Effect of Co-Inoculation with Pichia fermentans and Pediococcus acidilactici on Metabolite Produced During Fermentation and Volatile Composition of Coffee Beans
Abstract
1. Introduction
2. Material and Methods
2.1. Microorganism and Inoculum Preparation
2.2. Farm Experiments
2.3. Sampling and pH Measurement
2.4. HPLC Analysis of Fermenting Coffee Pulp
2.5. GC Analysis of Fermenting Coffee Pulp
2.6. GC/MS Analysis of Fermented Coffee Beans
2.7. Statistical Analysis
3. Results and Discussion
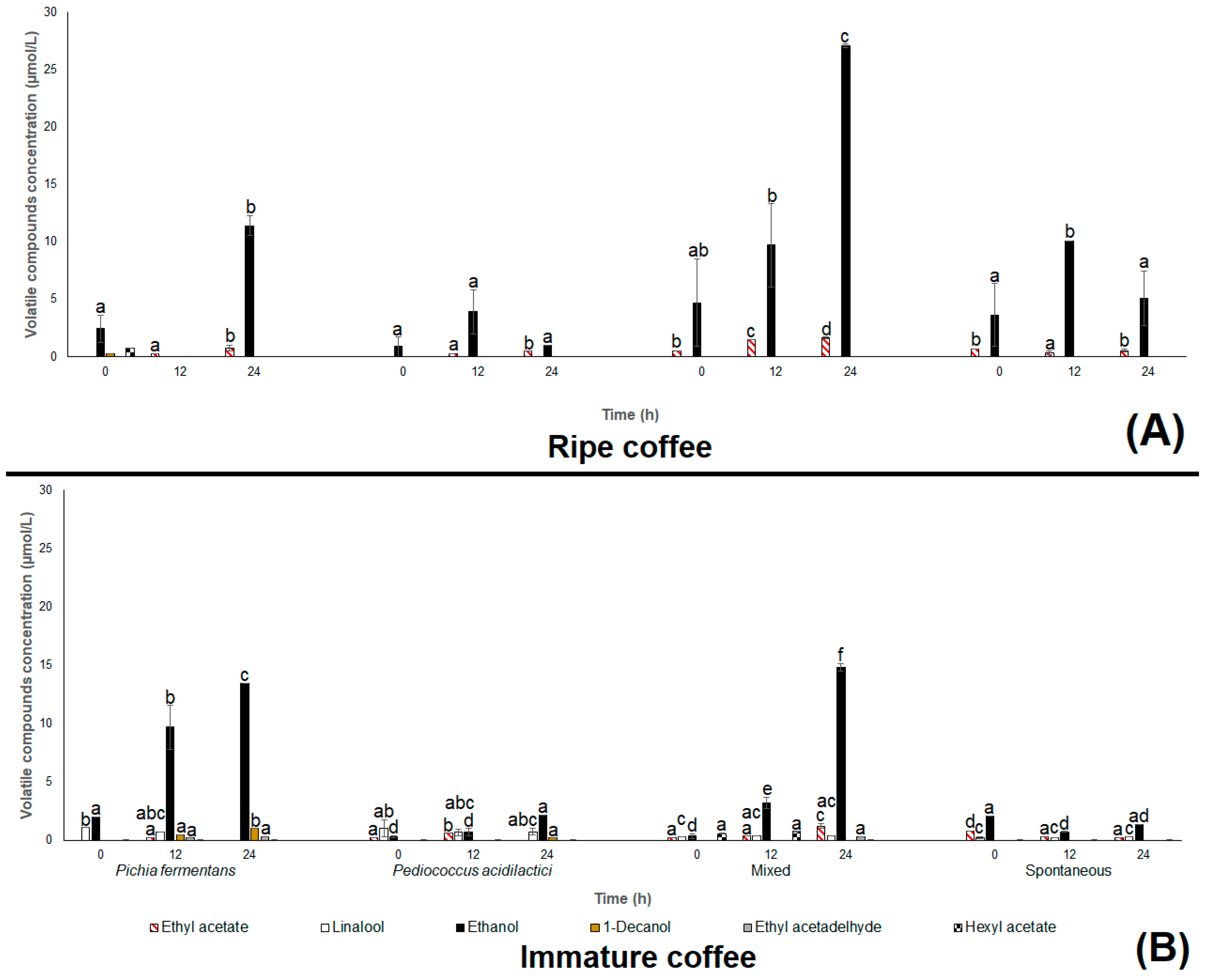
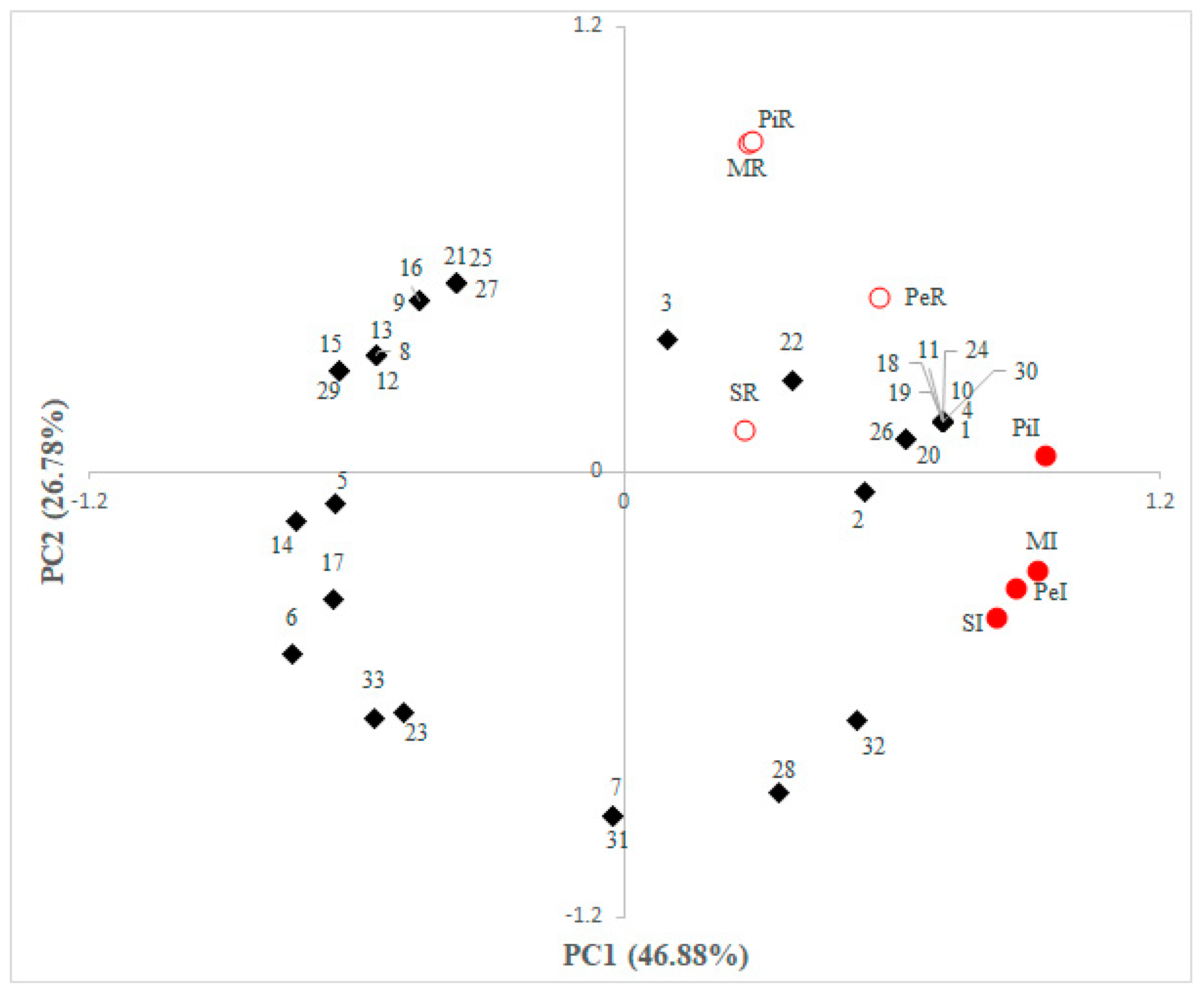
3.1. Field Experiment
3.2. Coffee Beans Chemical Composition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huch, M.; Franz, C.M.A.P. Coffee: Fermentation and microbiota. In Advances in Fermented Foods and Beverages: Improving Quality, Technologies and Health Benefits, 1st ed.; Holzapfel, W., Ed.; Woodhead Publishing: Sawston, UK, 2015; Volume 4, pp. 501–513. [Google Scholar]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Ecophysiology of coffee growth and production. Braz. J. Plant Physiol. 2007, 19, 485–510. [Google Scholar] [CrossRef]
- Mesquita, C.M.; Rezende, J.E.; De Carvalho, J.S.; Junior, M.A.F.; Moraes, N.C.; Dias, P.T.; Carvalho, R.M.; de Araújo, W.G. Manual do café: Colheita e preparo; Emater: Belo Horizonte, Brazil, 2016; pp. 6–21. [Google Scholar]
- Franca, A.S.; Oliveira, L.S.; Mendonça, J.C.F.; Silva, X.A. Physical and chemical attributes of defective crude and roasted coffee beans. Food Chem. 2005, 90, 89–94. [Google Scholar] [CrossRef]
- Oliveira, L.S.; Franca, A.S.; Mendonça, J.C.F.; Barros-Júnior, M.C. Proximate composition and fatty acids profile of green and roasted defective coffee beans. LWT Food Sci. Technol. 2006, 39, 235–239. [Google Scholar] [CrossRef]
- Bee, S.; Brando, C.H.J.; Brumen, G.; Carvalhaes, N.; Kölling-Speer, I.; Speer, K.; Liverani, F.S.; Teixeira, A.A.; Thomaziello, R.A.; Viani, R.; et al. The raw coffee. In Expresso Coffee: The Science of Quality; Illy, A., Vinai, R., Eds.; Elsevier Academic Press: London, UK, 2005; pp. 87–178. [Google Scholar]
