Abstract
The production of volatile compounds has become one of the major technological features for yeast selection. In fact, although the aromatic profile of the wine is the sum of varietal-, pre-, post-, and fermentative-aroma compound, yeasts affect the quality of the grape from maturation throughout fermentation, metabolizing sugars and other components into alcohols, esters, organic acids, and aldehydes. Among the new technological features, the production of mannoproteins has gained interest. From this perspective, the main aim of this work was to characterize 9 strains of Saccharomyces cerevisiae and 1 of Saccharomyces bayanus for their volatile profiles and the release of mannoproteins. The strains were inoculated in Trebbiano musts and incubated at 15 °C; at the end of fermentation the wines were evaluated by GC/MS/SPME for their volatile profiles and mannoprotein content by enzymatic assay. The strains were inoculated at level ranging between 4.9 and 6.3 log CFU/mL but only the strains L318 and 12233X6167 were able to reach values of 7.5 log CFU/mL. The aromatic profiles resulted in a strain-specific fingerprinting. According to the principal component analysis, the wines produced by the strains L288, L234, and L318 were characterized by the presence of propanoic acid, butanol, octanoic acid, and 3 methyl pentanol while the wine obtained by the strain 12233x35G2 was characterized by the presence of propanoic acid, butanol, octanoic acid and 3 methyl pentanol while the strain 12233x35G2 was characterized by the presence of decanoic acid ethyl ester, heptanoic acid ethyl ester, and acetic acid 2 phenetyl ester. Regarding mannoproteins, the highest concentration was achieved by strain12233x6167 (104 mg/L). The data allowed to select the strains endowed with the best fermentation performances in terms of aroma and mannoproteins release.
1. Introduction
Saccharomyces cerevisiae and the other strictly related species like Saccharomyces bayanus are considered the “wine yeasts” with the highest oenological potential and are commonly used in the wine making [1]. Although, they are not the numerically prevalent species on the raw material, their strong alcohol tolerance and fermentative aptitude represent a great ecological advantage allowing these species to take over quickly on the grape yeast microbiota [1]. In addition to their efficient conversion of fermentable sugars into ethanol and carbon dioxide during alcoholic fermentation, these species produce many secondary metabolites (alcohols, aldehydes, ketones, esters, carbonyl compounds, organic acids, and volatile sulfur molecules) which specifically contribute to the volatile molecular profiles and the organoleptic properties of wines. The amount and the perception of these aroma compounds in the final product depend on several factors such as the Vitis vinifera grape cultivar, the final wine physiochemical properties, the interaction between chemicals and or with solids suspended in the matrix. According to the literature, the study of these profiles and their behavior in relation the to the grape’s origin is used as a fingerprint tool to correlate a specific product to its production area. Gas chromatography combined with mass spectrometry and solid phase microextraction (SPME) represent one of the most used strategies to highlight the relation between fermentation agents and volatile molecular profile of wine [2,3,4] and represents a useful tool for the selection of new starters able to enhance sensorial properties of final products, due to the production of specifics aroma compounds [3,5,6]. Although oenological yeasts are selected on the base of traditional features such as alcohol tolerance, fermentative vigor, SO2 resistance, production of killer toxins [7], in the last decade the selection was also focused on “unconventional traits”, that significantly improve the wine technological, organoleptic, and sensorial properties. These new criteria include yeasts ability to improve the color of wine through the formation of stable pigments; yeast β-glucosidasic activity against must-derived monoterpenes and the consequent improvement of varietal bouquet of wines [8]; production of volatile compounds able to enhance the aroma of wines such as esters, terpinols, poli-alcohols; the ability to produce and release during the alcoholic fermentation specific macromolecules able to promote wines properties like mannoproteins (MPs). MPs are mainly constituted by mannose (85–90%) and proteins (10–15%) [9,10], and they are the yeast-derived glycoprotein complexes more present in wine and could have some positive effects on the technological and sensorial properties of wines [11]. In fact, they are reported to improve the wine colloidal stability, to reduce the tannin self-aggregation preventing the reduction of the wine acidity [12,13,14], to reduce the protein precipitation [15] preventing the protein haze [14] and allowing a better foam quality of sparkling wines [16,17], to promote the color stabilization. Moreover, due to their chemicals structure and properties MPs adsorb different anthocyanin and tannin derived complexes preventing their polymerization and aggregation [18] especially when mannoproteins are released from yeast autolysis during the alcoholic fermentation [19]. In addition, they can contribute to improve the wine safety, reducing the amounts of some toxic compounds present in grape juices and wines like the ochratoxin A [20,21,22]. As showed from different Authors, the presence of these polysaccharides can also stimulate the growth of different lactic acid bacteria including Oneococcus oeni [23,24] and positively interact with flor yeasts [25,26]. Moreover, MPs also promote the wine palatability and mouth feel [27] by increasing the sweetness [28], [29,30,31] and improving the aroma persistence and complexity [32,33]. Yeasts can release mannoproteins, thanks to a controlled hydrolysis [9], even during the fermentation and the aging process.
