Towards Sustainable Bioinoculants: A Fermentation Strategy for High Cell Density Cultivation of Paraburkholderia sp. SOS3, a Plant Growth-Promoting Bacterium Isolated in Queensland, Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Media Composition
2.3. Fermentations in 1 L Bioreactor
2.4. Assays for Plant-Growth Promotion: Seed Germination and Vigour Index
Sorghum (Sorghum bicolor L.) and Maize (Zea mays L.) Seeds Tretament
2.5. Glasshouse Trials–Full Term Growth
2.6. Polyhydroxybutyrate (PHB) Extraction
2.7. Proteomics Analysis
2.8. HPLC Analysis
3. Results
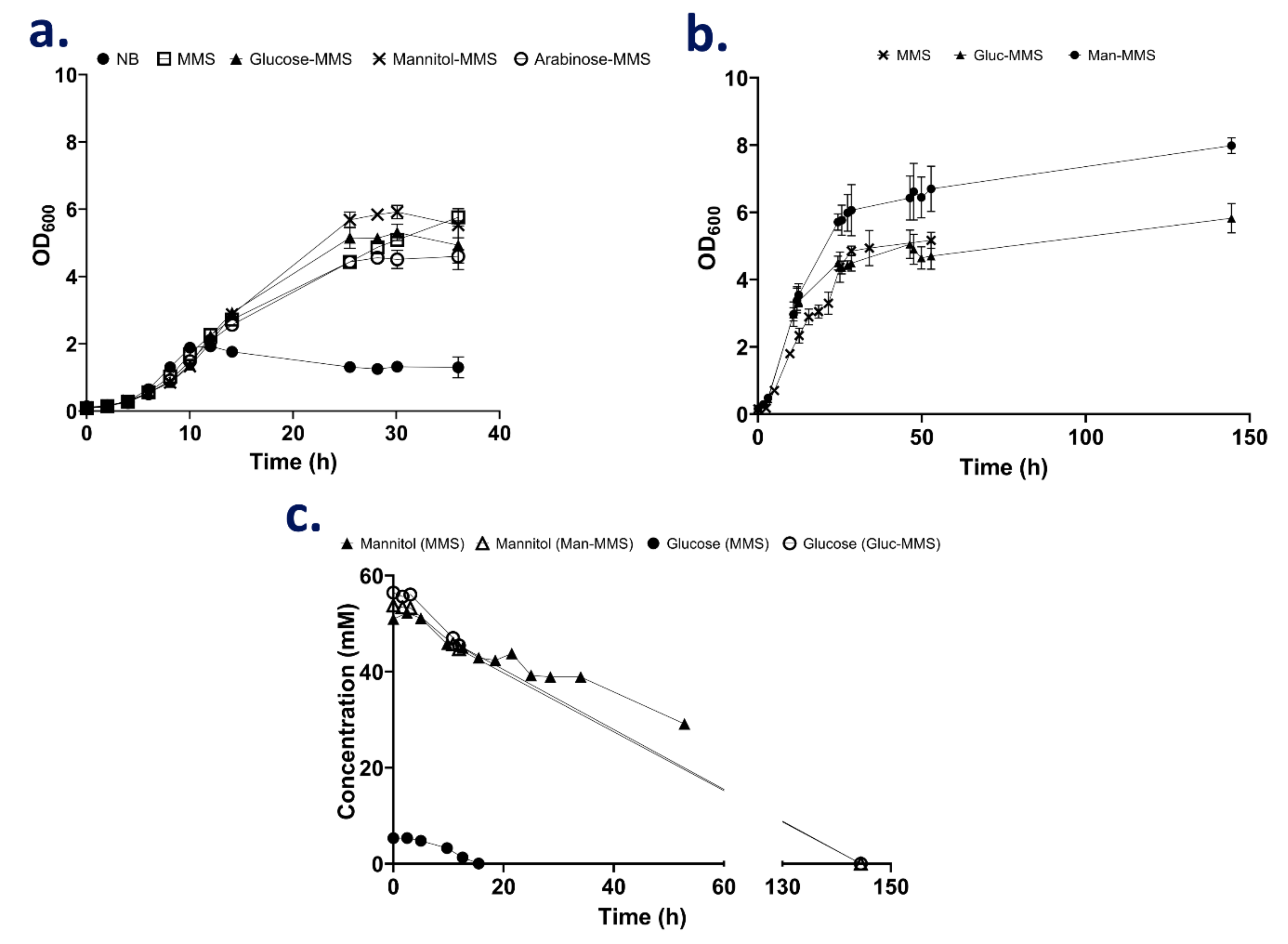
3.1. Growth of SOS3 in Complex Media
3.2. The Use of Semi-Defined Media Increases Final Biomass Yield in Batch Cultivation
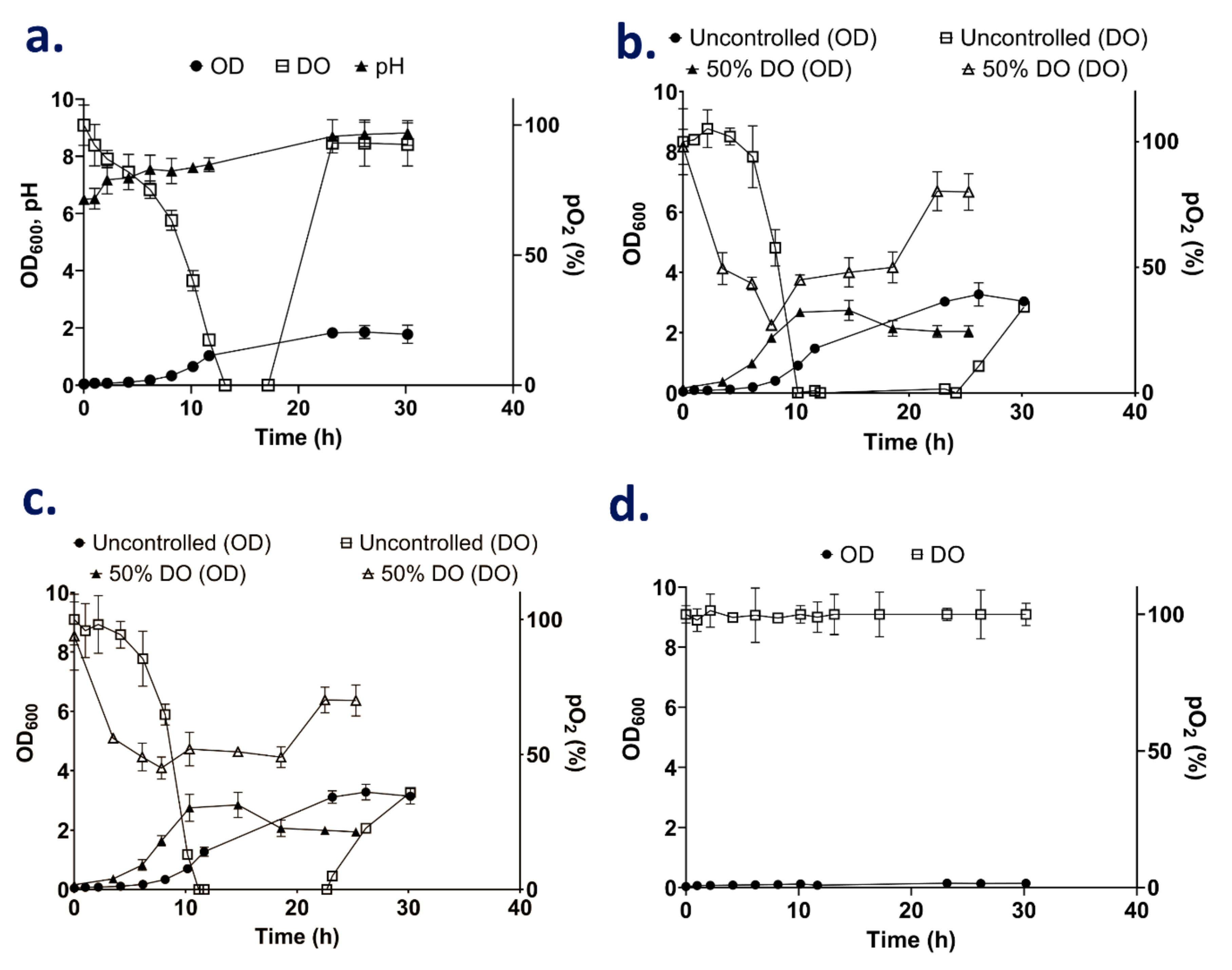
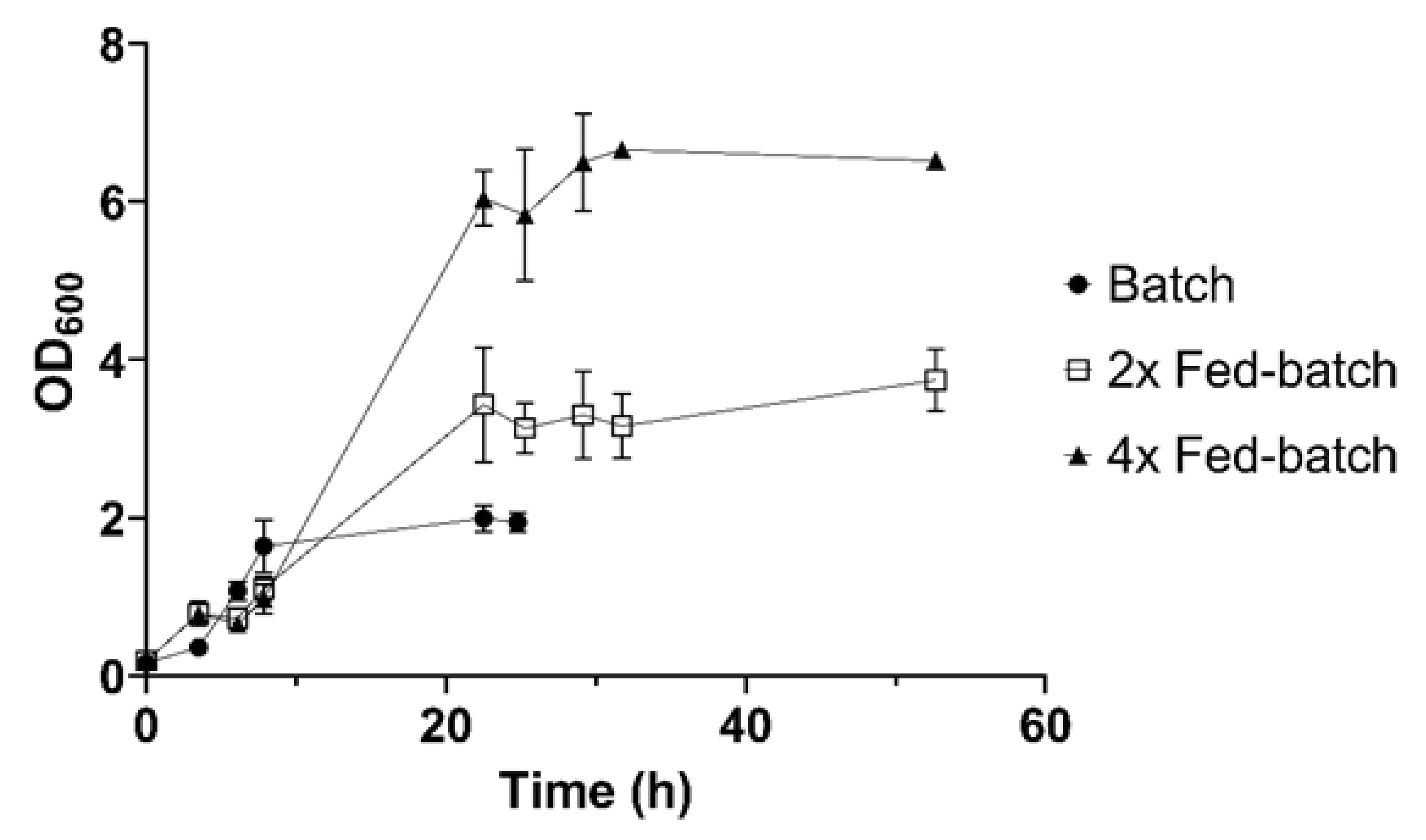
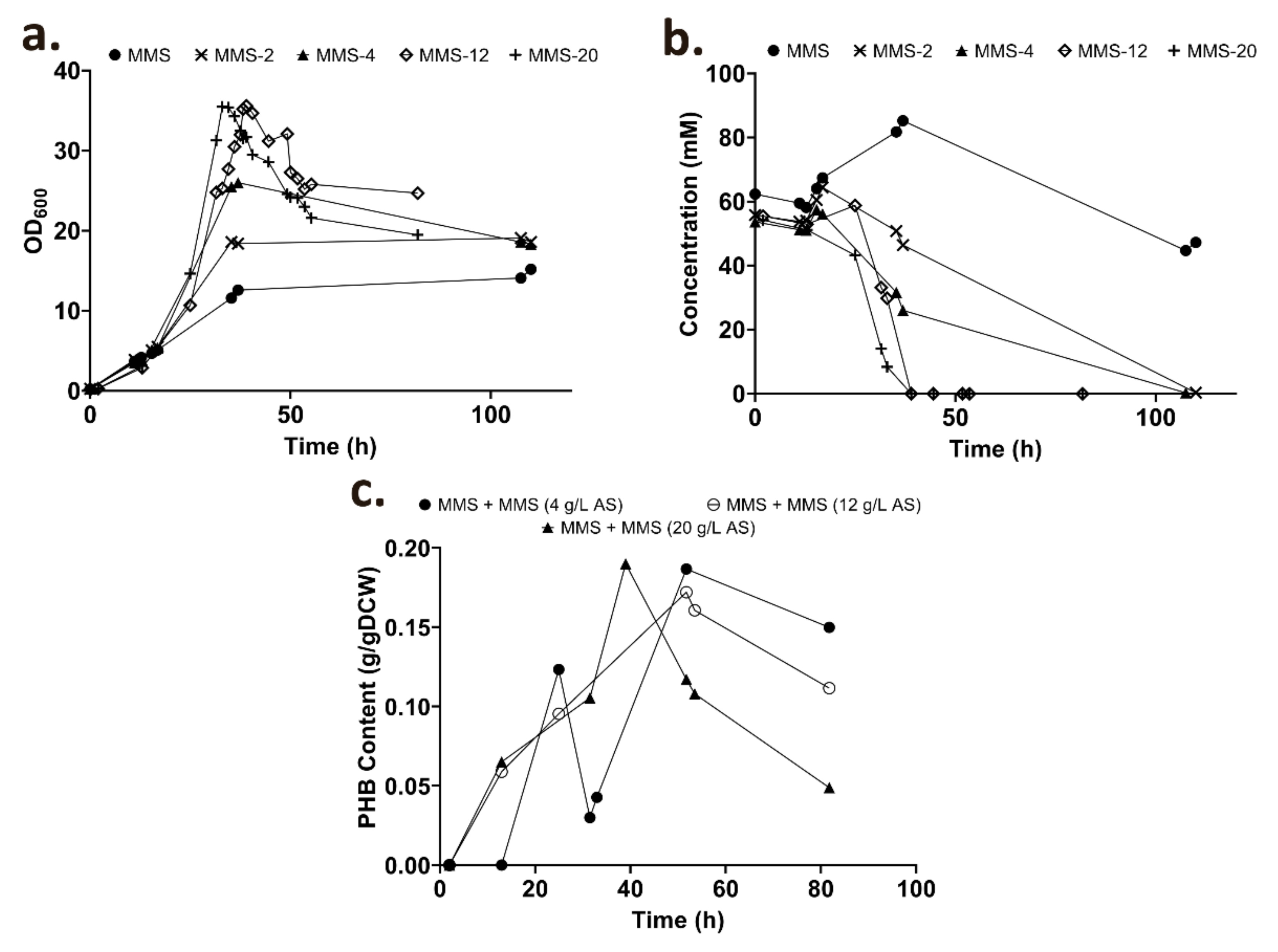
3.3. Fed-Batch Strategy for High Cell Density Cultures
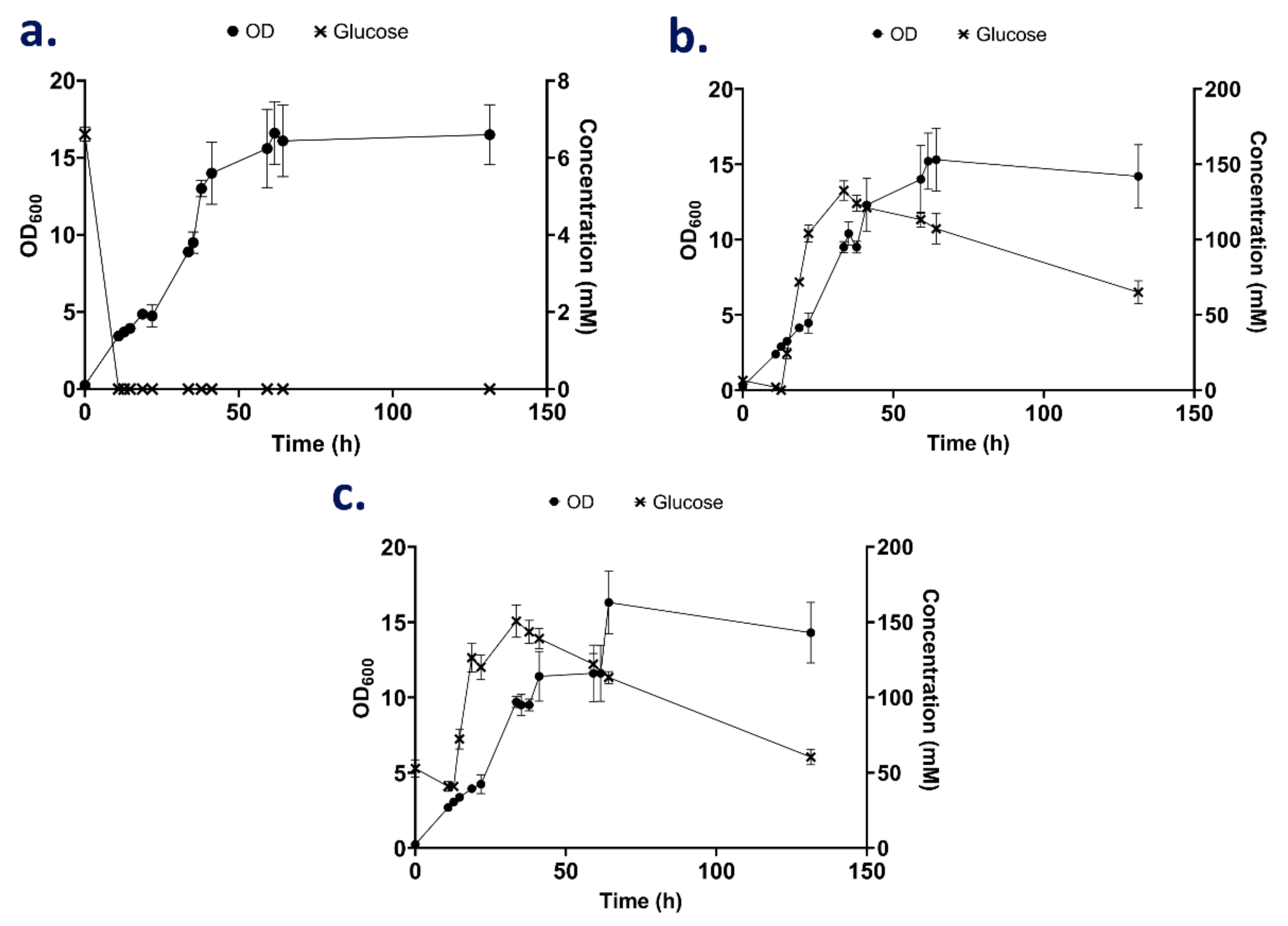
3.3.1. The Effect of Nitrogen Supplementation during High Cell Cultivation
PGPBs Are Complex
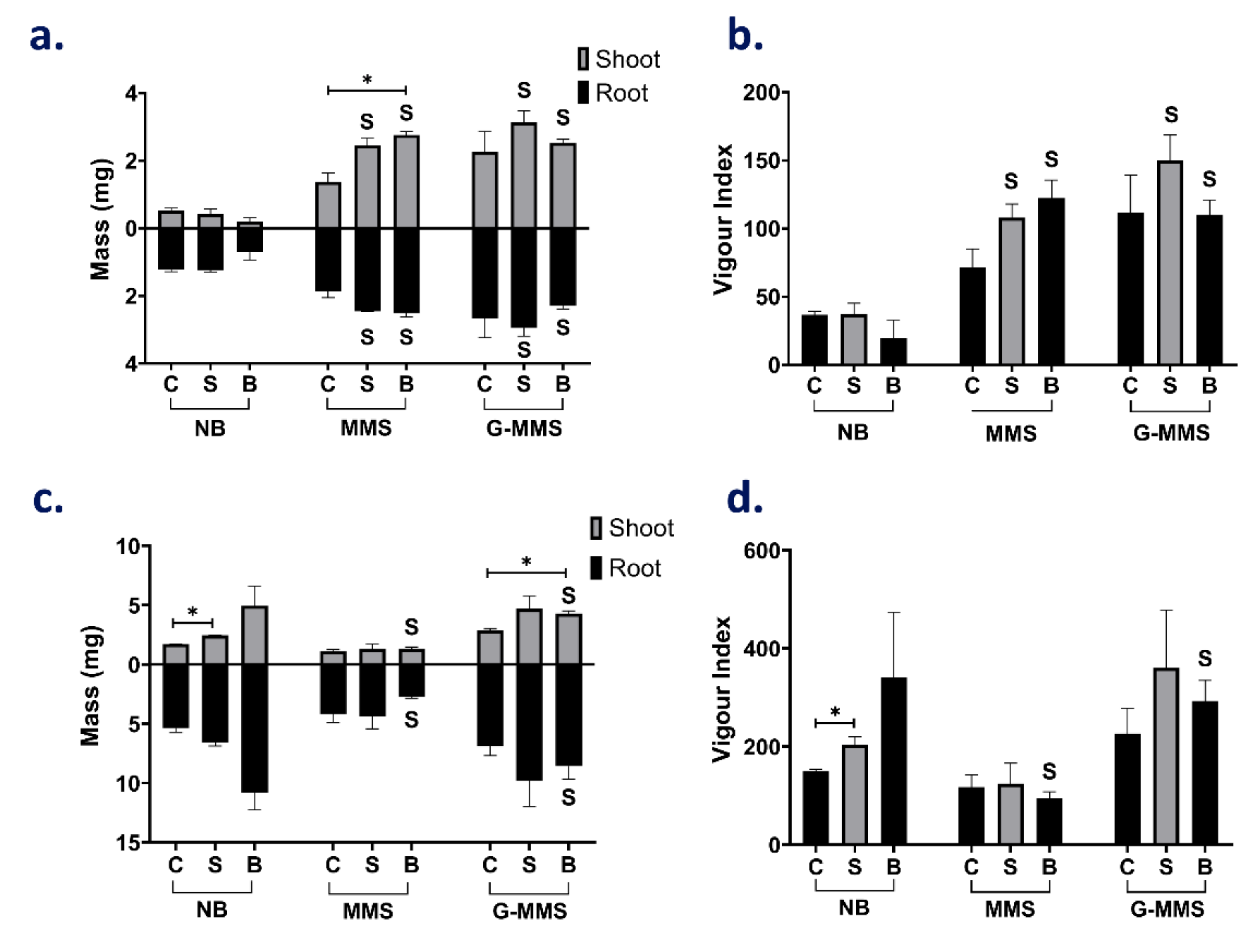
3.4. Plant Growth-Promoting Effects from High-Cell Density Cultures
3.4.1. SOS3 Maintains Its Growth-Promoting Effect under Control and Glasshouse Conditions
3.4.2. Proteomics Fingerprint of SOS3 Cultures as a Quality Measure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 1–16. [Google Scholar] [CrossRef]