- Illy, E. The complexity of coffee. Sci. Am. 2002, 286, 86–91. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, F.; Zhang, S.J.; Pothakos, V.; Torres, J.; Lambot, C.; Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. Exploring the impacts of postharvest processing on the microbiota and metabolite profiles during green coffee bean production. Appl. Environ. Microbiol. 2017, 83, e02398-16. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.M.; Soccol, V.T.; Brar, S.K.; Neto, E.; Soccol, C.R. Microbial ecology and starter culture technology in coffee processing. Crit. Rev. Food Sci. Nutr. 2017, 57, 2775–2788. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.M.; Carvalho Neto, D.P.; Júnior, A.I.M.; Vásquez, Z.S.; Medeiros, A.B.P.; Vandenberghe, L.P.S.; Soccol, C.R. Exploring the impacts of postharvest processing on the aroma formation of coffee beans—A review. Food Chem. 2019, 272, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Sun, J.; Lassabliere, B.; Yu, B.; Zhao, F.; Zhao, F.; Chen, Y.; Liu, S.Q. Potential of lactic acid bacteria to modulate coffee volatiles and effect of glucose supplementation: Fermentation of green coffee beans and impact of coffee roasting. J. Sci. Food Agric. 2019, 99, 409–420. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Modulation of coffee aroma via the fermentation of green coffee beans with Rhizopus oligosporus: I. Green coffee. Food Chem. 2016, 211, 916–924. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Carvalho Neto, D.P.; Medeiros, A.B.P.; Soccol, V.T.; Neto, E.; Woiciechowski, A.L.; Soccol, C.R. Potential of lactic acid bacteria to improve the fermentation and quality of coffee during on-farm processing. Int. J. Food Sci. Technol. 2016, 51, 1689–1695. [Google Scholar] [CrossRef]
- Pereira, G.V.M.; Neto, E.; Soccol, V.T.; Medeiros, A.B.P.; Woiciechowski, A.L.; Soccol, C.R. Conducting starter culture-controlled fermentations of coffee beans during on-farm wet processing: Growth, metabolic analyses and sensorial effects. Food Res. Int. 2015, 75, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Muynarsk, E.S.M.; Pereira, G.V.M.; Mesa, D.; Thomaz-Soccol, V.; Carvalho, J.C.; Pagnoncelli, M.G.B.; Soccol, C.R. Draft genome sequence of Pediococcus acidilactici strain LPBC161, isolated from mature coffee cherries during natural fermentation. Microbiol. Resour. Announc. 2019, 8, e00332-19. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.V.M.; Soccol, V.T.; Pandey, A.; Medeiros, A.B.P.; Lara, J.M.R.A.; Gollo, A.L.; Soccol, C.R. Isolation, selection and evaluation of yeasts for use in fermentation of coffee beans by the wet process. Int. J. Food Microbiol. 2014, 188, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Luo, Y.; Zhang, Q.; Huang, Y.; Zhang, B. Mixed starter culture regulates biogenic amines formation via decarboxylation and transamination during Chinese rice wine fermentation. J. Agric. Food Chem. 2018, 66, 6348–6356. [Google Scholar] [CrossRef] [PubMed]