In this context, the selection of new Saccharomyces cerevisiae strains or hybrids or S. bayanus strains able to release a significant concentration of mannoproteins represents one of the emerging challenges in the oenological sector. For this, 9 Saccharomyces cerevisiae and 1 Saccharomyces bayanus strains were evaluated for their aptitude to generate good volatile profiles and to release mannoproteins during the alcoholic fermentation in Trebbiano must incubated at 15 °C.
2. Results
2.1. Yeast Growth and Fermentation Kinetics
In Table 1, the yeast cell load (log CFU/mL) evolution during the fermentation of Trebbiano must is reported. Among the tested strains, only S. cerevisiae L318 and hybrid S. cerevisiae × S. cerevisiae 12233x6167 were able to reach cell loads higher than 7 log CFU/mL within 4 days of incubation at 15 °C. Otherwise, the remaining strains reached values no higher than 6.5 log CFU/mL in the same time considered. However, within 11 days of incubation, most of the tested strains were able to reach values higher than 7 log CFU/mL and to maintain high cell load values until the end of the fermentation. After 26 days of incubation at 15 °C, the strains considered decreased their cell loads.

Table 1.
Evolution of yeast cell loads (CFU/mL) during alcoholic fermentation at 15 °C of Trebbiano musts in relation to the fermenting agent used.
The fermentation kinetic data indicate that the greatest reduction in weight (%) were recorded for the L288, L318, and 12233 strains (Figure 1) which, at the end of fermentation, recorded the highest alcoholic degrees but not the lowest sugar residues (Table 2).
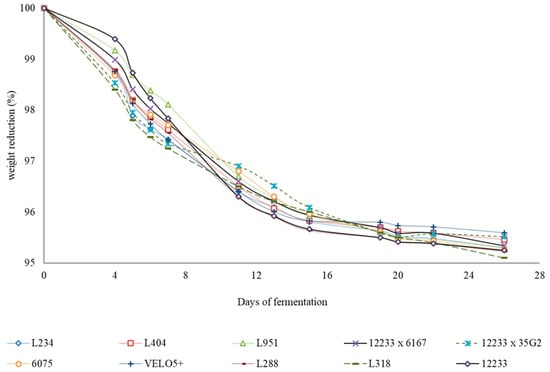
Figure 1.
Fermentation kinetics (weight reduction %) of Trebbiano must inoculated with the different strains and hybrids considered in the experimentation.

Table 2.
Total Alcoholometric Volumic title (TAV) and residual sugars (g/L) of Trebbiano wines after 26 days of fermentation in relation to the strain considered.
2.2. Total Alcoholometric Volumic Title and Residual Sugar
As reported in Table 2, the strains L12233, L288, and VELO 5+ originated wines having a TAV higher than 11 with residual sugar level ranging between 1 and 2 g/L. Also the strains 6075 and L318 originated wine having TAV higher than 11 but with residual sugars of 4 and 2 g/L, respectively. The strain L234, L404, and L951 presented the highest residual sugar levels.
2.3. Volatile Molecular Profiles Analysis
The effects of the considered strains on the volatile molecular profiles of Trebbiano samples were analyzed by gas-chromatography combined with mass spectrometry and solid phase micro extraction (SPME). About 60–65 molecules belonging to different classes of compounds, such as alcohols, aldehydes, ketones, acids, and esters were detected. In Figure 2, the equivalent ppm of alcohols and esters are reported. The strain 12233x35G2 produced the highest amount of alcohols (over 250 e-ppm) and esters (150 e-ppm). In particular, among alcohols, although in different amounts, 2 methyl propanol, 3 methyl 1 butanol, butanol, nonanol, 3 methyl pentanol, 3 etoxi propanol, (Z)-3-hexen-1-ol, 2 ethyl hexanol, (3S)-3,7-Dimethyl-6-octen-1-ol, hexanol, heptanol, phenethyl alcohol, and octanol were found. Regarding esters, butanol 3 methyl acetate, ethyl acetate, butanoic acid ethyl ester, hydroxy butyl ethyl ester, hexanoic acid ethyl ester, heptanoic acid ethyl ester, octanoic acid ethyl ester, decanoic acid ethyl ester, benzenacetic acid ethyl ester, and acetic acid 2 phenyl ethyl ester were detected.
