sterile propagation wort and placed on the orbital shaker table as above for twenty-four hours at room temperature. The contents of that flask were then homogenized and transferred to a 250 mL Erlenmeyer flask containing 100 mL of fresh sterile propagation wort and placed the same shaker table for 24 h. The contents of that flask were then homogenized and transferred to a 1 L glass bottle containing an additional 200 mL fresh sterile propagation wort and placed on the shaker table for 48 h. at room temperature. This final step was repeated two additional times, after which an additional 200 mL of sterile propagation wort was added and the total volume was split between two sterile bottles before both were placed back on the same shaker for 24 h. at room temperature. The propagation for each yeast was completed over 11 days (Figure 2).
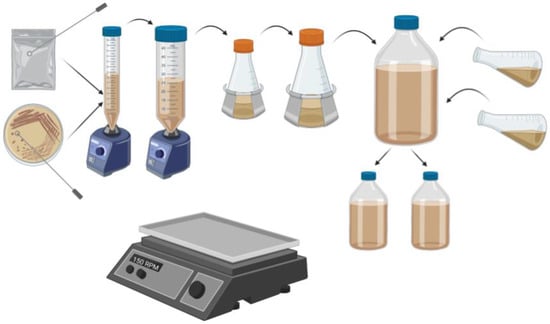
Figure 2.
Yeast was transferred for propagation as shown in this schematic diagram. Yeasts were propagated to a desired total amount of 40 × 1010 billion cells in each bottle with 390 mL of propagation wort, equivalent to the standard ale pitch rate of 10.0 × 105 cells per mL per °P [16] for the 40 L pilot fermentation. Figure created on BioRender.com, accessed on 26 April 2021, not to scale.
2.6. Sample Collection and Preparation
Beers were aseptically sampled daily within a two-hour window of knockout time. 50 mL conical tubes of each sample were centrifuged (ThermoFisher Scientific; Waltham, MA, USA) at 20.0 °C and 3000× g RCF for 5 min. The clarified supernatant was then degassed for 5 min. using the degas setting on a VWR B1500A-DTH 1.9 L ultrasonic cleaner (Radnor, PA, USA). Degassed samples were then decanted into the sample tubes of the Anton Paar (Graz, Austria) auto-sampling carousel for immediate analysis.
2.7. Pilot Fermentations
Pilot fermentations of 40 L were set to 20 °C with each unique Saccharomyces species or strain (Table 1) being transferred to its own fermenter in duplicate, totaling sixty-four fermentations. One fermenter in each yeast pair received 10 g/L (equivalent to 2.59 lbs./BBL) as a dry-hop when the fermentation reached between 3.0 and 4.0 °P gravity, or at seven days into fermentation, whichever occurred first. This amount of dry-hopping has become standard practice among craft breweries today, with many brewers far exceeding this amount at times [5,6,7,12,36]. End of fermentation at a commercial brewery is delineated as “terminal gravity” and defines when the yeast has assimilated all the available fermentable sugars [14]. Terminal gravity in this study was defined a change of less than 0.1 °P gravity for two simultaneous days following dry-hop, similar to methods utilized in commercial breweries.
2.8. Bench-Top Fermentations
Bench-top fermentations were performed to preemptively determine the amount of dry-hop creep to be experienced with each pilot scale fermentation [31,32]. 48 h. after initial yeast pitch to fermenter, 100 mL of high krausen green beer was aseptically sampled into two sterilized 250 mL Erlenmeyer flasks with magnetic stir bars. Hops were added to one of the flasks at double the dry-hopping rate of the pilot brews, equivalent to 20 g/L, and the openings for all flasks were covered with aluminum foil. Flasks were placed on a stir plate set to 225 rpm in a laminar flow hood for two days at room temperature, after which the samples were clarified as above. Degassing was deemed unnecessary as these samples were under constant agitation at room temperature and not under pressure. Centrifuged supernatant was transferred to the Anton-Paar sample tubes as above.