- Englezos, V.; Torchio, F.; Cravero, F.; Marengo, F.; Giacosa, S.; Gerbi, V.; Rantsiou, K.; Rolle, L.; Cocolin, L. Aroma profile and composition of Barbera wines obtained by mixed fermentations of Starmerella bacillaris (synonym Candida zemplinina) and Saccharomyces cerevisiae. LWT Food Sci. Technol. 2016, 73, 567–575. [Google Scholar] [CrossRef]
- Feltrin, V.P.; Sant’Anna, E.S.; Porto, A.C.S.; Torres, R.C.O. Produção de Lactobacillus plantarum em melaço de cana-de-açûcar. Braz. Arch. Biol. Technol. 2000, 43, 119–124. [Google Scholar] [CrossRef]
- Carvalho Neto, D.P.; Pereira, G.V.M.; Tanobe, V.O.A.; Thomaz-Soccol, V.; da Silva, B.G.J.; Rodrigues, C.; Soccol, C.R. Yeast diversity and physicochemical characteristics associated with coffee bean fermentation from the Brazilian cerrado mineiro region. Fermentation 2017, 3, 11. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Cinai, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef]
- Gaden, E.L., Jr.; Bokanga, M.; Harlander, S.; Hesseltine, C.W.; Steinkraus, K.H. Applications of Biotechnology to Traditional Fermented Foods; National Academy Press: Washington, DC, USA, 1992. [Google Scholar]
- Eira, M.T.S.; da Silva, E.A.A.; de Castro, R.D.; Dussert, S.; Walters, C.; Bewley, J.D.; Hilhorst, W.M. Coffee seed physiology. Braz. J. Plant Physiol. 2006, 18, 149–163. [Google Scholar] [CrossRef]
- Marques, W.L.; Raghavendran, V.; Stambuk, B.U.; Gombert, A.K. Sucrose and Saccharomyces cerevisiae: A relationship most sweet. FEMS Yeast Res. 2016, 16, fov107. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M. Improvement of Robusta coffee fermentation with microbial enzymes. Eur. J. Appl. Sci. 2011, 3, 130–139. [Google Scholar]
- Evangelista, S.R.; Miguel, M.G.C.P.; Cordeiro, C.S.; Silva, C.F.; Pinheiro, A.C.M.; Schwan, R.F. Inoculation of starter cultures in a semi-dry coffee (Coffea arabica) fermentation process. Food Microbiol. 2014, 44, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, S.R.; Silva, C.F.; Miguel, M.G.P.C.; Cordeiro, C.S.; Pinheiro, A.C.M.; Duarte, W.F.; Schwan, R.F. Improvement of coffee beverage quality by using selected yeasts strains during the fermentation in dry process. Food Res. Int. 2014, 61, 183–195. [Google Scholar] [CrossRef]
