The Second-Generation Biomethane from Mandarin Orange Peel under Cocultivation with Methanogens and the Armed Clostridium cellulovorans
Abstract
1. Introduction
2. Materials and Methods
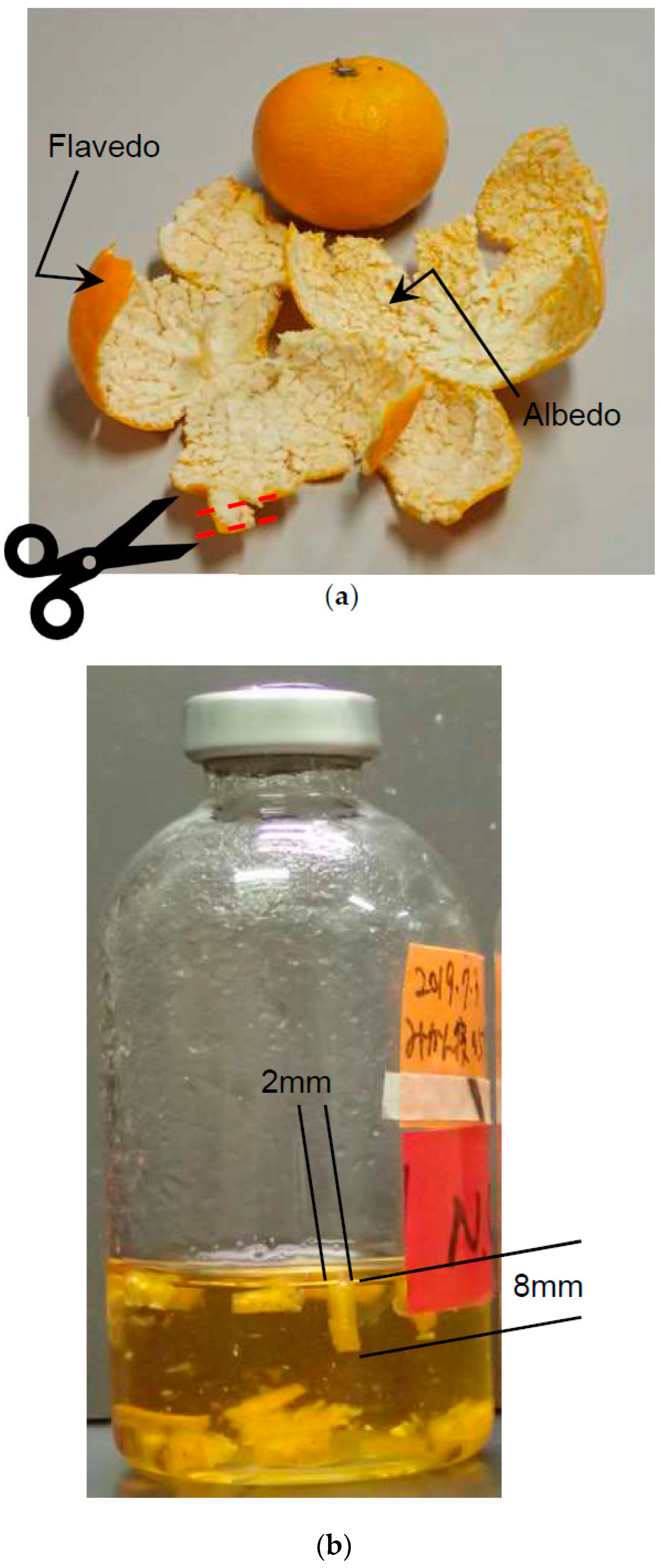
2.1. Materials
2.2. Microorganism and Culture Condition
2.3. Data Deposition
2.4. Measurement of Total Sugar Concentration
2.5. Gas Concentration
2.6. Organic Acid Concentration
2.7. Cell Growth
2.8. Statistics
3. Results
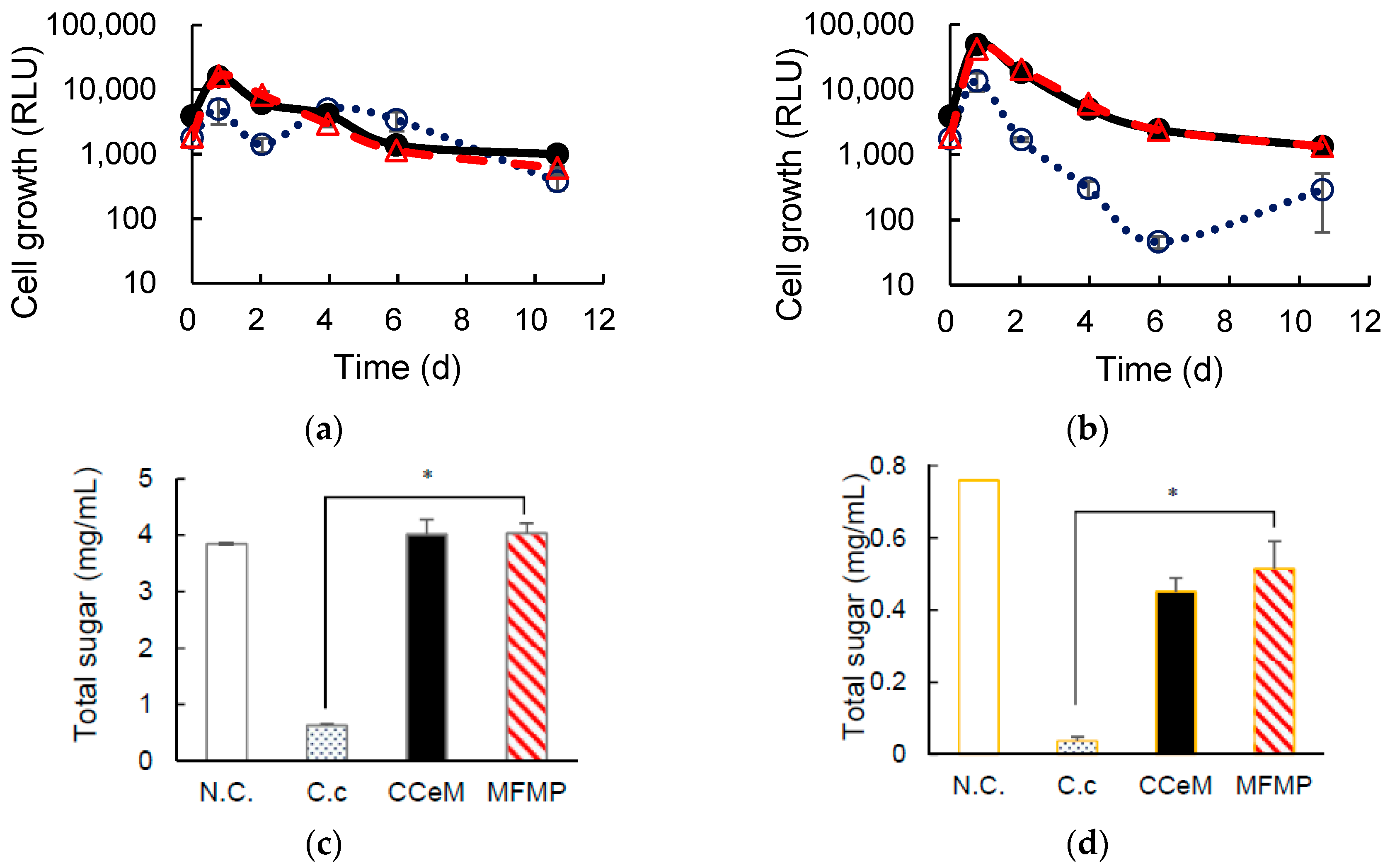
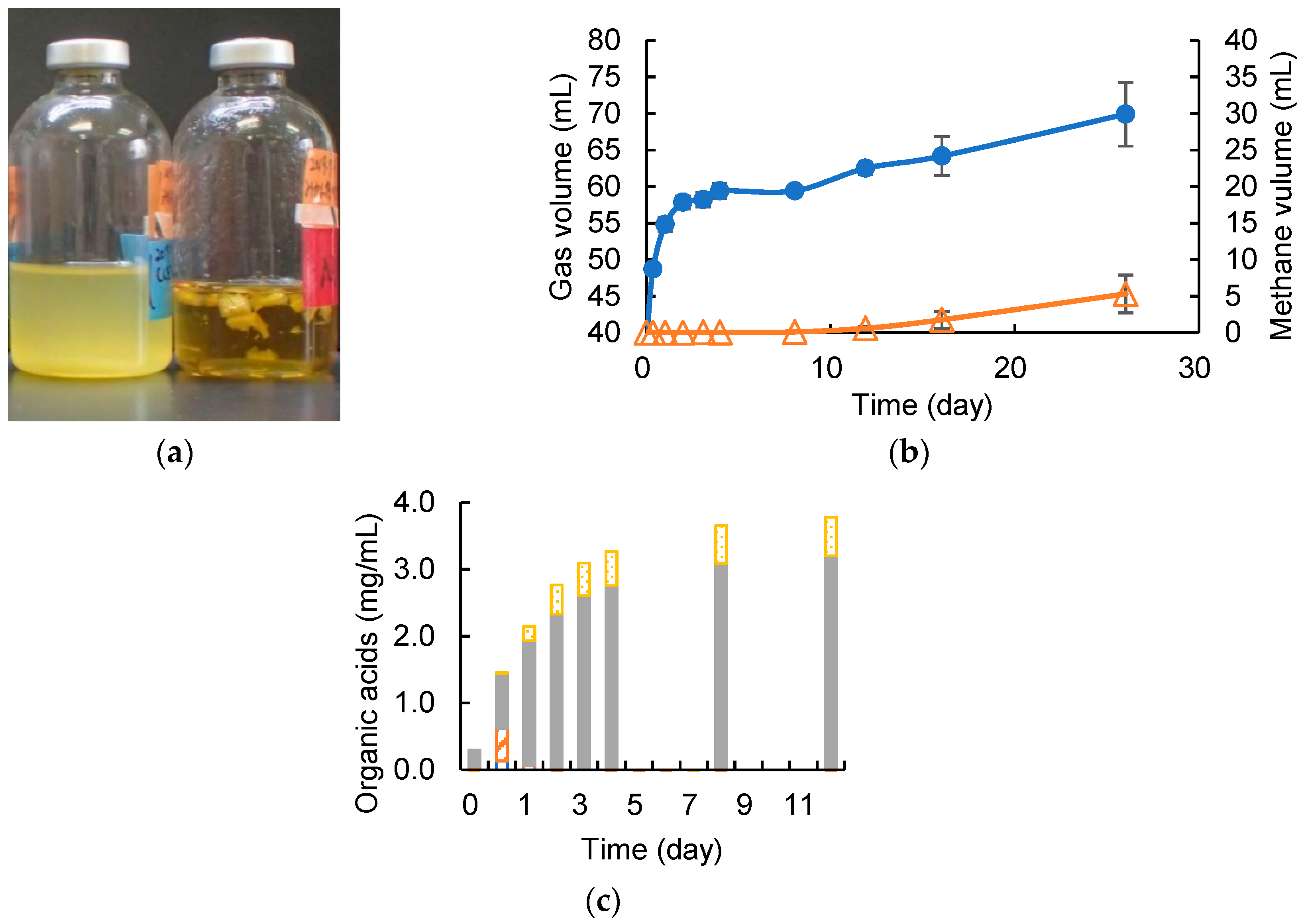
3.1. Degrading Cellulose and Removed Peel under Cocultivation with Methanogens and Non-Armed C. cellulovorans
3.2. Cellulose Degradation and Methane Production under Cocultivation with Methanogens and the Armed C. cellulovorans
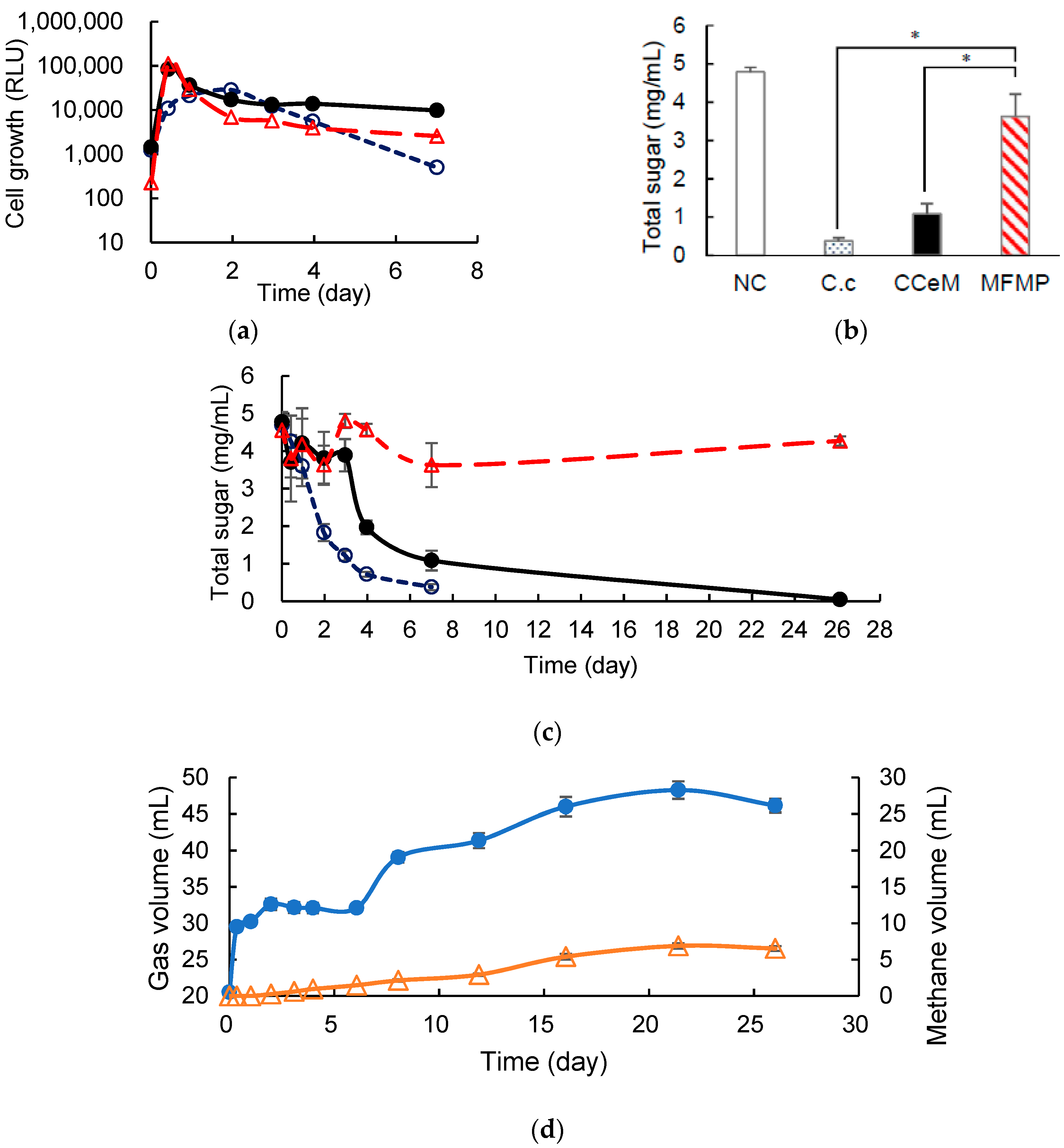
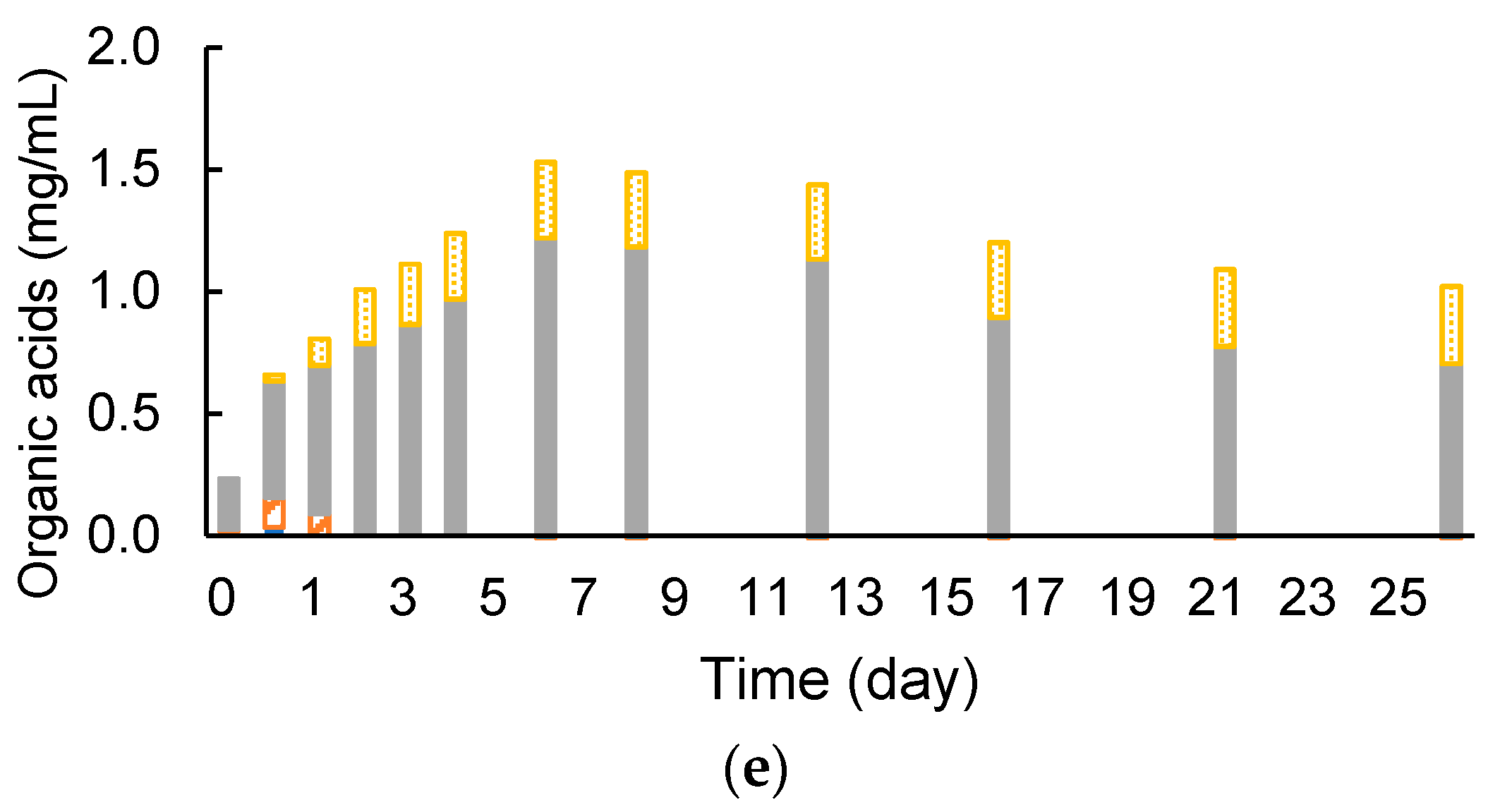
3.3. Removed Peel Degradation and Methane Production under Cocultivation with Methanogens and the Armed C. cellulovorans
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Schenk, P.M.; Thomas-Hall, S.R.; Stephens, E.; Marx, U.C.; Mussgnug, J.H.; Posten, C.; Kruse, O.; Hankamer, B. Second generation biofuels: High-efficiency microalgae for biodiesel production. Bioenergy Res. 2008, 1, 20–43. [Google Scholar] [CrossRef]
