Saccharomyces cerevisiae Fermentation of 28 Barley and 12 Oat Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Fermentation Conditions
2.2. NMR Spectroscopy
2.3. Statistical Analysis
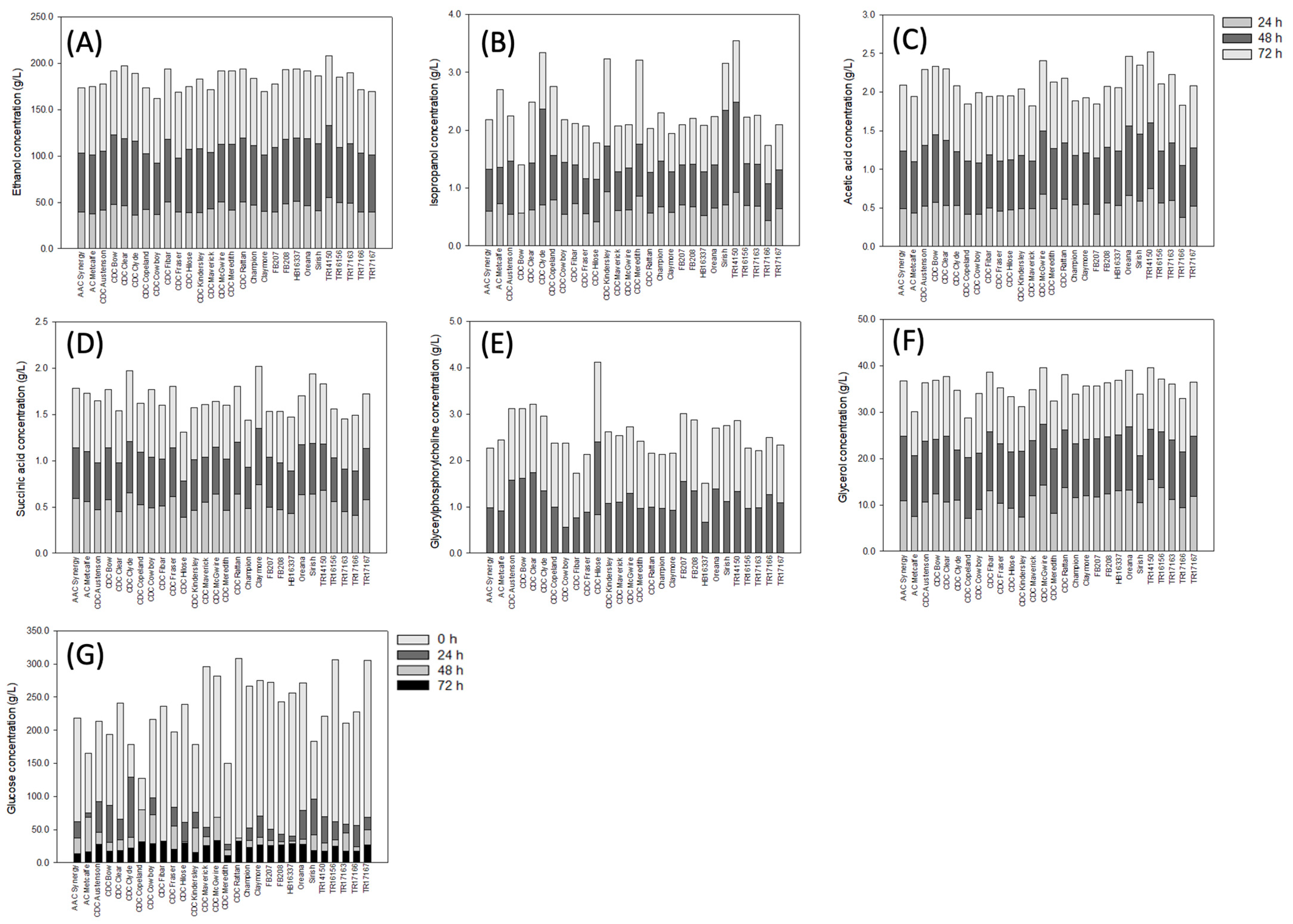
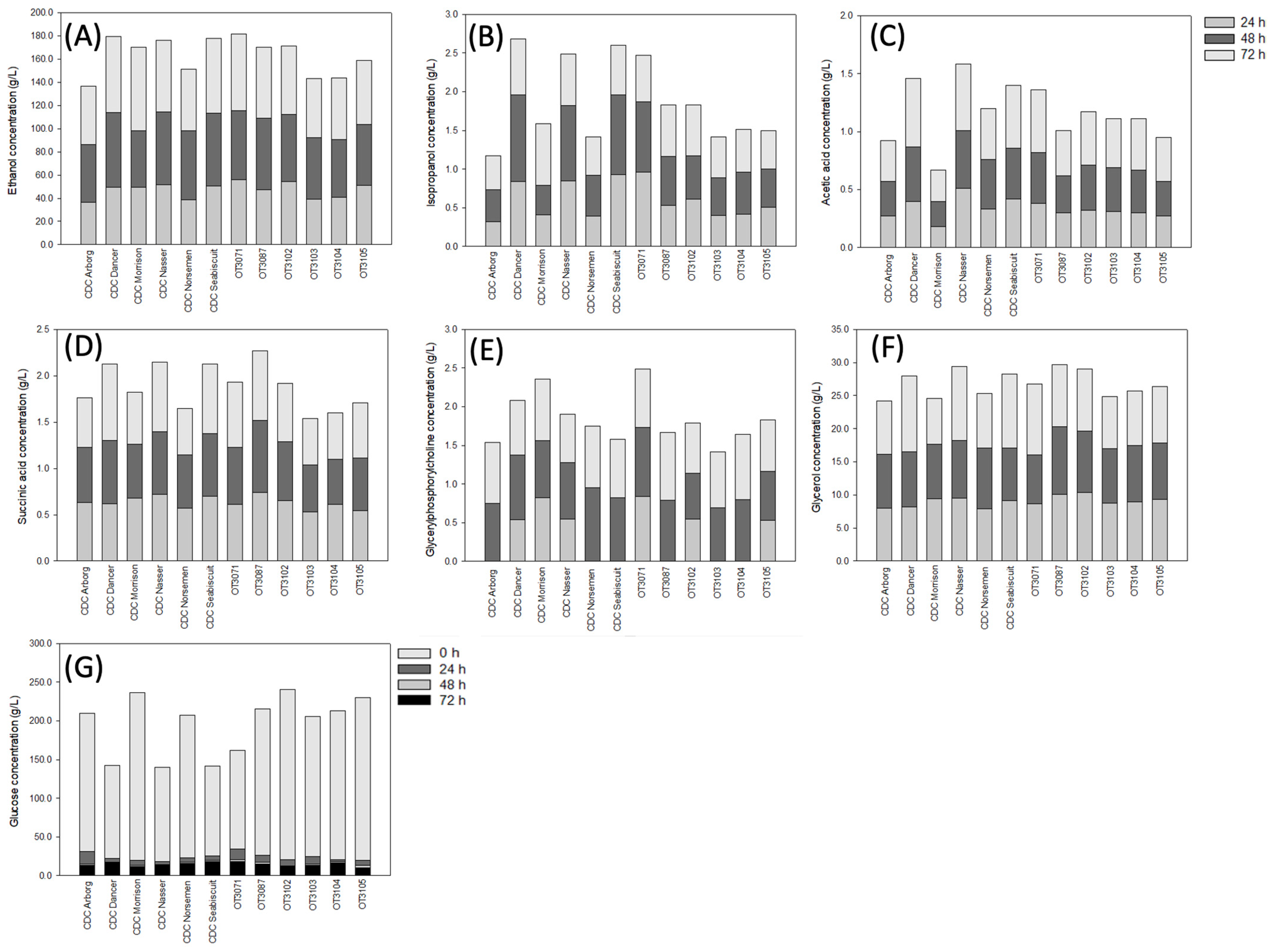