2.9. Analytical Measurements
Samples were then measured for extract, gravity, alcohol [26], and RDF using an Anton Paar Density Meter (DMA 5000 M) and alcolyzer (Alcolyzer Beer M) with an auto sampling carousel. The DMA 5000M instrument measures density by means of the built-in oscillating density meter and the Alcolyzer Beer M separately measures absorbance at NIR wavelengths (750 nm to 2500 nm) to calculate the alcohol content of the sample. From these two values, alcohol (% v/v and w/w), apparent and real extract, original extract, specific gravity, and RDF are calculated and reported from each sample. The DMA 5000 M has a repeatability within 0.000001 g/mL and the Alcolyzer Beer M has a repeatability within 0.03 °P and 0.01% v/v alcohol. pH was measured on a ThermoFisher Scientific benchtop pH meter that received three-point calibration weekly.
2.10. Statistical Analysis
In order to correlate the two concepts of flocculation and attenuation for this study, a numerical value was assigned to these (type converting): a “very low” value equating to a number of 0, “low” to a value of 1, “medium-low” to 2, “medium” to 3, “medium-high” to 4, “high” to 5, and “very high” to a value of 6 (Table 1). Standard deviation for RDF and pH values, correlation (R2 and Pearson’s) values for flocculation and RDF, as well as one-tailed statistical analysis (t-test) and corresponding p value were performed in Microsoft® Excel 2019, Version 2102 (Build 13801.20360).
3. Results and Discussion
3.1. Pilot Fermentations
Real degree of fermentation (RDF) is a measure expressing the degree to which the available extract was fermented and reported as a percentage calculated from the ethanol content and gravity of the remaining extract. RDF was chosen as the basis of comparison for these yeasts due to its ability to relate all fermentations, regardless of the variability in starting gravities. The potential limitation of the RDF calculation is that the enzymatic degradation of the dextrins to fermentable sugars in hop creep can only be quantified if those sugars are fermented by yeast to produce more ethanol. Without a change in density and alcohol, the effect on attenuation cannot be measured, but this study’s main focus was on the fermentation parameters of variable yeasts, therefore the RDF calculation is appropriate.
With two exceptions, all fermentations in this study showed an increased attenuation when comparing dry-hopped to non-dry-hopped beers, ranging from 0.24% to 12.5% increased RDF with dry-hops added (Table 2). All yeasts, with one exception, reached terminal gravity within two weeks. These kinetics indicated all yeasts were appropriately pitched with an adequate cell count to complete fermentations within a typical ale production schedule. The exception was UCDFST 01-157, a S. pastorianus strain isolated originally by Emil Christian Hansen from Carlsberg Brewery in 1888 [16]. The long lag phase of UCDFST 01-157 may be from the relative age of the culture, as most production breweries have switched from the type I Saaz strains to type II Frohberg strains due to its increased fermentation kinetics and cleaner profile [37]. The sluggish fermentation may also be due to its increased adaptation to ferment at colder lager fermentation temperatures, as has been shown previously [37,38].

Table 2.
Heat Map of the Real Degrees of Fermentation (RDF), expressed as a percentage based on the color values associated to the scale at left, for all paired dry-hopped and non-dry-hopped 40 L pilot fermentations. The difference in RDF between the samples that were dry-hopped and those that were not is also reported. A negative difference value represents a lower RDF in the dry-hopped treatment when compared to the non-hopped and vice versa.
The two exceptions from the average in this study that showed negative difference in RDF between the paired dry-hopped and non-hopped fermentations are SafAle™ BE-134 and UCDFST 11-510. SafAle™ BE-134 is a Belgian yeast strain known to be S. cerevisiae var. diastaticus, a variant of brewing yeast that contains the extracellular glucoamylase STA1 gene, capable of hydrolyzing dextrins without the presence of the enzymes from dry-hopping [39,40]. The var. diastaticus strain used in this study fermented rapidly, reaching predetermined dry-hop conditions in less than two days, and completing fermentation in just one week, with 76.3% and 75.3% RDF for the non-hopped and dry-hopped beers, respectively. Both of these RDFs were the highest values reported in this study, as expected from the manufacturer’s specifications for attenuation.
The other exception, UCDFST 11-510, is a S. mikatae species of yeast, which has only been used in alcoholic beverage fermentations, specifically wine, as a hybrid with S. cerevisiae [41,42]. On its own, this strain of S. mikatae was a poor fermenter, attenuating only 14.2% and 13.5% RDF between the non-hopped and dry-hopped beers in this study, respectively. Neither of these yeasts presented themselves as appropriate yeasts to use in order to reduce the effects of hop creep. Due to fermentation characteristics, both present other challenges in commercial production, but are both unique yeasts that can offer desirable beer profiles if used appropriately.