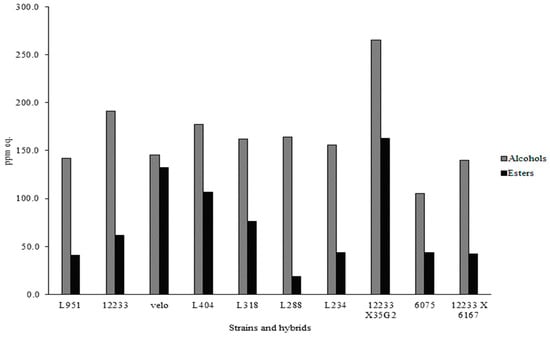
Figure 2.
Production of alcohols and esters (equivalent ppm) detected in wines at the end of fermentation in relation to the fermenting agent. The data are mean of three replicates and the coefficient of variance was between 5% and 10%.
According to the detected data, wine samples showed both qualitative (number of detected compounds) and quantitative (ppm equivalent) differences in volatile molecule profiles.
To highlight differences among the samples, a principal component analysis (PCA) was carried out taking into consideration all the molecules detected since the final wine volatile aroma profile is the result of the interaction of different molecules. PCA mapped wine samples in the space described by the first two principal component PC1 versus PC2 (Figure 3 and Figure 4). PC1 accounted the 20.28% of the total variability, while the PC2 for the 20.48%. Figure 3 describes the projection of samples in the space and the clusterization of the samples. On the basis of PCA analysis, samples were clustered into 4 different groups and were divided along the PC1 and PC2. Cluster 1 obtained from S. cerevisiae L 288, L 234, and L 318 strains was separated from other groups both along PC1 and PC2 as well as the Cluster 2 formed by the wine volatile profiles from the S. cerevisiae 12233×35G2 hybrid. Cluster 3 grouped samples from S. cerevisiae 12233 and S. bayanus L 951 strains. Cluster 4 was formed by S. cerevisiae strains L 404, velo 5+, 12233x6167 and 6075 samples (Figure 3).
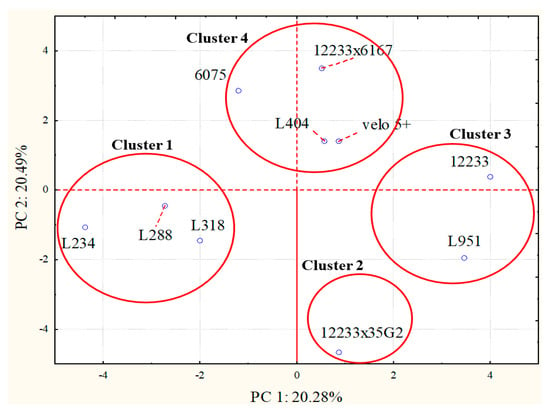
Figure 3.
Principal component analysis loading plot of volatile molecular profiles of Trebbiano wines after 26 days of fermentation at 15 °C in relation to the fermenting agent considered.
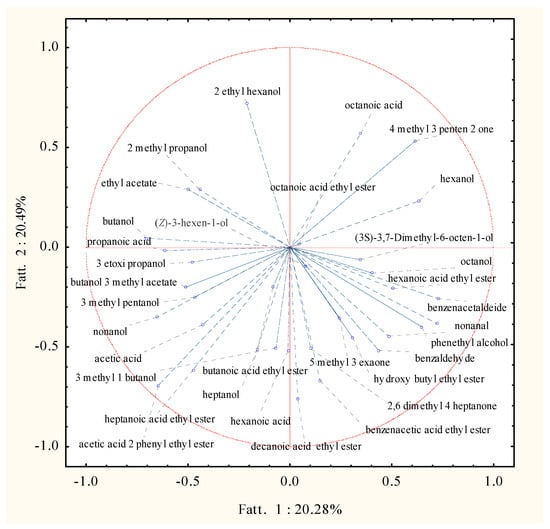
Figure 4.
Principal component analysis factor coordinates for the two-first factors of volatile molecular profiles of Trebbiano wines after 26 days of fermentation at 15 °C in relation to the fermenting agent considered.