- Ramakrishna, W.; Yadav, R.; Li, K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Appl. Soil Ecol. 2019, 138, 10–18. [Google Scholar] [CrossRef]
- Prasad, M.; Srinivasan, R.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. In PGPR Amelioration in Sustainable Agriculture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–157. [Google Scholar] [CrossRef]
- Wani, S.P.; Gopalakrishnan, S. Plant Growth-Promoting Microbes for Sustainable Agriculture. In Plant Growth Promoting Rhizobacteria (PGPR): Prospects for Sustainable Agriculture; Sayyed, R.Z., Reddy, M.S., Antonius, S., Eds.; Springer: Singapore, 2019; pp. 19–45. [Google Scholar] [CrossRef]
- Mutturi, S.; Bisaria, V.S. Bacterial Biofertilizers: High Density Cultivation. In Emerging Areas in Bioengineering; Chang, H.N., Ed.; Wiley-VCH Verlag GmbH & Co, KGaA: Weinheim, Germany, 2018; pp. 429–439. [Google Scholar] [CrossRef]
- Lobo, C.B.; Tomás, M.S.J.; Viruel, E.; Ferrero, M.A.; Lucca, M.E. Development of low-cost formulations of plant growth-promoting bacteria to be used as inoculants in beneficial agricultural technologies. Microbiol. Res. 2019, 219, 12–25. [Google Scholar] [CrossRef]
- Bashan, Y.; Trejo, A.; de-Bashan, L.E. Development of two culture media for mass cultivation of Azospirillum spp. and for production of inoculants to enhance plant growth. Biol. Fertil. Soils 2011, 47, 963–969. [Google Scholar] [CrossRef]