On average, fermentations with all yeasts experienced an increased attenuation from the addition of dry-hops 3.54% ± 3.19 (Figure 3). Excluding the slow fermenter of UCDFST 01-157 and the low attenuator UCDFST 11-510 discussed above, a p value of <0.01 was calculated for days two to terminal following dry-hop; even with those two yeasts included, there were significant changes for days two through five following dry-hop (p < 0.01). This means there is a significantly different value for the average RDF in the dry-hopped versus non-hopped fermentations.
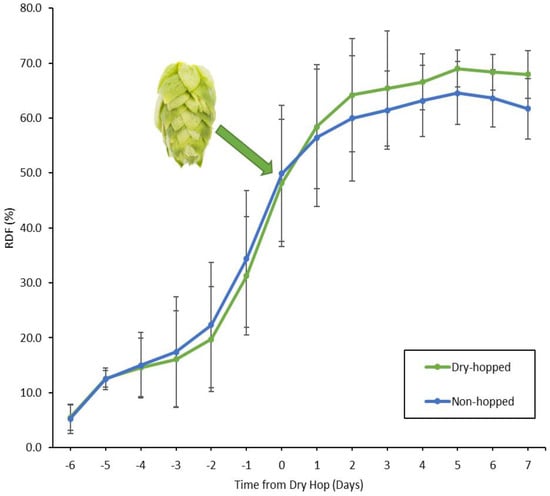
Figure 3.
Average of the RDF for all yeasts in this study, excluding UCDFST 01-157 and UCDFST 11-510, expressed as a percentage, relative to the days from performing of the dry-hop, with day 0 as dry-hop day. The green hop cone signifies the point of dry-hop, where a clear increase in RDF of the dry-hopped beers is observed. The average RDFs begin to decrease five days after dry-hop due to the relationship between fermentation kinetics and attenuation, meaning if the beer took longer than twelve days to fully ferment, the degree of fermentation tended to be lower overall. Error bars indicate standard deviation.
3.2. Bench-Top Fermentations
Most fermentations at bench scale reached terminal gravities lower than the pilot scale counterparts, but not all strains were effective when using the method previously devised to predict the amount of hop creep in unknown fermentations [31,32]. This perhaps help illuminate the inherent flaw of the methods, but still indicates that a bench-top fermentation with dry-hops is a promising tool for assessing the potential extent of hop creep when trialing a new beer or ingredient. It is suggested additional yeast is added to bench top fermentations in order to ensure maximum attenuation; this approach, called an end fermentation measurement, is taken from ASBC method Beer—16 [43].
Clear outliers exist in comparisons of pilot vs. benchtop fermentations of BY881, SafAle™ K-97, UCDFST 11-512, UCDFST 11-515, and UCDFST 96-12 on the non-hopped samples, and UCDFST 01-157 on the dry-hopped sample (Table 3), signifying sluggish fermentations according to the ASBC End of Fermentation method. The lowered RDF seen on the bench fermentations for these strains signal the yeast pitch volume did not meet recommended guidelines or was low vitality upon initial pitch [43,44], but the pilot fermentations all finished with acceptable RDFs (Table 2), with only one extended lag period previously discussed with UCDFST 01-157.

Table 3.
Heat Map of the Real Degrees of Fermentation (RDF), expressed as a percentage expressed as a percentage based on the color values associated to the scale at left, for all paired dry-hopped and non-dry-hopped 100 mL benchtop fermentations. The difference between the samples that were dry-hopped and those that were not is also reported. A negative difference value represents a lower RDF in the dry-hopped treatment when compared to the non-hopped and vice versa.
Another species of interest, UCDFST 11-161 S. paradoxus, showed a slight decrease of −0.37% RDF in bench top fermentations (Table 3) and a slight increase of 0.54% RDF in the pilot scale fermentations (Table 2). While not an effective yeast for hop creep mitigation, low relative difference between the dry-hopped and hon-dry-hopped fermentations, without over or under attenuation like SafAle™ BE-134 var. diastaticus or UCDFST 11-510 S. mikatae, show that it may be of interest for future trials. Research remains on the brewing potential and flavor characteristics of S. paradoxus, but initial insights from research in this lab to be published at a later date, as well as research from VTT Technical Research Centre in Finland [45] show that this species has great prospects in beer fermentation.