- Avallone, S.; Brillouet, J.M.; Guyot, B.; Olguin, E.; Guiraud, J.P. Involvement of pectolytic micro-organisms in coffee fermentation. Int. J. Food Sci. Technol. 2002, 37, 191–198. [Google Scholar] [CrossRef]
- Muñoz, R.; Moreno-Arribas, M.V.; de las Rivas, B. Lactic acid bacteria. In Molecular Wine Microbiology, 1st ed.; Carrascosa, A.V., Muñoz, R., González, R., Eds.; Academic Press: Burlington, VT, USA, 2011; pp. 191–226. [Google Scholar]
- Endo, A.; Dicks, L.M.T. Physiology of the LAB. In Lactic Acid Bacteria: Biodiversity and Taxonomy; Holzapfel, W.H., Wood, B.J.B., Eds.; Wiley Blackwell: Chichester, UK, 2014; pp. 13–30. [Google Scholar]
- Rantsiou, K.; Dolci, P.; Giacosa, S.; Torchio, F.; Tofalo, R.; Torriani, S.; Suzzi, G.; Rolle, L.; Cocolin, L. Candida zemplinina can reduce acetic acid produced by Saccharomyces cerevisiae in sweet wine fermentations. Appl. Environ. Microbiol. 2012, 78, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Neto, D.P.; Pereira, G.V.M.; Finco, A.M.O.; Letti, L.A.J.; Silva, B.J.G.; Vandenberghe, L.P.S.; Soccol, C.R. Efficient coffee beans mucilage layer removal using lactic acid fermentation in a stirred-tank bioreactor: Kinetic, metabolic and sensorial studies. Food Biosci. 2018, 26, 80–87. [Google Scholar] [CrossRef]
- Velmourougane, K. Impact of natural fermentation on physicochemical, microbiological and cup quality characteristics of Arabica and Robusta coffee. Proc. Natl. Acad. Sci. USA India Sect. B Biol. Sci. 2013, 83, 233–239. [Google Scholar] [CrossRef]
- Jackels, S.C.; Jackels, C.F. Characterization of the coffee mucilage fermentation process using chemical indicators: A field study in Nicaragua. Food Chem. Toxicol. 2005, 70, 321–325. [Google Scholar] [CrossRef]
- Sun, S.Y.; Gong, H.S.; Zhao, K.; Wang, X.L.; Wang, X.; Zhao, X.H.; Yu, B.; Wang, H.X. Co-inoculation of yeast and lactic acid bacteria to improve cherry wines sensory quality. Int. J. Food Sci. Technol. 2013, 48, 1783–1790. [Google Scholar] [CrossRef]