- Camelo-Rusinque, M.; Moreno-Galván, A.; Romero-Perdomo, F.; Bonilla-Buitrago, R. Development of a liquid fermentation system and encystment for a nitrogen-fixing bacterium strain having biofertiliser potential. Rev. Argent. Microbiol. 2017, 49, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Bai, Z.; Jin, B.; Xiao, R.; Zhuang, G. Bioconversion of wastewater from sweet potato starch production to Paenibacillus polymyxa biofertiliser for tea plants. Sci. Rep. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, L.; Zhao, J.; Huang, R.; Li, R.; Shen, Q. Utilization of different waste proteins to create a novel PGPR-containing bio-organic fertiliser. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Glick, B.R. Introduction to plant growth-promoting bacteria. In Beneficial Plant-Bacterial Interactions; Glick, B.R., Ed.; Springer: Berlin, Germany, 2020; pp. 1–37. [Google Scholar] [CrossRef]
- Vassilev, N.; Vassileva, M.; Lopez, A.; Martos, V.; Reyes, A.; Maksimovic, I.; Eichler-Löbermann, B.; Malusa, E. Unexploited potential of some biotechnological techniques for biofertiliser production and formulation. Appl. Microbiol. Biotechnol. 2015, 99, 4983–4996. [Google Scholar] [CrossRef] [PubMed]
- Paungfoo-Lonhienne, C.; Redding, M.; Pratt, C.; Wang, W. Plant growth promoting rhizobacteria increase the efficiency of fertilisers while reducing nitrogen loss. J. Environ. Manag. 2019, 233, 337–341. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Watanarojanaporn, N.; Jaemsaeng, R. Plant Growth Promoting Rhizobacteria Enhance the Efficiency of the Combination of Organic and Chemical Fertilisers in Sugarcane. Open J. Ecol. 2020, 10, 440–444. [Google Scholar] [CrossRef]
- Kanjanasopa, D.; Aiedhet, W.; Thitithanakul, S.; Paungfoo-Lonhienne, C. Plant Growth Promoting Rhizobacteria as Biological Control Agent in Rice. Agric. Sci. 2021, 12, 1. [Google Scholar] [CrossRef]
- Matkin, O.A.; Chandler, P.A. The U.C.-Type Soil Mixes. In The U.C. System for Producing Healthy Container-Grown Plants; Baker, K.F., Ed.; The University of California: Los Angeles, LA, USA, 1957; pp. 68–85. [Google Scholar]
- Lemgruber, R.d.S.P.; Valgepea, K.; Tappel, R.; Behrendorff, J.B.; Palfreyman, R.W.; Plan, M.; Hodson, M.P.; Simpson, S.D.; Nielsen, L.K.; Köpke, M. Systems-level engineering and characterisation of Clostridium autoethanogenum through heterologous production of poly-3-hydroxybutyrate (PHB). Metab. Eng. 2019, 53, 14–23. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, R.A.; McCubbin, T.; Turner, M.S.; Nielsen, L.K.; Marcellin, E. Engineering Escherichia coli for propionic acid production through the Wood–Werkman cycle. Biotechnol. Bioeng. 2020, 117, 167–183. [Google Scholar] [CrossRef]
- Weuster-Botz, D. Experimental design for fermentation media development: Statistical design or global random search? J. Biosci. Bioeng. 2000, 90, 473–483. [Google Scholar] [CrossRef]