- Brethauer, S.; Studer, M.H. Biochemical conversion processes of lignocellulosic biomass to fuels and chemicals—A review. Chimia 2015, 69, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Gray, K.A.; Zhao, L.; Emptage, M. Bioethanol. Curr. Opin. Chem. Biol. 2006, 10, 141–146. [Google Scholar] [CrossRef]
- Grohmann, K.; Baldwin, E.A.; Buslig, B.S. Production of ethanol from enzymatically hydrolyzed orange peel by the yeast Saccharomyces cerevisiae. Appl. Environ. Microbiol. 1994, 45, 315–327. [Google Scholar]
- Winniczuk, P.P.; Parish, M.E. Minimum inhibitory concentrations of antimicrobials against microorganisms related to citrus juice. Food Microbiol. 1997, 14, 373–381. [Google Scholar] [CrossRef]
- Lane, A.G. Removal of peel oil from citrus peel press liquors before anaerobic digestion. Environ. Technol. Lett. 1983, 4, 65–72. [Google Scholar] [CrossRef]
- Pourbafrani, M.; Talebnia, F.; Niklasson, C.; Taherzade, M.J. Protective effect ofencapsulation in fermentation of limonene-contained media and orange peel hydrolyzate. Int. J. Mol. Sci. 2007, 8, 777–787. [Google Scholar] [CrossRef]
- Tomita, H.; Tamaru, Y. Direct IBE fermentation from mandarin orange wastes by combination of Clostridium cellulovorans and Clostridium beijerinckii. AMB Express 2019, 9, 1. [Google Scholar] [CrossRef]