3.3. Flocculation and Attenuation
Flocculation is defined as the likelihood of yeast cells of a particular strain or species to cluster together during fermentation, forming a multicellular mass that eventually precipitates to the bottom of a lager fermenter or aggregates at the surface of an ale fermentation. It is a complex process that involves several genetic, environmental, and physiological parameters, but it is of great importance as beer is the only fermented beverage whose industry serially re-pitches its yeast [46,47,48]. Of importance to this study, there is a conventionally held belief amongst craft brewers that dry-hop creep may be mitigated with a more flocculant yeast strain [30]. As flocculation involves yeast health variables controlled by the brewer, such as nutrient conditions, dissolved oxygen, pH, fermentation temperature, and yeast handling and storage conditions, it is generally reported by commercial suppliers on a scale of “low” to “high”.
The given supplier references were type converted to numerical values, then visualized against the difference in RDF between the dry-hopped and standard fermentations for each yeast. This study found no correlation between flocculation and hop creep, with an extremely low R2 value of 0.0001 (Figure 4). The Pearson’s Correlation Coefficient was also calculated to be −0.0102, suggesting no reliable relationship. As an alternative to the intensity scale type converting the informal flocculation amounts from the yeast supplier catalogues, future research could use the accepted method of the Helm’s sedimentation test [49], and relate it to genetic research of the yeasts [47,48] to determine a more accurate flocculation numerical value.
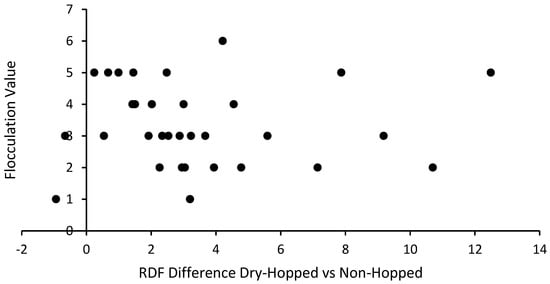
Figure 4.
Type converted flocculation numerical values plotted against the RDF difference between dry-hopped and standard fermentations for each beer using pilot scale fermentation data. Negative RDF difference indicates lower RDF in dry-hopped fermentations vs. non-hopped. A linear regression of these data produces an R2 value of 0.0001, indicating that the flocculation value is not correlated to the excess attenuation from hop creep.
3.4. pH and Dry-Hopping
An observed increase in pH following dry-hopping has been shown in several studies [19,50,51]. Following a hop addition of 4 g/L (1 lbs./BBL) of dry-hop, green beer pH has been shown to increase by 0.1 pH unit. In our study, a similar trend was observed: dry-hopping increased the pH an average of 0.2 at the dry-hop rate of 10 g/L. A clear increase in pH towards the end of fermentation was observed in dry-hopped when compared to standard fermentations for all yeasts studied (Figure 5), with the largest separation being observed at four days from dry-hop. Significant differences were shown between the two treatments following dry-hopping, with a p value of <0.05 one day after dry-hop and a p value of <0.01 for all subsequent days of fermentation until terminal. No significant difference was shown for days prior to and including the day of dry hopping. Large error bars in Figure 5 are likely due to the large variability in pH in fermentations in this study, as different yeast strains in this study fermented to variable pH and with different buffering capacities amongst the pilot beers due to variable starting gravities and ion contents.
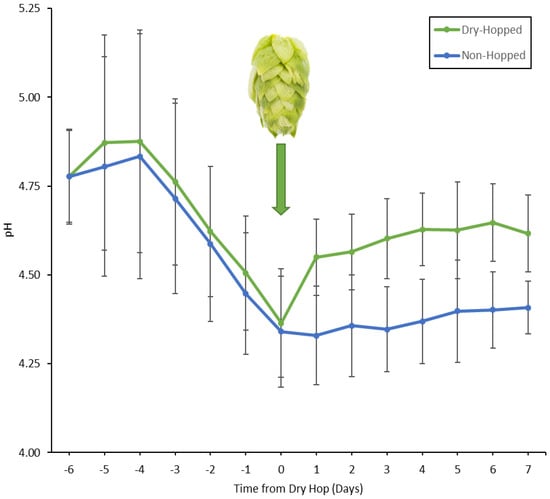
Figure 5.