The projection of the variables which affected the clusterization is reported in Figure 4. In particular, cluster 1 was affected by butanol, propanoic acid, 3 ethoxy propanol, 3 methyl butanol, nonanol; for the second group mainly ethyl or methyl esters like heptanoic acid ethyl ester, acetic acid 2 phenyl ethyl ester, decanoic acid ethyl ester, and benzenacetic acid ethyl ester affected the clusterization. The third cluster differed for a greater production of benzenacetaldehyde, octanol, hexanol and hexanoic acid ethyl ester; while 2-ethyl hexanol, octanoic acid, 4-methyl-3-penten-2-one contributed to the grouping of samples 6075, 12233x6167, velo 5+ and L404 in cluster 4.
In order to increase the significance of the variability among the samples, raw data concerning alcohols and esters were analyzed by PCA separately. In Figure 5, the projection of the samples, according to the used strains and the production of alcohols is reported. The PC1 and PC2 explained more than 50% of the variability among the samples. On the other side, the PC1 and PC2, considering the detection of esters, were able to explain more than 60% of variability among the obtained wines (Figure 6).
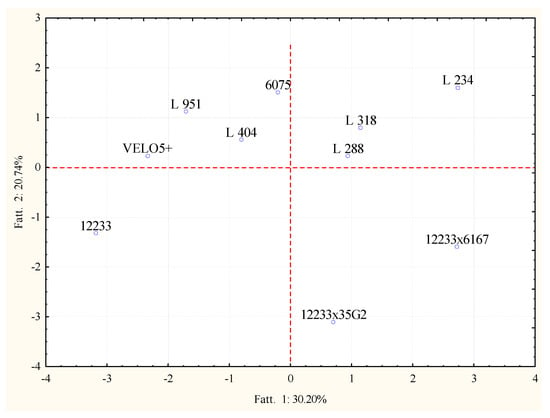
Figure 5.
Principal component analysis loading plot of alcohols profiles of Trebbiano wines after 26 days of fermentation at 15 °C in relation to the fermenting agent considered.
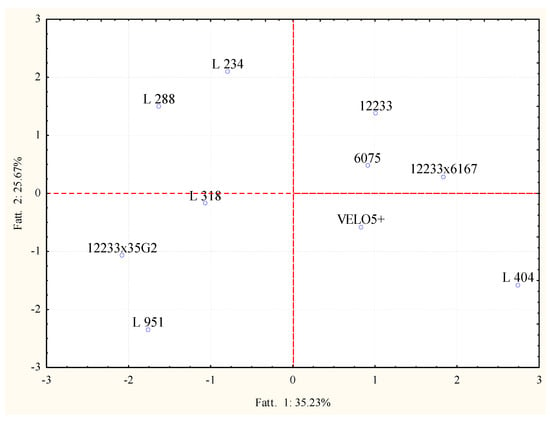
Figure 6.
Principal component analysis loading plot of esters profiles of Trebbiano wines after 26 days of fermentation at 15 °C in relation to the fermenting agent considered.
The concentrations of mannoproteins (MPs) detected by the enzymatic method at the end of fermentation in relation to the starter agent are reported in Table 3. The strains that allowed the highest concentrations of MPs at the end of the fermentation were both the S. cerevisiae hybrids 12233x6167, 12233x35G2 and the 12233, L951 strains (83–104 mg/L) On the other hand, Trebbiano wines obtained using S. cerevisiae 6075 and S. cerevisiae L234 as fermenting agent, resulted with the lowest amounts of mannoproteins respectively of 56 and 57 mg/L.

Table 3.
Concentrations of mannoproteins (mg/L) detected in Trebbiano wines after 26 days of fermentation at 15 °C in relation to the fermenting agent considered.