- Jones, D.T.; Woods, D.R. Acetone-Butanol Fermentation Revisited. Microbiol. Rev. 1986, 50, 484–524. [Google Scholar]
- Vieiraa, C.F.S.; Filhob, F.M.; Filhoa, R.M.; Marianoa, A.P. Acetone-free biobutanol production: Past and recent advances in the Isopropanol-Butanol-Ethanol (IBE) fermentation. Bioresour. Technol. 2019, 287, 121425. [Google Scholar] [CrossRef] [PubMed]
- Doi, R.H.; Kosugi, A. Cellulosomes: Plant-cell-wall-degrading enzyme complexes. Nat. Rev. Microbiol. 2004, 2, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Sleat, R.; Mah, R.A.; Robinson, R. Isolation and characterization of an anaerobic, cellulolytic bacterium, Clostridium cellulovorans sp. nov. Appl. Environ. Microbiol. 1984, 48, 88–93. [Google Scholar] [PubMed]
- Koukiekolo, R.; Cho, H.Y.; Kosugi, A.; Inui, M.; Yukawa, H.; Doi, R.H. Degradation of corn fiber by Clostridium cellulovorans cellulases and hemicellulases and contribution of scaffolding protein CbpA. Appl. Environ. Microbiol. 2005, 71, 3504–3511. [Google Scholar] [CrossRef]
- Beukes, N.; Chan, H.; Doi, R.H.; Pletschke, B.I. Synergistic associations between Clostridium cellulovorans enzymes XynA, ManA and EngE against sugarcane bagasse. Enzym. Microb. Technol. 2008, 42, 492–498. [Google Scholar] [CrossRef]