Average of the pH for all fermentations in this study relative to the days from brewing each beer. Error bars are ± standard deviation. The green hop cone signifies the point of dry-hop at day 0, where a clear increase in pH of the dry-hopped beers is observed (p < 0.05 one day after dry-hop, p < 0.01 for all following days).
pH is an important brewing and fermentation parameter, as there are optimal pH levels at which enzymes are active in the mash [16] and that are necessary to control the extraction of polyphenols and tannins in wort [52], as well as pH optimums for yeast during fermentation [53]. There is evidence that Saccharomyces species replicate more readily at a pH of 5.0 than at pH of 4.2 [53,54], which could cause more available biomass to ferment any available sugars. The observed increase in pH from dry-hopping could be creating more yeast to not only ferment the newly hydrolyzed sugars, but also any residual sugars from the primary fermentation that were not consumed by the lower amount of available yeast. Additionally, Amylase enzymes from malt have an optimum pH of 5.4–5.5 in the mash [3], and it could be postulated that when the pH of the fermenting beer is increased from the addition of dry-hops, there is increased activity from those similar glucoamylase hop enzymes concurrently.
3.5. Biological Replicates
SafAle™ US-05 is a yeast ubiquitous among the craft beer industry and serves as a form of quality control to assess variation and change from brew to brew in the study. All values reported for this yeast throughout this study are the mean of the three biological replicates from three separate brews, totaling six fermentation using US-05. Variance was within an acceptable range as standard deviation among values remained low. RDF and pH values followed the same trends as all yeasts in this study. A one-tailed t-test was performed and showed statistical significance only on days following dry hopping on pH (p < 0.01) and RDF (p < 0.05) when comparing dry-hopped and non-hopped fermentations.
4. Conclusions
This study found a statistically increased average RDF in the dry-hopped versus non-hopped treatments for all the yeasts in this study, with the exception of two outliers in pilot scale and six bench-top fermentations. SafAle™ BE-134 S. cerevisiae var. diastaticus and UCDFST 11-510 S. mikatae were the only two yeasts that showed decreased RDF when dry-hopped in pilot scale fermentations, but both have other unique considerations when used for beer fermentation, as described above. Bench top fermentations proved effective at predictively determining the likelihood of potential hop creep in pilot scale fermentations, but modifications to the method are suggested for increased predictive power. Unfortunately, no yeasts in this study present themselves as effective strategies for mitigation of hop creep, but this research serves as a first look at many yeasts and a new perspective on how the impact of hop creep can vary in different fermentations. Further investigation should be done on the correlation of yeast flocculation and over-attenuation from dry-hop creep. Instead of using a type-correlated intensity scale to correspond to the degrees of flocculation reported in commercial yeast supplier catalogues, future research could genetic information of the yeasts to determine a quantifiable flocculation value. The observed increase in pH from adding dry-hops implies altered parameters that optimized yeast growth and enzyme activity, both of which may actively contribute to hop creep. As there is no effective yeast derived mitigation strategy for hop creep, it still stands that the best strategies to completely avoid hop creep are to pasteurize after fermentation is complete and denature the diastatic enzymes of the hops while rendering remaining yeast inactive, or to sterile filter the beer and remove all possible fermentative organisms. Dry-hopping early, during active fermentation, so that the fermentable sugars produced by the enzymes in the hops could be assimilated by yeast is also effective, but this could volatilize the desired aromatics from dry-hopping. Ultimately, the brewer must understand the way in which their chosen yeast strain interacts with dry-hops during fermentation.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/fermentation7020066/s1, Table S1. Malt analysis provided by Admiral Maltings.