3. Discussion
Trebbiano is an Italian white wine, obtained from a neutral variety named Trebbiano Romagnolo, whose aroma is particularly affected by the yeasts used during the fermentation process rather than the presence of free monoterpenes [34]. For this, the selection of yeasts able to impart good volatile profiles, in addition to specific β-glucosidase activities, has become an important challenge and criterion for the strain choice to improve the final quality of this product. The strains employed in the present research fitted the primary selection criteria of yeast strain for alcoholic beverage, ending the fermentation and converting efficiently the grape sugars to alcohol. In fact, the total alcoholometric volumic title detected in the different micro-vinifications are in line with those detected in commercial and similar products. Each strain employed was able to impart specific features in volatile molecule profiles to the wine obtained, generating a specific aromagramma which, in its turns, depends on several factors. The first ones are the molecules initially present in the must and later the yeasts employed as well as the adopted winemaking conditions. The choice of the yeast strain largely affects the array of the generated volatile substances (e.g. higher alcohols, acids, esters, carbonyls, and thiols). In our experimental conditions, no terpenic molecules were founds, leading to suppose that the S. cerevisiae strains employed are not endowed of β-glucosidase activity. On the other hand, this feature is more pronounced in non-Saccharomyces yeasts as reported by several Authors [35,36,37,38]. Regarding mannoproteins, their accumulation has been studied in recent years given several of these molecules’ beneficial aspects in wine environments [39]. Thus, wine researchers have focused on finding yeast strains that have the ability to release large amounts of mannoproteins during winemaking. The experimental data have underlined that the strains 951 and 12233x6167 produced the highest amounts of mannoproteins while the strains velo 5+ and 6075 released the lowest amount. On the other hand, the quantity of mannoproteins released can vary extremely in relation to the strain and to the chemical–physical and compositional conditions of the system [33,40,41]. Their presence can also affect the release of volatile compounds, affecting the final perception of the wine [33]. In winemaking, the addition of commercial products rich in mannoproteins can be performed, but the challenge to find mannoprotein producing yeast can represent a great advantage also from an economical point of view. Generally, the use of mannoproteins and fine lees increased the levels of fruity esters such as ethyl hexanoate, methyl, and ethyl hexadecanoate probably due to the esterification of fatty acids released by yeasts during fermentation or autolysis. Particularly the strain 12233x35G2, although not producing the highest amount of mannoproteins, was characterized by the highest amount of ethyl hexadecanoate as for the strain 951. Also, Pérez-Través et al., [42] investigated the ability of natural hybrids of S. cerevisiae × S. krudriavzevii to release mannoproteins founding that the strains, at the adopted operating conditions, was able to produce higher quantity of mannoproteins with respect the only S. cerevisiae. Moreover, the author found that the genome interaction in hybrids generates a physiological environment that enhances the release of mannoproteins.
4. Material and Methods
4.1. Strains
In this study, 9 strains of S. cerevisiae and 1 strain of S. bayanus, belonging to the collection of the Department of Agricultural and Food Sciences, University of Bologna (Bologna, Italy), were used.
All the S. cerevisiae strains were isolated by Prof. Carlo Zambonelli, University of Bologna, from grapes of the Emilia-Romagna region (Bologna region, Italy). All these strains were characterized by prof. Zambonelli for the fermentation ability, SO2 resistance, alcohol tolerance, acetic acid production, SO2 production, glycerol production. S. bayanus 951 was isolated by Prof. Carlo Zambonelli from Lambrusco grapes [3].
All strains were grown on YPD medium (1% w/v yeast extract, 2% peptone, and 2% glucose; all chemicals provided by Oxoid, Milan, Italy) and incubated at 25 °C for 48 h before using.
4.2. Micro-Vinifications
The fermentations were carried out in flasks containing 5L of Trebbiano must vintage 2012 and inoculated with the different yeast strains at levels between 5–6 Log CFU/mL. The pre-cultures (48 hours) were used to inoculate the pasteurized must and fermentations were carried out at 15 °C. the fermentation was monitored daily by measurement of weight loss. The fermentations were considered finished when no variation of weight was observed for four consecutive days. Afterwards, the wines were clarified with bentonite (1 g/L) by stirring them very slightly for about 30 min at room temperature, then separated by filtration. Three replicates for each trial were set.
4.3. Microbiological Analysis
Viable cell counts were evaluated at different sampling times by plate counting using YPD agar (Oxoid, Basingstoke, UK)) incubated at 25 °C for 48 h. Three repetitions for each sample were considered.
4.4. Ethanol and WineRreducing Sugars
The ethanol determination was performed according to the EC2000 official EU method. Samples were considered statistically different at p < 0.05. Sugars (glucose and fructose) were detected by HPLC using a Jasco HPLC system with a pump (PU980) (Jasco Inc, Tokyo, Japan) equipped with a refractive index detector (RI830) according to the method proposed by [43]. Peak qualitative and quantitative analysis were based on retention times (Rt) and spiking technique using the external standard method. Analysis were performed in triplicates.
4.5. Mannoproteins Analysis
Polysaccharides were isolated by means of a HCl−ethanol solution and subsequent centrifugations according to [28], then mannoproteins were separated based on the OENO 26/2004 resolution [44] method and quantified by an enzymatic assay (Megazyme, Astori, Italy).