- Fratelli, F.; Siquini, T.J.; Prado, S.M.A.; Higashi, H.G.; Converti, A.; de Carvalho, J.C.M. Effect of Medium Composition on the Production of Tetanus Toxin by Clostridium tetani. Biotechnol. Prog. 2005, 21, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Murzin, D.Y.; Daigue, E.; Slotte, R.; Sladkovskiy, D.A.; Salmi, T. Techno-economic analysis for production of L-arabitol from L-arabinose. Chem. Eng. Technol. 2020, 43, 1260–1267. [Google Scholar] [CrossRef]
- Dessbesell, L.; Souzanchi, S.; Venkateswara Rao, K.T.; Carrillo, A.A.; Bekker, D.; Hall, K.A.; Lawrence, K.M.; Tait, C.L.; Xu, C. Production of 2, 5-furandicarboxylic acid (FDCA) from starch, glucose, or high-fructose corn syrup: Techno-economic analysis. Biofuels. Bioprod. Biorefining 2019, 13, 1234–1245. [Google Scholar] [CrossRef]
- Saha, B.C.; Racine, F.M. Biotechnological production of mannitol and its applications. Appl. Microbiol. Biotechnol. 2011, 89, 879–891. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Petersen, I.A. Fermentation of a Rhizosphere-Associated Bacterium for Agricultural Applications; The University of Queensland: St. Lucia, QLD, Australia, 2020. [Google Scholar]
- Oliveri, D.A.; Ferrerira, S.D.; Carrera, D.L.; Serrao, C.P.; Callegari, D.M.; Barros, N.L.; Coelho, F.M.; Souza, C.R. Characterization of Pseudomonas bacteria of Piper tuberculatum regarding the production of potentially bio-stimulating compounds for plant growth. Acta Amaz. 2021, 51, 1. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Silva, C.E.; Carvalho, G.; Reis, M.A. Strategies for efficiently selecting PHA producing mixed microbial cultures using complex feedstocks: Feast and famine regime and uncoupled carbon and nitrogen availabilities. New Biotechnol. 2017, 37, 69–79. [Google Scholar] [CrossRef]
- Stritzler, M.; Diez Tissera, A.; Soto, G.; Ayub, N. Plant growth-promoting bacterium Pseudomonas fluorescens FR1 secrets a novel type of extracellular polyhydroxybutyrate polymerase involved in abiotic stress response in plants. Biotechnol. Lett. 2018, 40, 1419–1423. [Google Scholar] [CrossRef]
- Santos, M.S.; Hungria, M.; Nogueira, M.A. Production of polyhydroxybutyrate (PHB) and biofilm by Azospirillum brasilense aiming at the development of liquid inoculants with high performance. Embrapa Soja-Artigo em Periódico Indexado (ALICE) 2017, 16, 1855–1862. [Google Scholar] [CrossRef]
- Sanhueza, C.; Diaz-Rodriguez, P.; Villegas, P.; González, Á.; Seeger, M.; Suárez-González, J.; Concheiro, A.; Alvarez-Lorenzo, C.; Acevedo, F. Influence of the carbon source on the properties of poly-(3)-hydroxybutyrate produced by Paraburkholderia xenovorans LB400 and its electrospun fibers. Int. J. Biol. Macromol. 2020, 152, 11–20. [Google Scholar] [CrossRef]