- Cañas, P.M.I.; Romero, E.G.; Pérez-Martín, F.; Seseña, S.; Palop, M.L. Sequential inoculation versus co-inoculation in Cabernet Franc wine fermentation. Food Sci. Technol. Int. 2015, 21, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H.; Guilloux-Benatier, M. Yeast autolysis in sparkling wine—A review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Junqueira, A.C.O.; Pereira, G.V.M.; Medina, J.D.C.; Alvear, M.C.R.; Rosero, R.; Carvalho Neto, D.P.; Enríquez, H.G.; Soccol, C.R. First description of bacterial and fungal communities in Colombian coffee beans fermentation analysed using Illumina-based amplicon sequencing. Sci. Rep. 2019, 9, 8794. [Google Scholar] [CrossRef] [PubMed]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Elías, L.G. Chemical composition of coffee-berry by-products. In Coffee Pulp: Composition, Technology, and Utilization; Braham, J.E., Bressani, R., Eds.; IDRC: Ottawa, ON, Canada, 1979; pp. 11–16. [Google Scholar]
- Toci, A.T.; Farah, A. Volatile fingerprint of Brazilian defective coffee seeds: Corroboration of potential marker compounds and identification of new low quality indicators. Food Chem. 2014, 153, 298–314. [Google Scholar] [CrossRef] [PubMed]
- Oestreich-Janzen, S. Chemistry of Coffee. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier Science: Kindlington, UK, 2013; Volume 3, pp. 1085–1117. [Google Scholar]
- Toledo, P.R.A.B.; Pezza, L.; Pezza, H.R.; Toci, A.T. Relationship between the different aspects related to coffee quality and their volatile compounds. Compr. Rev. Food Sci. Food Saf. 2016, 15, 705–719. [Google Scholar] [CrossRef]
- Gonzalez-Rios, O.; Suarez-Quiroz, M.L.; Boulanger, R.; Barel, M.; Guyot, B.; Guiraud, J.P.; Schorr-Galindo, S. Impact of “ecological” post-harvest processing on the volatile fraction of coffee beans: I. Green coffee. J. Food Compos. Anal. 2007, 20, 289–296. [Google Scholar] [CrossRef]
- Akiyama, M.; Murakami, K.; Hirano, Y.; Ikeda, M.; Iwatsuki, K.; Wada, A.; Tokuno, K.; Onishi, M.; Iwabuchi, H. Characterization of headspace aroma compounds of freshly brewed arabica coffees and studies on a characteristic aroma compound of Ethiopian coffee. J. Food Sci. 2008, 73, C335–C346. [Google Scholar] [CrossRef]
- Lee, L.W.; Cheong, M.W.; Curran, P.; Yu, B.; Liu, S.Q. Modulation of coffee aroma via the fermentation of green coffee beans with Rhizopus oligosporus: II. Effects of different roast levels. Food Chem. 2016, 211, 925–936. [Google Scholar] [CrossRef]
- Afriliana, A.; Pratiwi, D.; Giyarto; Belgis, M.; Harada, H.; Yushiharu, M.; Taizo, M. Volatile compounds change in unfermented Robusta coffee by re-fermentation using commercial kefir. Nutr. Food Sci. Int. J. 2019, 8, 555745. [Google Scholar] [CrossRef]