4.6. Volatile Molecule Profile
The sample volatile molecule profiles were determined by GC-MS-solid phase micro extraction (SPME). For this, five-milliliters of samples were placed in 10-mL glass vials, to which 1 g NaCl and 10 μL of 4-methyl-2-pentanol (as internal standard) were added. The samples were then equilibrated for 10 min at 45 °C, after that a 85 μm SPME fiber-polyacrylate coated fiber (Supelco Inc., Bellefonte, PA, USA) was exposed to each sample for 40 min. The equilibration and absorption phases were carried out under stirring condition. GC-MS analyses were performed using a Agilent 6890 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) equipped with an electron impact mass selective detector Agilent 5970 (ionization voltage, 70 eV) (Agilent Technologies, Palo Alto, CA, USA). CPWax52 CB capillary column (1.2 μm df; 0.32 mm i.d.; 50 m length) was used (Chrompack, Middelburg, The Netherlands). The molecule identification was performed by the comparison of NIST pure standards spectra library (version 2005) with the mass spectra obtained. The amounts of each compound identified was determined using a calibration curves method. Pure reference compounds were used to built calibration curves and the amounts of each compounds were determined by the interpolation of the relative areas versus the internal standard area. For Figure 3 the following alcohols were considered: 2 methyl propanol, 3 methyl 1 butanol, butanol, nonanol, 3 methyl pentanol, 3 etoxi propanol, (Z)-3-hexen-1-ol, 2 ethyl hexanol, (3S)-3,7-Dimethyl-6-octen-1-ol, hexanol, heptanol, phenethyl alcohol, and octanol. Also the following esters were included: butanol 3 methyl acetate, ethyl acetate, butanoic acid ethyl ester, hydroxy butyl ethyl ester, hexanoic acid ethyl ester, heptanoic acid ethyl ester, octanoic acid ethyl ester, decanoic acid ethyl ester, benzenacetic acid ethyl ester, and acetic acid 2 phenyl ethyl ester.
4.7. Statistical Analysis
Alcoholometric, residual sugars and mannoprotein data were compared using one-way ANOVA, followed by Tukey test, while raw volatile data were analyzed with a principal component analysis (PCA) using STATISTICA software (version 8.0, Statsoft, TULSA, OK, USA).
5. Conclusions
The present research permitted to highlight the potential of some Saccharomyces strains to produce specific volatile molecule fingerprintings and to release mannoproteins during fermentation time. Although, the production of mannoproteins need to be further investigated also from a molecular point of view, the selection of proper strains can represent a tool to increase the final quality of Trebbiano wine, especially from an aromatic point of view.
Author Contributions
F.P., R.L., L.G., A.V. contributed to the set u pof the experimental plan and the writing oft he manuscript. G.B. and A.R. contributed tot he experimental analyses.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Sagratini, G.; Maggi, F.; Caprioli, G.; Cristalli, G.; Ricciutelli, M.; Torregiani, E.; Vittori, S. Comparative study of aroma profile and phenolic content of Montepulciano monovarietal red wines from the Marches and Abruzzo regions of Italy using HS-SPME–GC–MS and HPLC–MS. Food Chem. 2012, 132, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, F.; Ndagijimana, M.; Vernocchi, P.; Gianotti, A.; Riponi, C.; Gardini, F.; Lanciotti, R. High-Pressure homogenization to modify yeast performance for sparkling wine production according to traditional methods. Am. J. Enol. Vitic. 2013, 64, 258–267. [Google Scholar] [CrossRef]
- Di Gianvito, P.; Perpetuini, G.; Tittarelli, F.; Schirone, M.; Arfelli, G.; Piva, A.; Patrignani, F.; Lanciotti, R.; Olivastri, L.; Suzzi, G.; et al. Impact of Saccharomyces cerevisiae strains on traditional sparkling wines production. Food Res. Int. 2018, 109, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama, A.; Fernandez, J.; Iniguez, M.; Souto, J.; De Saja, J. Discrimination of wine aroma using an array of conducting polymer sensors in conjunction with solid-phase micro-extraction (SPME) technique. Sens. Actuators B Chem. 2001, 77, 401–408. [Google Scholar] [CrossRef]