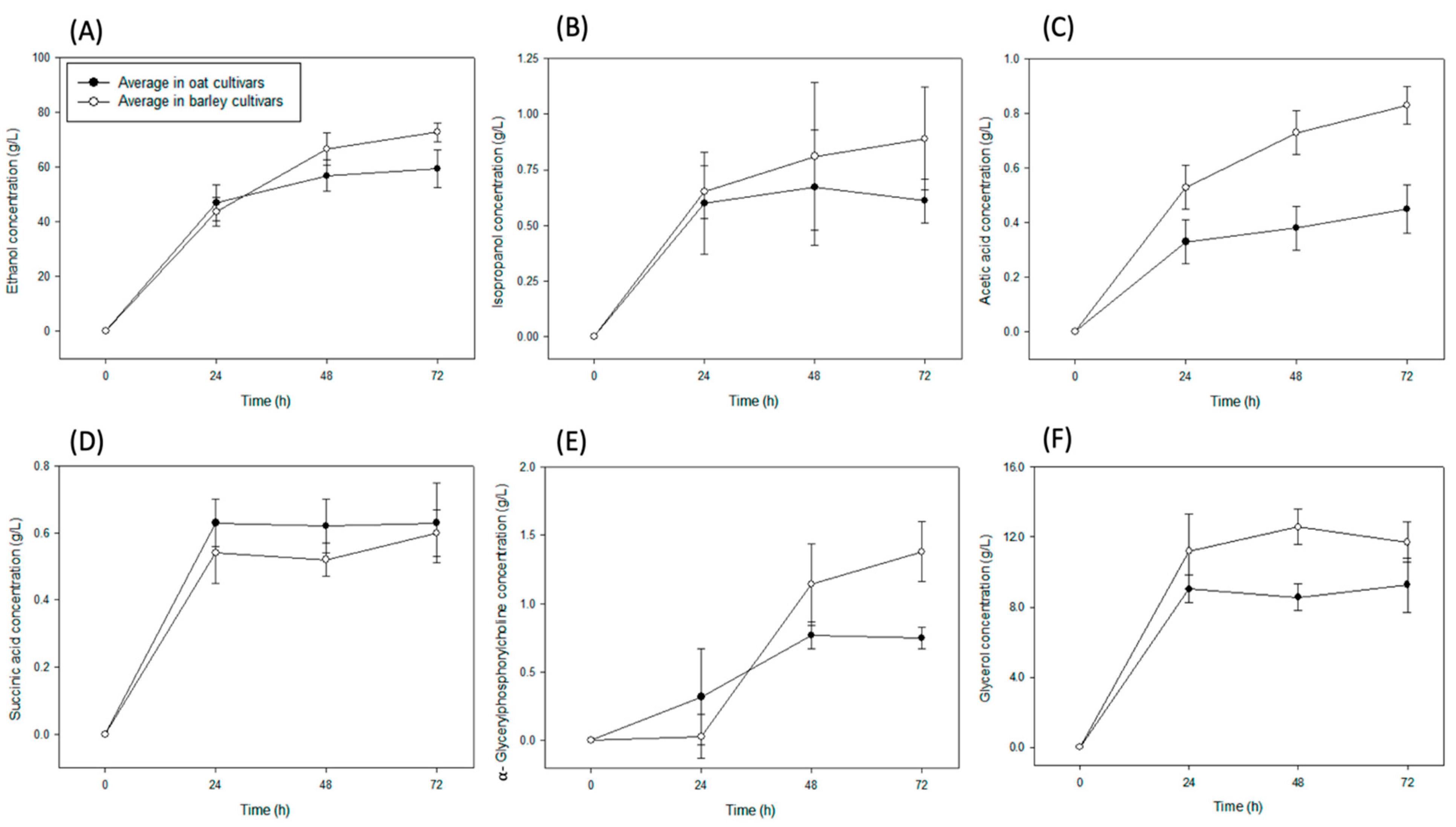
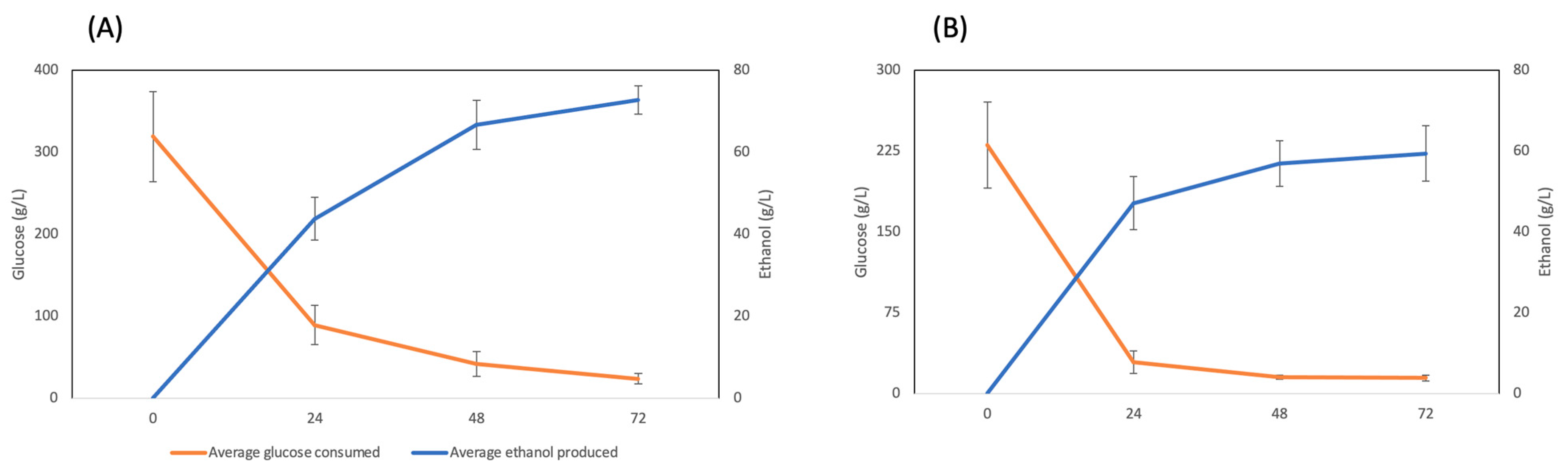
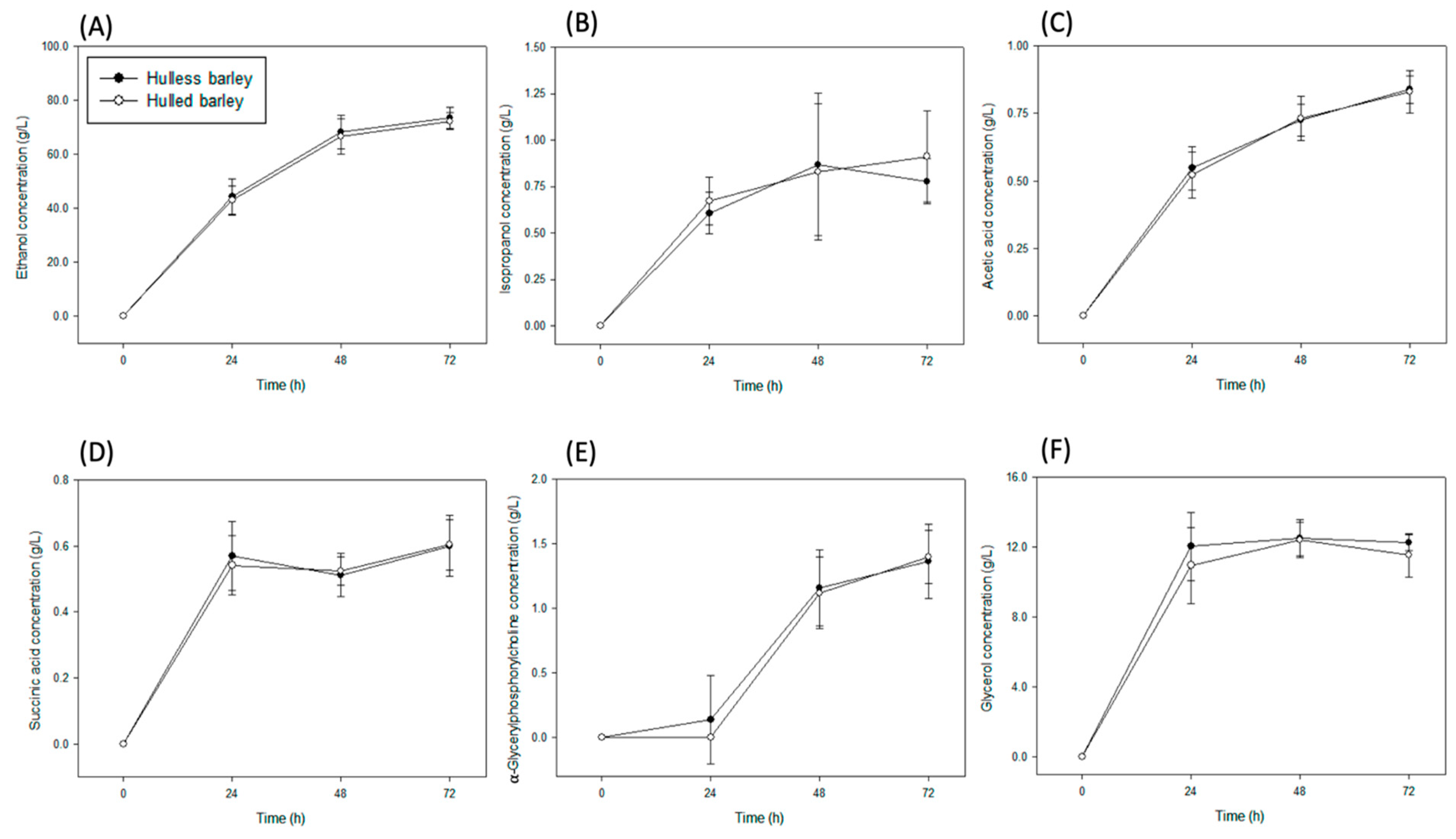
3. Results
3.1. Concentration of Organic Solutes in Barley
3.2. Concentration of Organic Solutes in Oat Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics Canada. Production of Principal Field Crops, November 2019. The Daily, 6 December 2019. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/191206/dq191206b-eng.htm (accessed on 8 April 2021).
- Canadian Agri-Food Trade Alliance (CAFTA). Barley. 2017. Available online: http://cafta.org/agri-food-exports/cafta-exports/ (accessed on 5 November 2020).
- Statistics Canada. Principal Field Crop Areas. June 2019. Available online: https://www150.statcan.gc.ca/n1/daily-quotidien/190626/dq190626b-eng.htm (accessed on 24 November 2020).