- Gundi, J.S.; Santos, M.S.; Oliveira, A.L.; Nogueira, M.A.; Hungria, M. Development of liquid inoculants for strains of Rhizobium tropici group using response surface methodology. Embrapa Soja-Artigo em Periódico Indexado (ALICE) 2018, 17, 411–421. [Google Scholar] [CrossRef][Green Version]
- Trujillo-Roldán, M.A.; Valdez-Cruz, N.A.; Gonzalez-Monterrubio, C.F.; Acevedo-Sánchez, E.V.; Martínez-Salinas, C.; García-Cabrera, R.I.; Gamboa-Suasnavart, R.A.; Marín-Palacio, L.D.; Villegas, J.; Blancas-Cabrera, A. Scale-up from shake flasks to pilot-scale production of the plant growth-promoting bacterium Azospirillum brasilense for preparing a liquid inoculant formulation. Appl. Microbiol. Biotechnol. 2013, 97, 9665–9674. [Google Scholar] [CrossRef]
- Berninger, T.; González López, Ó.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for Plant Growth and Mitigation of Abiotic Stresses: A Metabolomics Perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.A.; Rothballer, M.; Chowdhury, S.P.; Nussbaumer, T.; Gutjahr, C.; Falter-Braun, P. Systems biology of plant-microbiome interactions. Mol. Plant 2019, 12, 804–821. [Google Scholar] [CrossRef] [PubMed]
- Rilling, J.I.; Acuña, J.J.; Nannipieri, P.; Cassan, F.; Maruyama, F.; Jorquera, M.A. Current opinion and perspectives on the methods for tracking and monitoring plant growth—promoting bacteria. Soil Biol. Biochem. 2019, 130, 205–219. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petersen, I.; Paungfoo-Lonhienne, C.; Marcellin, E.; Nielsen, L.K.; Gonzalez, A. Towards Sustainable Bioinoculants: A Fermentation Strategy for High Cell Density Cultivation of Paraburkholderia sp. SOS3, a Plant Growth-Promoting Bacterium Isolated in Queensland, Australia. Fermentation 2021, 7, 58. https://doi.org/10.3390/fermentation7020058
Petersen I, Paungfoo-Lonhienne C, Marcellin E, Nielsen LK, Gonzalez A. Towards Sustainable Bioinoculants: A Fermentation Strategy for High Cell Density Cultivation of Paraburkholderia sp. SOS3, a Plant Growth-Promoting Bacterium Isolated in Queensland, Australia. Fermentation. 2021; 7(2):58. https://doi.org/10.3390/fermentation7020058
Chicago/Turabian StylePetersen, Ian, Chanyarat Paungfoo-Lonhienne, Esteban Marcellin, Lars Keld Nielsen, and Axayacatl Gonzalez. 2021. "Towards Sustainable Bioinoculants: A Fermentation Strategy for High Cell Density Cultivation of Paraburkholderia sp. SOS3, a Plant Growth-Promoting Bacterium Isolated in Queensland, Australia" Fermentation 7, no. 2: 58. https://doi.org/10.3390/fermentation7020058
APA StylePetersen, I., Paungfoo-Lonhienne, C., Marcellin, E., Nielsen, L. K., & Gonzalez, A. (2021). Towards Sustainable Bioinoculants: A Fermentation Strategy for High Cell Density Cultivation of Paraburkholderia sp. SOS3, a Plant Growth-Promoting Bacterium Isolated in Queensland, Australia. Fermentation, 7(2), 58. https://doi.org/10.3390/fermentation7020058