- Lanciotti, R.; Patrignani, F.; Iucci, L.; Saracino, P.; Guerzoni, M.E. Potential of high pressure homogenization in the control and enhancement of proteolytic and fermentative activities of some Lactobacillus Species. Food Chem. 2007, 102, 542–550. [Google Scholar] [CrossRef]
- Henschke, P. Wine Yeast. In Yeast Sugar Metabolism; Zimmermann, F.K., Entian, K.D., Eds.; Technomic Publishing: Lancaster, PA, USA, 1997. [Google Scholar]
- Jolly, N.; Augustyn, O.; Pretorius, I. The role and use of non-Saccharomyces yeasts in wine production. S. Afri. J. Enol. Vitic. 2006, 27, 15–39. [Google Scholar] [CrossRef]
- Fleet, G. In the Yeasts: Yeast Organelles; Rose, A.H., Harrison, J.S., Eds.; Academic Press: London, UK, 1991. [Google Scholar]
- Klis, F.M.; Boorsma, A.; De Groot, P.W. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley and Sons: Hoboken, NJ, USA, 2006; Volume 1. [Google Scholar]
- Riou, V.; Vernhet, A.; Doco, T.; Moutounet, M. Aggregation of grape seed tannins in model wine—Effect of wine polysaccharides. Food Hydrocoll. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- Rodrigues, A.; Ricardo-da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of commercial mannoproteins on wine colour and tannins stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, D.; Cebollero, E.; Gonzalez, R. A recombinant Saccharomyces cerevisiae strain overproducing mannoproteins stabilizes wine against protein haze. Appl. Environ. Microbiol. 2008, 74, 5533–5540. [Google Scholar] [CrossRef]
- Da Silva Araujo, V.; de Melo, A.N.F.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; de Souza, E.L.; Magnani, M. Followed extraction of β-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- Vincenzi, S.; Crapisi, A.; Curioni, A. Foamability of Prosecco wine: Cooperative effects of high molecular weight glycocompounds and wine PR-proteins. Food Hydrocoll. 2014, 34, 202–207. [Google Scholar] [CrossRef]
- Núñez, Y.P.; Carrascosa, A.V.; González, R.; Polo, M.C.; Martínez-Rodríguez, A. Isolation and characterization of a thermally extracted yeast cell wall fraction potentially useful for improving the foaming properties of sparkling Wines. J. Agric. Food Chem. 2006, 54, 7898–7903. [Google Scholar] [CrossRef]
- Escot, S.; Feuillat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Pérez-Mestre, C.; Ferreras-Charro, R.; Rivero, F.J.; Heredia, F.J.; Escribano-Bailon, M.T. The addition of mannoproteins and/or seeds during winemaking and their effects on pigment composition and color stability. J. Agric. Food Chem. 2019, 67, 4031–4042. [Google Scholar] [CrossRef]
- Moruno, E.G.; Sanlorenzo, C.; Boccaccino, B.; Di Stefano, R. Treatment with yeast to reduce the concentration of ochratoxin A in red wine. Am. J. Enol. Vitic. 2005, 56, 73–76. [Google Scholar]
- Caridi, A. New perspectives in safety and quality enhancement of wine through selection of yeasts based on the parietal adsorption activity. Int. J. Food Microbiol. 2007, 120, 167–172. [Google Scholar] [CrossRef]
- Cecchini, F.; Morassut, M.; Moruno, E.G.; Di Stefano, R. Influence of yeast strain on ochratoxin A content during fermentation of white and red must. Food Microbiol. 2006, 23, 411–417. [Google Scholar] [CrossRef]
- Jamal, Z.; Miot-Sertier, C.; Thibau, F.; Dutilh, L.; Lonvaud-Funel, A.; Ballestra, P.; Le Marrec, C.; Dols-Lafargue, M. Distribution and functions of phosphotransferase system genes in the genome of the lactic acid bacterium Oenococcus oeni. Appl. Environ. Microbiol. 2013, 79, 3371–3379. [Google Scholar] [CrossRef]
- Diez, L.; Guadalupe, Z.; Ayestarán, B.N.; Ruiz-Larrea, F. Effect of yeast mannoproteins and grape polysaccharides on the growth of wine lactic acid and acetic acid bacteria. J. Agric. Food Chem. 2010, 58, 7731–7739. [Google Scholar] [CrossRef]
- Legras, J.-L.; Moreno-Garcia, J.; Zara, S.; Zara, G.; Garcia-Martinez, T.; Mauricio, J.C.; Mannazzu, I.; Coi, A.L.; Bou Zeidan, M.; Dequin, S. Flor yeast: New perspectives beyond wine aging. Front. Microbiol. 2016, 7, 503. [Google Scholar] [CrossRef]