- Boczkowska, M.; Podyma, W.; Łapiński, B. Oat. In Genetic and Genomic Resources for Grain Cereals Improvement; Academic Press: Cambridge, MA, USA, 2016; pp. 159–225. [Google Scholar]
- Punia, S.; Sandhu, K.S.; Dhull, S.B.; Siroha, A.K.; Purewal, S.S.; Kaur, M.; Kidwai, M.K. Oat starch: Physico-chemical, morphological, rheological characteristics and its applications—A review. Int. J. Biol. Macromol. 2020, 154, 493–498. [Google Scholar] [CrossRef]
- Chibbar, R.N.; Ganeshan, S.; Båga, M.; Khandelwal, R.L. Carbohydrate Metabolism. In Encylopedia of Grain Science; Wrigley, C., Ed.; Elsevier: Oxford, UK, 2004; pp. 168–179. [Google Scholar]
- Sayar, S.; White, P.J. Oat starch: Physicochemical properties and function. In Otas: Chemistry and Technology, 2nd ed.; American Association of Cereal Chemists, Inc. (AACC): Saint Paul, MN, USA, 2011; pp. 109–122. [Google Scholar]
- Bhatty, R.S.; Rossnagel, B.G. Comparison of pearled and unpearled Canadian and Japanese barleys. Cereal Chem. 1998, 75, 15–21. [Google Scholar] [CrossRef]
- Oyeneye, A.; Shen, J.; Shim, Y.Y.; Tse, T.J.; Reaney, M.J.T. Production of α-glycerylphosphorylcholine and other compounds from wheat fermentation. ACS Omega 2020, 5, 12486–12494. [Google Scholar] [CrossRef]
- Ratanapariyanuch, K.; Shin, Y.Y.; Emami, S.; Reaney, M.J.T. Production of protein concentrate and 1,3-propanediol by wheat-based thin stillage fermentation. J. Agric. Food Chem. 2017, 65, 3858–3867. [Google Scholar] [CrossRef] [PubMed]
- Tse, T.J.; Shen, J.; Shim, Y.Y.; Reaney, M.J.T. Changes in bacterial populations and their metabolism over 90 sequential cultures on wheat-based thin stillage. J. Agric. Food Chem. 2020, 68, 4717–4729. [Google Scholar] [CrossRef]
- Aslankoohi, E.; Rezaei, M.N.; Vervoort, Y.; Courtin, C.M.; Verstrepen, K.J. Glycerol production by fermenting yeast cells is essential for optimal bread dough fermentation. PLoS ONE 2015, 10, e0119364. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Vance, D.E. Phosphatidylcholine and choline homeostasis. J. Lipid Res. 2008, 49, 1187–1194. [Google Scholar] [CrossRef] [PubMed]
- Shabtai, Y.; Bendelac, L.; Jubran, H.; Hirschberg, J.; Fainsod, A. Acetaldehyde inhibits retinoic acid biosynthesis to mediate alcohol teratogenicity. Sci. Rep. 2018, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Sentheshanmuganathan, S. The mechanism of the formation of higher alcohols from amino acids by Saccharomyces cerevisiae. Biochem. J. 1960, 74, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Janssen, P.H. Propanol as an end product of threonine fermentation. Arch. Microbiol. 2004, 182, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Pielech-Przybylska, K.; Balcerek, M.; Dziekońska-Kubczak, U.; Pacholczyk-Sienicka, B.; Ciepielowski, G.; Albrecht, Ł.; Patelski, P. The role of Saccharomyces cerevisiae yeast and lactic acid bacteria in the formation of 2-propanol from acetone during fermentation of rye mashes obtained using thermal-pressure method of starch liberation. Molecules 2019, 24, 610. [Google Scholar] [CrossRef] [PubMed]
- Narendranath, N.V.; Hynes, S.H.; Thomas, K.C.; Ingledew, W.M. Effects of lactobacilli on yeast-catalyzed ethanol fermentations. Appl. Environ. Microbiol. 1997, 63, 4158–4163. [Google Scholar] [CrossRef] [PubMed]
- Pielech-Przybylska, K.; Balcerek, M.; Ciepielowski, G.; Pacholczyk-Sienicka, B.; Albrecht, Ł.; Dziekońska-Kubczak, U.; Bonikowski, R.; Patelski, P. Effect of Co-Inoculation with Saccharomyces cerevisiae and Lactic Acid Bacteria on the Content of Propan-2-ol, Acetaldehyde and Weak Acids in Fermented Distillery Mashes. Int. J. Mol. Sci. 2019, 20, 1659. [Google Scholar] [CrossRef]
- Walker, G.M.; Stewart, G.G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Kleff, S.; Schwegmann, S. Succinic acid: Technology development and commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Ahn, J.H.; Jang, Y.-S.; Lee, S.Y. Production of succinic acid by metabolically engineering microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Raab, A.M.; Lang, C. Oxidative versus reductive succinic acid production in the yeast Saccharomyces cerevisiae. Bioeng. Bugs 2011, 2, 120–123. [Google Scholar] [CrossRef]
- Ferone, M.; Raganati, F.; Ercole, A.; Olivieri, G.; Salatino, P.; Marzochella, A. Continuoous succinic acid fermentation by Actinobacillus succinogenes in a packed-bed biofilm reactor. Biotechnol. Biofuels 2018, 11, 138. [Google Scholar] [CrossRef]
- Nissen, T.L.; Hamann, C.W.; Kielland-Brandt, M.C.; Nielsen, J.; Villadsen, J. Anaerobic and aerobic batch cultivations of Saccharomyces cerevisiae mutants impaired in glycerol synthesis. Yeast 2000, 16, 463–474. [Google Scholar] [CrossRef]
- André, L.; Hemming, A.; Adler, L. OOsmoregulation in Saccharomyces cerevisiae. Studies on the osmotic induction of glycerol production and glycerol-3-phosphate dehydrogenase (NAD+). FEBS Lett. 1991, 286, 13–17. [Google Scholar] [CrossRef]
- Larsson, K.; Ansell, R.; Eriksson, P.; Adler, L. A gene encoding sn-glycerol 3-phosphate dehydrogenase (NAD+) complements an osmosensitive mutant of Saccharomyces cerevisiae. Mol. Microbiol. 1993, 10, 1101–1111. [Google Scholar] [CrossRef]
- Ansell, R.; Granath, K.; Hohmann, S.; Thevelein, J.M.; Adler, L. The two isoenzymes for yeast NAD+-depedent glycerol 3-phosphate dehydrogenase encoded by GPD1 and GPD2 hav distinct roles in osmoadaptation and redox regulation. EMBO J. 1997, 16, 2179–2187. [Google Scholar] [CrossRef]
- Pagliardini, J.; Hubmann, G.; Alfenore, S.; Nevoigt, E.; Bideaux, C.; Guillouet, S.E. The metabolic costs of improving ethanol yield by reducing glycerol formation capacity under anaerobic conditions in Saccharomyces cerevisiae. Microb. Cell Fact. 2013, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- AC Immune. Alzheimer’s Disease. Available online: http://www.acimmune.com/en/alzheimer-s-disease/ (accessed on 12 February 2020).