Author Contributions
J.B. conceived the study, performed the research, gathered and transcribed data, and wrote the manuscript. A.M. assisted in the brewing of beer and sample collection, data curation, figure manipulation and statistics, and assisted with the final editing of the manuscript. G.F. supervised the work, offered insight, and assisted with final editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Funding provided by the UC Davis Food Science and Technology Department, as well as funds from the H.A. Jastro-Shields Research Award, Margrit Mondavi Graduate Fellowship, George F. Stewart Memorial Fund, and Michael J. Lewis Endowment.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Acknowledgments
Many thanks to Kyria Boundy-Mills and Irnayuli Sitepu from the UC Davis Phaff Collection for advice in yeast selection and subsequent revival of cryogenically stored yeast. Thanks to Kelly Scott and Joe Williams of the UC Davis Food Science and Anheuser-Busch Research Pilot Brewery for guidance and training on equipment in the brewery. Much gratitude to Nick Harris of Berkeley Yeast, Kara Taylor of White Labs, Anne Flesch and Kevin Lane of Fermentis, and Rebecca Newman of Lagunitas for advice on yeast selection. Additional thanks to Berkeley Yeast, White Labs, Fermentis, and the Phaff Collection for donating the yeast for this study, as well as Hopsteiner for providing the hops.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Moritz, E.R.; Morris, G.H. A Text-Book of the Science of Brewing; Spon: London, UK, 1891. [Google Scholar]
- Schönberger, C.; Kostelecky, T. 125th Anniversary Review: The Role of Hops in Brewing. J. Inst. Brew. 2011, 117, 259–267. [Google Scholar] [CrossRef]
- Kunze, W. Technology Brewing & Malting; 6th Revised English; Hendel, O., Ed.; Versuchs- und Lehranstalt für Brauerei in Berlin (VLB): Berlin, Germany, 2019. [Google Scholar]
- Brown, H.; Morris, G. On Certain Functions of Hops Used in the Dry-Hopping of Beers. Trans. Inst. Brew 1893, 6, 94–106. [Google Scholar]
- How Hoppy Beer Production Has Redefined Hop Quality and a Discussion of Agricultural and Processing Strategies to Promote It. Tech. Q. 2019. [CrossRef]
- Lafontaine, S.R.; Shellhammer, T.H. Investigating the Factors Impacting Aroma, Flavor, and Stability in Dry-Hopped Beers. MBAA Tech. Q. 2019, 56, 13–23. [Google Scholar] [CrossRef]
- Hauser, D.G.; Van Simaeys, K.R.; Lafontaine, S.R.; Shellhammer, T.H. A Comparison of Single-Stage and Two-Stage Dry-Hopping Regimes. J. Am. Soc. Brew. Chem. 2019, 77, 251–260. [Google Scholar] [CrossRef]
- Dykstra, J. The Beer Connoisseur; CafeMedia: New York, NY, USA, 2020; pp. 18–29. [Google Scholar]
- National Beer Sales & Production Data|Brewers Association. Available online: https://www.brewersassociation.org/statistics-and-data/national-beer-stats/ (accessed on 11 January 2020).
- Bud Light Crisp. Available online: https://www.budlight.com/en/our-beers/crisp.html (accessed on 31 March 2021).
- Guinness® Nitro IPA|Guinness®. Available online: https://www.guinness.com/en/our-beers/guinness-nitro-ipa/ (accessed on 30 March 2021).
- Salanță, L.C.; Coldea, T.E.; Ignat, M.V.; Pop, C.R.; Tofană, M.; Mudura, E.; Borșa, A.; Pasqualone, A.; Zhao, H. Non-Alcoholic and Craft Beer Production and Challenges. Processes 2020, 8, 1382. [Google Scholar] [CrossRef]
- Otter, G.E.; Taylor, L. Determination of the sugar composition of wort and beer by gas liquid chromatography. J. Inst. Brew. 1967, 73, 570–576. [Google Scholar] [CrossRef]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2001; p. 656. [Google Scholar]
- He, Y.; Dong, J.; Yin, H.; Zhao, Y.; Chen, R.; Wan, X.; Chen, P.; Hou, X.; Liu, J.; Chen, L. Wort composition and its impact on the flavour-active higher alcohol and ester formation of beer—A review. J. Inst. Brew. 2014, 120, 157–163. [Google Scholar] [CrossRef]