- Peñuela-Martínez, A.E.; Zapata-Zapata, A.D.; Durango-Restrepo, D.L. Performance of different fermentation methods and the effect on coffee quality (Coffea arabica L.). Coffee Sci. 2018, 13, 465–476. [Google Scholar] [CrossRef]
- Mendes-Ferreira, A.; Barbosa, C.; Falco, V.; Leão, C.; Mendes-Faia, A. The production of hydrogen sulphide and other aroma compounds by wine strains of Saccharomyces cerevisiae in synthetic media with different nitrogen concentrations. J. Ind. Microbiol. Biotechnol. 2009, 36, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Yvon, M.; Rijnen, L. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 2001, 11, 185–201. [Google Scholar] [CrossRef]
- Plessas, S.; Bekatorou, A.; Gallanagh, J.; Nigam, P.; Koutinas, A.A.; Psarianos, C. Evolution of aroma volatiles during storage of sourdough breads made by mixed cultures of Kluyveromyces marxianus and Lactobacillus delbrueckii ssp. bulgaricus or Lactobacillus helveticus. Food Chem. 2008, 107, 883–889. [Google Scholar] [CrossRef]
- Patrignani, F.; Chinnici, F.; Serrazanetti, D.I.; Vernocchi, P.; Ndagijimana, M.; Riponi, C.; Lanciotti, R. Production of volatile and sulfur compounds by 10 Saccharomyces cerevisiae strains inoculated in trebbiano must. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Leclercq-Perlat, M.-N.; Corrieu, G.; Spinnler, H.-E. Comparison of volatile compounds produced in model cheese medium deacidified by Debaryomyces hansenii or Kluyveromyces marxianus. J. Dairy Sci. 2010, 87, 1545–1550. [Google Scholar] [CrossRef]

| Compounds | Aroma Perception | Fermented Ripe Coffee Beans | |||
|---|---|---|---|---|---|
| Spontaneous | P. fermentans | P. acidilactici | P. fermentans + P. acidilactici | ||
| Organic acids (3) | |||||
| Butanoic acid, 3-methyl | - | 7.02 ± 1.00 a | 6.88 ± 1.48 a | 6.10 ± 1.74 a | 6.91 ± 0.18 a |
| Butanoic acid, 2-methyl | Fruity, dirty, acidic with a dairy buttery | 1.77 ± 0.35 a | 1.98 ± 0.28 a | ND | 1.79 ± 0.28 a |
| Hexanoic acid | Sour, fatty, sweat, cheesy | ND | 1.34 ± 0.61 a | 0.99 ± 0.23 a | 1.67 ± 0.88 a |
| Higher alcohols (7) | |||||
| 2-Heptanol | Fresh, lemon grass, herbal | 10.21 ± 0.00 a | ND | ND | 12.12 ± 0.10 b |
| 5-Methyl-2-Hexanol | - | 6.09 ± 2.66 | ND | ND | ND |
| 1-Hexanol | Green, fruity, apple-skin and oily | 15.90± 2.36 a | 16.95 ± 2.23 a | 17.10 ± 2.36 a | 18.24 ± 0.17 a |