- Lopez, C.M.; Govoni, S.; Battaini, F.; Bergamaschi, S.; Longoni, A.; Giaroni, C.; Trabucchi, M. Effect of a new cognition enhancer, alpha-glycerylphosphorylcholine, on scopolamine-induced amnesia and brain acetylcholine. Pharmacol. Biochem. Behav. 1991, 39, 835–840. [Google Scholar] [CrossRef]
- Bellar, D.; LeBlanc, N.R.; Campbell, B. The effect of 6 days of alpha glycerylphosphorylcholine on isometric strength. J. Int. Soc. Sports Nutr. 2015, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.G.; Byars, A.; Purpura, M.; Jäger, R. Effect of alpha-glycerylphosphorylcholine, caffeine or placebo on markers of mood, cognitive function, power, speed, and agility. J. Int. Soc. Sports Nutr. 2015, 21, 41. [Google Scholar] [CrossRef]
- DeFina, P.A.; Moser, R.S.; Glenn, M.; Lichtenstein, J.D.; Fellus, J. Alzheimer’s Disease Clinical and Research Update for Health Care Practitioners. J. Aging Res. 2013, 2013, 207178. [Google Scholar] [CrossRef]
- Miljić, U.; Puškaš, V.; Vučurović, V.; Muzalevski, A. Fermentation characteristics and aromatic profile of plum wines produced with indigeous microbiota and pure cultures of selected yeasts. J. Food Sci. 2017, 82, 1443–1450. [Google Scholar] [CrossRef]
- Sonkar, K.; Ayyappan, V.; Tressler, C.M.; Adelaja, O.; Cai, R.; Cheng, M.; Glunde, K. Focus on the glycerophosphocholine pathway in choline phospholipid metabolism of cancer. NMR Biomed. 2019, 32, e4112. [Google Scholar] [CrossRef]
- Fernández-Murray, J.P.; McMaster, C.R. Glycerophosphocholine catabolism as a new route for choline formation for phosphatidylcholine syntehsis by the Kennedy Pathway. J. Biol. Chem. 2005, 280, 38290–38296. [Google Scholar] [CrossRef]
- Wood, L. Global Brain Health Supplements Market, 2017 to 2025—ResearchAndMarkets.com. Available online: https://www.businesswire.com/news/home/20180404005510/en/Global-Brain-Health-Supplements-Market-2017-2025 (accessed on 8 January 2019).
- Ratanapariyanuch, K.; Shen, J.; Jia, Y.; Tyler, R.T.; Shin, Y.Y.; Reaney, M.J.T. Rapid NMR method for the quantification of organic compounds in thin stillage. J. Agric. Food Chem. 2011, 59, 10454–10460. [Google Scholar] [CrossRef] [PubMed]
- Renewable Fuels Association. 2019 Ethanol Industry Outlook. 2019. Available online: https://ethanolrfa.org/wp-content/uploads/2019/02/RFA2019Outlook.pdf (accessed on 3 December 2020).
- U.S. Energy Information Adminstration (EIA). Biofuels Explained—Ethanol. 2020. Available online: https://www.eia.gov/energyexplained/biofuels/ethanol.php (accessed on 3 December 2020).
- Government of Canada. Ethanol. 2020. Available online: https://www.nrcan.gc.ca/energy-efficiency/energy-efficiency-transportation/alternative-fuels/biofuels/ethanol/3493 (accessed on 3 December 2020).