- Bamforth, C.W. Scientific Principles of Malting and Brewing; American Society of Brewing Chemists, Ed.; American Society of Brewing Chemists: St. Paul, MN, USA, 2006; p. 246. [Google Scholar]
- Takoi, K.; Koie, K.; Itoga, Y.; Katayama, Y.; Shimase, M.; Nakayama, Y.; Watari, J. Biotransformation of Hop-Derived Monoterpene Alcohols by Lager Yeast and Their Contribution to the Flavor of Hopped Beer. J. Agric. Food Chem. 2010, 58, 5050–5058. [Google Scholar] [CrossRef]
- Kirkpatrick, K.R.; Shellhammer, T.H. Evidence of Dextrin Hydrolyzing Enzymes in Cascade Hops (Humulus lupulus). J. Agric. Food Chem. 2018, 66, 9121–9126. [Google Scholar] [CrossRef] [PubMed]
- Olodokun, O.; Cowley, T.; James, S.; Smart, K.A. Dry-hopping: The effects of temperature and hop variety on the bittering profiles and properties of resultant beers. Brew. Sci. 2017, 70, 187–196. [Google Scholar]
- Kirkendall, J.A.; Mitchell, C.A.; Chadwick, L.R. The Freshening Power of Centennial Hops. J. Am. Soc. Brew. Chem. 2018, 76, 178–184. [Google Scholar] [CrossRef]
- Janicki, J.; Kotasthane, W.V.; Parker, A.; Walker, T.K. The DIAStatic activity of hops, together with a note on maltase in hops. J. Inst. Brew. 1941, 47, 24–36. [Google Scholar] [CrossRef]
- U.S. Department of the Treasury. The Beverage Alcohol Manual (BAM): A Practical Guide; Basic Mandatory Labeling Information for Malt Bev-Erages; US Gov., Tax and Trade Bureau: Washington, DC, USA, 2007; Chapter 1; Volume 3, Section 5; p. 7.
- Otter, G.E.; Taylor, L. Estimation and occurrence of acetaldehyde in beer. J. Inst. Brew. 1971, 77, 467–472. [Google Scholar] [CrossRef]
- Wainwright, T. diacetyl-a review: Part i-analytical and biochemical considerations: Part ii-brewing experience. J. Inst. Brew. 1973, 79, 451–470. [Google Scholar] [CrossRef]
- Tian, J. Determination of several flavours in beer with headspace sampling–gas chromatography. Food Chem. 2010, 123, 1318–1321. [Google Scholar] [CrossRef]
- Technical Committee. Alcohol. In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Cutaia, A.J.; Munroe, J.H. A Method for the Consistent Estimation of Real Degree of Fermentation. J. Am. Soc. Brew. Chem. 1979, 37, 188–189. [Google Scholar] [CrossRef]
- Huerta-Zurita, R.; Horsley, R.D.; Schwarz, P.B. Is the Apparent Degree of Fermentation a Reliable Estimator of Fermentability? J. Am. Soc. Brew. Chem. 2019, 77, 1–9. [Google Scholar] [CrossRef]
- Kirkpatrick, K.R.; Shellhammer, T.H. A Cultivar-Based Screening of Hops for Dextrin Degrading Enzymatic Potential. J. Am. Soc. Brew. Chem. 2018, 76, 247–256. [Google Scholar] [CrossRef]
- Shellhammer, T.H.; Beauchamp, A.; Kravitz, M.; Vaughn, C.; Cilurzo, V. Hop Creep: What It Is and Approaches to Man-Aging It; Brewers’ Association: Boulder, CO, USA, 2020. [Google Scholar]
- Bruner, J.; Williams, J.; Fox, G. Further Exploration of Hop Creep Variability with Humulus lupulus Cultivars and Proposed Method for Determination of Secondary Fermentation. Tech. Q. 2020, 57, 57. [Google Scholar] [CrossRef]
- Stokholm, A.; Lindsey, N.R.; Shellhammer, T.H. Evaluating a benchtop fermentation method for estimating dextrin degra-dation by hop’ ‘ diastatic enzymes during dry-hopping. Brew. Sci. 2020, 73, 140–148. [Google Scholar]
- Gallagher, L.W.; Silberstein, R.; Prato, L.; Vogt, H. ‘Butta 12’, a two-rowed malting barley adapted to the California Central Valley with proven floor-malting success and craft brewer acceptance. J. Plant Regist. 2020, 14, 250–265. [Google Scholar] [CrossRef]
- Bruner, J.; Fox, G. Novel Non-Cerevisiae Saccharomyces Yeast Species Used in Beer and Alcoholic Beverage Fermentations. Fermentation 2020, 6, 116. [Google Scholar] [CrossRef]