- Dredge, R.; Radloff, S.E.; van Dyk, J.S.; Pletschke, B.I. Lime pretreatment of sugar beet pulp and evaluation of synergy between ArfA, ManA and XynA from Clostridium cellulovorans on the pretreated substrate. 3 Biotech 2011, 1, 151–159. [Google Scholar] [CrossRef][Green Version]
- Tamaru, Y.; Miyake, H.; Kuroda, K.; Ueda, M.; Doi, R.H. Comparative genomics of the mesophilic cellulosome-producing Clostridium cellulovorans and its application to biofuel production via consolidated bioprocessing. Environ. Technol. 2010, 31, 889–903. [Google Scholar] [CrossRef]
- Matsui, K.; Bae, J.; Esaka, K.; Morisaka, H.; Kuroda, K.; Ueda, M. Exoproteome profiles of Clostridium cellulovorans on various carbon sources. Appl. Environ. Microbiol. 2013, 79, 6576–6584. [Google Scholar] [CrossRef]
- Tamaru, Y.; Ui, S.; Murashima, K.; Kosugi, A.; Chan, H.; Doi, R.H.; Liu, B. Formation of protoplasts from cultured tobacco cells and Arabidopsis thaliana by the action of cellulosomes and pectate lyase from Clostridium cellulovorans. Appl. Environ. Microbiol. 2002, 68, 2614–2618. [Google Scholar] [CrossRef]
- Blair, B.G.; Anderson, K.L. Comparison of staining techniques for scanning electron microscopic detection of ultrastructural protuberances on cellulolytic bacteria. Biotech. Histochem. 1998, 73, 107–113. [Google Scholar] [CrossRef]