- Ingledew, W.M.; Jones, A.M.; Bhatty, R.S.; Rossnagel, B.G. Fuel alcohol production from hull-less barley. Cereal Chem. 1995, 72, 147–150. [Google Scholar]
- Holtekjølen, A.K.; Uhlen, A.K.; Bråthen, E.; Sahlstrøm, S.; Knutsen, S.H. Contents of starch and non-starch polysaccharides in barley varieties of different origin. Food Chem. 2006, 94, 348–358. [Google Scholar] [CrossRef]
- Gous, P.W.; Warren, F.; Mo, O.W.; Gilbert, R.G.; Fox, G.P. The effects of variable nitrogen application on barley starch structure under drought stress. J. Inst. Brew. 2015, 121, 502–509. [Google Scholar] [CrossRef]
- Tester, R.F. Influence of growth conditions on barley starch properties. Int. J. Biol. Macromol. 1997, 21, 37–45. [Google Scholar] [CrossRef]
- Brown, G.D.; Bauer, J.; Osborn, H.M.I.; Kuemmerle, R. A solution NMR approach to determine the chemical structures of carbohydrates using the hydroxyl groups as starting points. ACS Omega 2018, 3, 17957–17975. [Google Scholar] [CrossRef]
- Stolz, M.; Schlawne, C.; Hoffmann, J.; Hartman, V.; Marini, I.; Fritsche, A.; Peter, A.; Bakchoul, T.; Schick, F. Feasibility of precise and reliable glucose quantification in human whole blood samples by 1 tesla benchtop NMR. NMR Biomed. 2020, 33, e4358. [Google Scholar] [CrossRef]
- Rudd, T.; Skidmore, M.A.; Yates, E.A. Chapter 12—Surface-Based Studies of Heparin/Heparan Sulfate-Protein Interactions: Considerations for Surface Immobilisation of HS/Heparin Saccharides and Monitoring Their Interactions with Binding Proteins. In Chemistry and Biology of Heparin and Heparana Sulfate; Garg, H.G., Linhardt, R.J., Hales, C.A., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2005; pp. 345–366. [Google Scholar]
- Öhgren, K.; Bura, R.; Lesnicki, G.; Saddler, J.; Zacchi, G. A comparison between simultaneous saccharification and fermentation seperate hydrolysis and fermentation using steam-pretreated corn stover. Process Biochem. 2007, 42, 834–839. [Google Scholar] [CrossRef]
- Öhgren, K.; Rudolf, A.; Galbe, M.; Zacchi, G. Fuel ethanol production from steam-pretreated corn stover using SSF at higher dry matter content. Biomass Bioenergy 2006, 30, 863–869. [Google Scholar] [CrossRef]
- Latif, F.; Rajoka, M.I. Production of ethanol and xylitol from corn cobs by yeasts. Bioresour. Technol. 2001, 77, 57–63. [Google Scholar] [CrossRef]
- Djordjevic, V.; Willaert, R.; Gibson, B.; Nedovic, V. Immobilized yeast cells and secondary metabolites. In Fungal Metabolites; Merillion, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–40. [Google Scholar]
- Nikolic, S.; Mojovic, L.; Pejin, D.; Rakin, M.; Vukasinov, M. Production of bioethanol from corn meal hydrolyzates by free and immobilized cells of Saccharomyces cerevisiae var. ellipsoideus. Biomass Bioenergy 2010, 34, 1449–1456. [Google Scholar] [CrossRef]
- Berbegal, C.; Spano, G.; Tristezza, M.; Griego, F.; Capozzi, V. Microbial resources and innovation in the wine production sector. S. Afr. J. Enol. Vitic. 2017, 38, 156–166. [Google Scholar] [CrossRef]
- Du Toit, W.J.; Lambrechts, M.G. The enumeration and identification of acetic acid bacteria from south african red wine fermentatioons. Int. J. Food Microbiol. 2002, 74, 57–64. [Google Scholar] [CrossRef]
- Pinto, I.; Cardoso, H.; Leão, C. High enthalpy and low enthalpy death in Saccharomyces cerevisiae induced by acetic acid. Biotechnol. Bioeng. 1989, 33, 1350–1352. [Google Scholar] [CrossRef] [PubMed]
- Vilela-Mourra, A.; Schullar, D.; Mendes-Faia, A.; Silva, R.D.; Chaves, S.R.; Sousa, M.J.; Côrte-Real, M. The impact of acetate metabolism on yeast fermentative performance and wine quality: Reduction of volatile acidity of grape musts and wines. Appl. Microbiol. Biotechnol. 2011, 89, 271–280. [Google Scholar] [CrossRef]
- Sousa, M.J.; Ludovico, P.; Rodrigues, F.; Leão, C.; Côrte-Real, M. Stress and cell death in yeast induced by acetic acid. In Cell Metabolism—Cell Homeostasis and Stress Response; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Thoukis, G.; Ueda, M.; Wright, D. The formation of succinic acid during alcoholic fermentation. Am. J. Enol. Vitic. 1965, 16, 1–8. [Google Scholar]
- Lee, Y.Y.; Iver, P.; Torget, R.W. Dilute-acid hydrolysis of lignocellulosic biomass. Adv. Biochem. Eng. Biotechnol. 1999, 65, 93–115. [Google Scholar]