- Schisler, D.O. Comparison of Revised Yeast Counting Methods. J. Am. Soc. Brew. Chem. 1986, 44, 81–85. [Google Scholar] [CrossRef]
- Lafontaine, S.R.; Shellhammer, T.H. Impact of static dry-hopping rate on the sensory and analytical profiles of beer. J. Inst. Brew. 2018, 124, 434–442. [Google Scholar] [CrossRef]
- Magalhães, F.; Vidgren, V.; Ruohonen, L.; Gibson, B. Maltose and maltotriose utilisation by group I strains of the hybrid lager yeastSaccharomyces pastorianus. FEMS Yeast Res. 2016, 16. [Google Scholar] [CrossRef]
- Gibson, B.R.; Storgårds, E.; Krogerus, K.; Vidgren, V. Comparative physiology and fermentation performance of Saaz and Frohberg lager yeast strains and the parental speciesSaccharomyces eubayanus. Yeast 2013, 30, 255–266. [Google Scholar] [CrossRef]
- Yamashita, I.; Suzuki, K.; Fukui, S. Nucleotide sequence of the extracellular glucoamylase gene STA1 in the yeast Saccharomyces diastaticus. J. Bacteriol. 1985, 161, 567–573. [Google Scholar] [CrossRef]
- Sakai, K.; Fukui, S.; Yabuuchi, S.; Aoyagi, S.; Tsumura, Y. Expression of theSaccharomyces Diastaticus STA1Gene in Brewing Yeasts. J. Am. Soc. Brew. Chem. 1989, 47, 87–91. [Google Scholar] [CrossRef]
- Bellon, J.R.; Schmid, F.; Capone, D.L.; Dunn, B.L.; Chambers, P.J. Introducing a New Breed of Wine Yeast: Interspecific Hybridisation between a Commercial Saccharomyces cerevisiae Wine Yeast and Saccharomyces mikatae. PLoS ONE 2013, 8, e62053. [Google Scholar] [CrossRef]
- Bellon, J.; Schmidt, S.; Solomon, M. Case study: Development of Saccharomyces cerevisiae × Saccharomyces mikatae wine yeast hybrids and their potential to deliver alternative wine styles. AWRI Tech. Rev. 2019, 241, 6–11. [Google Scholar]
- Technical Committee. Yeast Fermentable Extract. In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Technical Committee. End fermentation (yeast fermentable extract). In ASBC Methods of Analysis; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Nikulin, J.; Vidgren, V.; Krogerus, K.; Magalhães, F.; Valkeemäki, S.; Kangas-Heiska, T.; Gibson, B. Brewing potential of the wild yeast species Saccharomyces paradoxus. Eur. Food Res. Technol. 2020, 246, 2283–2297. [Google Scholar] [CrossRef]
- D’Hautcourt, O.; Smart, K.A. Measurement of Brewing Yeast Flocculation. J. Am. Soc. Brew. Chem. 1999, 57, 123–128. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Verachtert, H.; Delvaux, F.R. Yeast flocculation: What brewers should know. Appl. Microbiol. Biotechnol. 2003, 61, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.G. Yeast Flocculation—Sedimentation and Flotation. Ferment 2018, 4, 28. [Google Scholar] [CrossRef]
- Bendiak, D.S. Quantification of the Helm’s Flocculation Test. J. Am. Soc. Brew. Chem. 1994, 52, 120–122. [Google Scholar] [CrossRef]
- Humulinone Formation in Hops and Hop Pellets and Its Implications for Dry Hopped Beers. Tech. Q. 2016, 53, 23–27. [CrossRef]
- Maye, J.P.; Smith, R.; Leker, J. Dry Hopping and Its Effect on Beer Bitterness, the IBU Test, and pH. BrauW. Int. 2018, 2018, 25–29. [Google Scholar]
- Vanbeneden, N.; Van Roey, T.; Willems, F.; Delvaux, F.; Delvaux, F.R. Release of phenolic flavour precursors during wort production: Influence of process parameters and grist composition on ferulic acid release during brewing. Food Chem. 2008, 111, 83–91. [Google Scholar] [CrossRef]
- García, A.I.; García, L.A.; Díaz, M. Prediction of ester production in industrial beer fermentation. Enzym. Microb. Technol. 1994, 16, 66–71. [Google Scholar] [CrossRef]
- García, A.I.; García, L.A.; Díaz, M. Fusel Alcohols Production in Beer Fermentation Processes. Process. Biochem. 1994, 29, 303–309. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).