- Blair, B.G.; Anderson, K.L. Regulation of cellulose inducible structures of Clostridium cellulovorans. Can. J. Microbiol. 1999, 45, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peng, Y.; Ni, B.J.; Han, X.; Fan, L.; Yuan, Z. Dissecting microbial community structure and methane-producing pathways of a full-scale anaerobic reactor digesting activated sludge from wastewater treatment by metagenomic sequencing. Microb. Cell Factories 2015, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Tamaru, Y. Biomethane production from sugar beet pulp under cocultivation with Clostridium cellulovorans and methanogens. AMB Express 2019, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Miyake, H.; Maeda, Y.; Ishikawa, T.; Tanaka, A. Calorimetric studies of the growth of anaerobic microbes. J. Biosci. Bioeng. 2016, 122, 364–369. [Google Scholar] [CrossRef]
- Joshi, S.M.; Waghmare, J.S.; Sonawane, K.D.; Waghmare, S.R. Bio-ethanol and bio-butanol production from orange peel waste. Biofuels 2015, 6, 55–56. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Madl, R.L.; Saida, L.; Abeykoon, J.P. Ethanol Production from Orange Peels: Two-Stage Hydrolysis and Fermentation Studies Using Optimized Parameters through Experimental Design. J. Agric. Food Chem. 2010, 58, 3422–3429. [Google Scholar] [CrossRef]