- Maiorella, B.; Blanch, H.W.; Wilke, C.R. By-product inhibition effects on ethanolic fermentation by Saccharomyces cereivisiae. Biotechnol. Bioeng. 1983, 25, 103–121. [Google Scholar] [CrossRef]
- Wu, K.J.; Lin, Y.H.; Lo, Y.C.; Chen, C.Y.; Chen, W.M.; Chang, J.S. Converting glycerol into hydrogen, ethanol, and diols with a Klebsiella sp. HE1 strain via anaerobic fermentation. J. Taiwan Inst. Chem. Eng. 2011, 42, 20–25. [Google Scholar] [CrossRef]
- Goold, H.D.; Kroukamp, H.; Williams, T.C.; Paulsen, I.T.; Varela, C.; Pretorius, I.S. Yeast’s balancing act between ethanol and glycerol production in low-alcohol wines. Microb. Biotechnol. 2017, 10, 264–278. [Google Scholar] [CrossRef]
- Bideaux, C.; Alfenore, S.; Cameleyre, X.; Molina-Jouve, C.; Uribelarrea, J.; Guillouet, S.E. Minimization of glycerol production during the high-performance fed-batch ethanolic fermentation process in Saccharomyces cerevisiae using a metabolic model as a prediction tool. Appl. Environ. Microbiol. 2006, 72, 2134–2140. [Google Scholar] [CrossRef]
- Baer, E.; Kates, M. L-α-Glycerylphosphorylcholine. J. Am. Chem. Soc. 1948, 70, 1394–1399. [Google Scholar] [CrossRef] [PubMed]
- Brockerhoff, H.; Yurkowski, M. Simplified preparation of L-α-Glycerylphosphoryl choline. Can. J. Biochem. 1965, 43, 1777. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, Y.S.; Song, E.S.; Kang, D.S.; Song, I.W.; Kang, P.G.; Oh, S.S.; Moon, S.C.; Lee, B.G. A Process for Preparation of I-Alpha-Glycerophosphoeyl Choline. W.O. Patent 2007145476 A1 21 December 2007. [Google Scholar]
- Zhang, K.; Wang, X.; Huang, J.; Liu, Y. Purification of L-alpha-glycerylphosphorylcholine by column chromatography. J. Chromatogr. A 2012, 1220, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Blasi, F.; Cossignani, L.; Simonetti, M.S.; Brutti, M.; Ventura, F.; Damiani, P. Enzymatic deacylation of of 1,2-diacyl-sn-glycero-3-phosphocholines to sn-glycerol-3-phosphocholine. Enzym. Microb. Technol. 2006, 39, 1405–1408. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Wang, X. Enzymatic preparation of L-α-Glycerylphosphorylcholine in an aqueous medium. Eur. J. Lipid Sci. Technol. 2012, 114, 1254–1260. [Google Scholar] [CrossRef]
- Jouhet, J.; Maréchal, E.; Bligny, R.; Joyard, J.; Block, M.A. Transient increase of phosphatidylcholine in plant cells in response to phosphate deprivation. FEBS Lett. 2003, 544, 63–68. [Google Scholar] [CrossRef]

| Barley Cultivars | Oat Cultivars | ||
|---|---|---|---|
| Cultivar | Hulled vs. Hull-less | Cultivar | Hulled |
| AAC Synergy | Hulled | CDC Arborg | Hulled |
| AC Metcalfe | Hulled | CDC Dancer | Hulled |
| CDC Austenson | Hulled | CDC Morrison | Hulled |
| CDC Bow | Hulled | CDC Nasser | Hulled |
| CDC Clear | Hull-less | CDC Norsemen | Hulled |
| CDC Clyde | Hulled | CDC Seabiscuit | Hulled |
| CDC Copeland | Hulled | OT3071 | Hulled |
| CDC Cowboy | Hulled | OT3087 | Hulled |
| CDC Fibar | Hull-less | OT3102 | Hulled |
| CDC Fraser | Hulled | OT3103 | Hulled |
| CDC Hilose | Hull-less | OT3104 | Hulled |
| CDC Kindersley | Hulled | OT3105 | Hulled |
| CDC Maverick | Hulled | ||
| CDC McGwire | Hull-less | ||
| CDC Meredith | Hulled | ||
| CDC Rattan | Hull-less | ||
| Champion | Hulled | ||
| Claymore | Hulled | ||
| FB207 | Hulled | ||
| FB208 | Hulled | ||
| HB16337 | Hull-less | ||
| Oreana | Hulled | ||
| Sirish | Hulled | ||
| TR14150 | Hulled | ||
| TR16156 | Hulled | ||
| TR17163 | Hulled | ||
| TR17166 | Hulled | ||
| TR17167 | Hulled | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tse, T.J.; Wiens, D.J.; Shen, J.; Beattie, A.D.; Reaney, M.J.T. Saccharomyces cerevisiae Fermentation of 28 Barley and 12 Oat Cultivars. Fermentation 2021, 7, 59. https://doi.org/10.3390/fermentation7020059
Tse TJ, Wiens DJ, Shen J, Beattie AD, Reaney MJT. Saccharomyces cerevisiae Fermentation of 28 Barley and 12 Oat Cultivars. Fermentation. 2021; 7(2):59. https://doi.org/10.3390/fermentation7020059
Chicago/Turabian StyleTse, Timothy J., Daniel J. Wiens, Jianheng Shen, Aaron D. Beattie, and Martin J. T. Reaney. 2021. "Saccharomyces cerevisiae Fermentation of 28 Barley and 12 Oat Cultivars" Fermentation 7, no. 2: 59. https://doi.org/10.3390/fermentation7020059
APA StyleTse, T. J., Wiens, D. J., Shen, J., Beattie, A. D., & Reaney, M. J. T. (2021). Saccharomyces cerevisiae Fermentation of 28 Barley and 12 Oat Cultivars. Fermentation, 7(2), 59. https://doi.org/10.3390/fermentation7020059