- Alexandre, H.; Blanchet, S.; Charpentier, C. Identification of a 49-KDa hydrophobic cell wall mannoprotein present in velum yeast which may be implicated in velum formation. FEMS Microbiol. Lett. 2000, 185, 147–150. [Google Scholar] [CrossRef][Green Version]
- Del Barrio-Galán, R.; Pérez-Magariño, S.; Ortega-Heras, M.; Guadalupe, Z.; Ayestarán, B. Polysaccharide characterization of commercial dry yeast preparations and their effect on white and red wine composition. LWT Food Sci. Technol. 2012, 48, 215–223. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Palacios, A.; Ayestarán, B. Maceration enzymes and mannoproteins: A possible strategy to increase colloidal stability and color extraction in red wines. J. Agric. Food Chem. 2007, 55, 4854–4862. [Google Scholar] [CrossRef]
- White, M.A.; Diffenbaugh, N.; Jones, G.V.; Pal, J.; Giorgi, F. Extreme heat reduces and shifts United States premium wine production in the 21st century. Proc. Natl. Acad. Sci. USA 2006, 103, 11217–11222. [Google Scholar] [CrossRef]
- Quijada-Morín, N.; Williams, P.; Rivas-Gonzalo, J.C.; Doco, T.; Escribano-Bailón, M.T. Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringency. Food Chem. 2014, 154, 44–51. [Google Scholar] [CrossRef]
- Rinaldi, A.; Coppola, M.; Moio, L. Aging of Aglianico and Sangiovese wine on mannoproteins: Effect on astringency and colour. LWT 2019, 105, 233–241. [Google Scholar] [CrossRef]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Costa, G.P.; Nicolli, K.P.; Welke, J.E.; Manfroi, V.; Zini, C.A. Volatile Profile of sparkling wines produced with the addition of mannoproteins or lees before second fermentation performed with free and immobilized yeasts. J. Braz. Chem. Soc. 2018, 29, 1866–1875. [Google Scholar] [CrossRef]
- Mateo, J.; Jiménez, M. Monoterpenes in grape juice and wines. J. Chromatogr. A 2000, 881, 557–567. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F. Chapter 4—Use of Non-Saccharomyces Yeasts in Red Winemaking. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 51–68. [Google Scholar] [CrossRef]
- Thongekkaew, J.; Fujii, T.; Masaki, K.; Koyama, K. Evaluation of Candida easanensis JK8 β-glucosidase with potentially hydrolyse non-volatile glycosides of wine aroma precursors. Nat. Prod. Res. 2018, 1–5. [Google Scholar] [CrossRef]
- Maturano, Y.P.; Assof, M.; Fabani, M.P.; Nally, M.C.; Jofré, V.; Assaf, L.A.R.; Toro, M.E.; De Figueroa, L.I.C.; Vazquez, F. Enzymatic activities produced by mixed Saccharomyces and non-Saccharomyces cultures: Relationship with wine volatile composition. Antonie Van Leeuwenhoek 2015, 108, 1239–1256. [Google Scholar] [CrossRef]
- Rodríguez, M.; Lopes, C.; Van Broock, M.; Valles, S.; Ramón, D.; Caballero, A. Screening and typing of Patagonian wine yeasts for glycosidase activities. J. Appl. Microbiol. 2004, 96, 84–95. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Doco, T.; Vuchot, P.; Cheynier, V.; Moutounet, M. Structural modification of wine arabinogalactans during aging on lees. Am. J. Enol. Vitic. 2003, 54, 150–157. [Google Scholar]
- Vidal, S.; Williams, P.; Doco, T.; Moutounet, M.; Pellerin, P. The polysaccharides of red wine: Total fractionation and characterization. Carbohydr. Polym. 2003, 54, 439–447. [Google Scholar] [CrossRef]
- Pérez-Través, L.; Querol, A.; Pérez-Torrado, R. Increased mannoprotein content in wines produced by Saccharomyces kudriavzevii× Saccharomyces cerevisiae hybrids. Int. J. Food Microbiol. 2016, 237, 35–38. [Google Scholar] [CrossRef]
- Vernocchi, P.; Ndagijimana, M.; Serrazanetti, D.I.; López, C.C.; Fabiani, A.; Gardini, F.; Guerzoni, M.E.; Lanciotti, R. Use of Saccharomyces cerevisiae strains endowed with β-glucosidase activity for the production of Sangiovese wine. World J. Microbiol. Biotechnol. 2011, 27, 1423–1433. [Google Scholar] [CrossRef]
- OIV. Resolution OENO 26/2004; OIV: Paris, France, 2004. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).