- Tamaru, Y.; Miyake, H.; Kuroda, K.; Nakanishi, A.; Matsushima, C.; Doi, R.H.; Ueda, M. Comparison of the mesophilic cellulosome-producing Clostridium cellulovorans genome with other cellulosome-related clostridial genomes. Microb. Biotechnol. 2011, 4, 64–73. [Google Scholar] [CrossRef]
- Lu, H.; Ng, S.K.; Jia, Y.; Cai, M.; Lee, P.K.H. Physiological and molecular characterizations of the interactions in two cellulose-to-methane cocultures. Biotechnol. Biofuels 2017, 10, 37. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA–Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Holden, J.M.; Eldridge, A.L.; Beecher, G.R.; Buzzard, I.M.; Bhagwat, S.; Davis, C.S.; Douglas, L.W.; Gebhardt, S.; Haytowitz, D.; Schakel, S. Carotenoid content of U.S. foods: An update of the database. J. Food Compos. Anal. 1999, 12, 169–196. [Google Scholar] [CrossRef]
- Sluijs, I.; Beulens, J.W.; Grobbee, D.E.; van der Schouw, Y.T. Dietary carotenoid intake is associated with lower prevalence of metabolic syndrome in middle-aged and elderly men. J. Nutr. 2009, 139, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, Y.; Azqueta, A.; Luna, L.; Bonilla, F.; Domínguez, G.; Collins, A.R. The carotenoid β-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis 2009, 30, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Burri, B.J. Beta-cryptoxanthin as a source of vitamin A. J. Sci. Food Agric. 2014, 95, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Hu, K.Q.; Russell, R.M.; Wang, X.D. β-Cryptoxanthin suppresses the growth of immortalized human bronchial epithelial cells and non-small-cell lung cancer cells and up-regulates retinoic acid receptor β expression. Cancer Cell Biol. 2006, 119, 2084–2089. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, H.; Tamaru, Y. The Second-Generation Biomethane from Mandarin Orange Peel under Cocultivation with Methanogens and the Armed Clostridium cellulovorans. Fermentation 2019, 5, 95. https://doi.org/10.3390/fermentation5040095
Tomita H, Tamaru Y. The Second-Generation Biomethane from Mandarin Orange Peel under Cocultivation with Methanogens and the Armed Clostridium cellulovorans. Fermentation. 2019; 5(4):95. https://doi.org/10.3390/fermentation5040095
Chicago/Turabian StyleTomita, Hisao, and Yutaka Tamaru. 2019. "The Second-Generation Biomethane from Mandarin Orange Peel under Cocultivation with Methanogens and the Armed Clostridium cellulovorans" Fermentation 5, no. 4: 95. https://doi.org/10.3390/fermentation5040095
APA StyleTomita, H., & Tamaru, Y. (2019). The Second-Generation Biomethane from Mandarin Orange Peel under Cocultivation with Methanogens and the Armed Clostridium cellulovorans. Fermentation, 5(4), 95. https://doi.org/10.3390/fermentation5040095