| 1-Octen-3-ol | Earthy, green, oily, umami sensation | 0.82 ± 0.00 a | 0.80 ± 0.17 a | ND | 0.86 ± 0.03 a |
| 3-Octanol | Musty, mushroom, earthy, creamy dairy | ND | 0.50 ±0.14 a | 0.58 ± 0.00 a | 0.67 ± 0.00 a |
| Benzylalcohol | Sweet, fruity with balsamic nuances | 2.58 ± 0.05 a | 3.26 ± 0.36 a | 3.20 ± 0.68 a | 4.31 ± 0.17 b |
| Phenylethyl alcohol | Floral, sweet and bready | 9.52 ± 0.73 a | 10.34 ± 0.41 a | 12.17 ± 0.25 b | 12.60 ± 1.31 b |
| Esters (3) | |||||
| Butanoic acid, 2-methyl, ethyl ester | - | 0.58 ± 0.26 a | 0.34 ± 0.00 a | ND | 0.54 ± 0.20 a |
| Butanoic acid, 3-methyl- ethyl ester | - | 4.62 ± 0.59 a | 3.66 ± 1.71 a | ND | 4.70 ± 0.57 a |
| Methyl salicylate | Wintergreen, mint-like | ND | 0.28 ± 0.01 | ND | ND |
| Aldehydes (6) | |||||
| 2-Heptenal | Sweet, fresh fruity apple skin nuances | ND | 0.80 ± 0.00 a | ND | 0.80 ± 0.00 a |
| Benzaldehyde | Fruity, cherry | ND | 0.32 ± 0.00 a | 2.15 ± 0.84 a | 2.56 ± 0.69 a |
| Dodecanal | Soapy, waxy, citrus, orange mandarin | ND | ND | 0.23 ± 0.14 | ND |
| Nonanal | With a fresh green lemon peel-like nuance | 0.95 ± 0.05 a | 0.93 ± 0.35 a | 0.81 ± 0.06 a | 0.84 ± 0.27 a |
| Benzeneacetaldehyde | Almond, fruity, powdery, nutty | 1.83 ± 0.29 a | 2.80 ± 0.73 a | 2.28 ± 0.04 a | 6.94 ± 0.00 b |
| Decanal | Sweet, aldehydic, orange, waxy and citrus rind | ND | 0.37 ± 0.11 a | 0.37 ± 0.09 a | 0.34 ± 0.07 a |
| Ketone (1) | |||||
| 2-Heptanone | Fruity, spice, herbal | 3.49 ± 0.69 a | 2.92 ± 0.28 a | 2.29 ± 0.90 a | 4.38 ± 1.75 a |
| Pyridine (2) | |||||
| Pyridine, 2,3-dimethyl | - | ND | 1.55 ± 0.94 a | 1.44 ± 0.26 a | 1.25 ± 0.63 a |
| Pyridine, 2,6-Lutidine | Nutty, amine, woody, bready and vegetable-like | 1.85 ± 0.19 | ND | ND | ND |
| Lactone (1) | |||||
| Butyrolactone | Creamy, oily, fatty, caramellic | 5.28 ± 0.10 a | 6.17 ± 1.04 a | 4.86 ± 1.13 a | 6.09 ± 0.13 a |
| Terpenes (3) | |||||
| Linalool | Citrus, orange, lemon | 2.36 ± 0.29 a | 2.27 ± 0.21 a | 2.22 ± 0.82 a | 2.92 ± 0.17 b |
| D-Limonene | Sweet, orange, citrus | ND | 1.29 ± 0.60 a | 1.37 ± 0.49 a | 1.42 ± 0.35 a |
| Anethole | - | 4.10 ± 1.00 b | 1.87 ± 0.26 a | 1.96 ± 0.66 a | 3.13 ± 0.20 a,b |
| Hydrocarbons (2) | |||||
| Styrene | Sweet, balsamic, floral | ND | 2.89 ± 0.00 a | ND | 3.15 ± 0.00 a |
| Tetradecane | Waxy | 0.91 ± 0.07 a | 0.82 ± 0.06 a | 0.88 ± 0.16 a | 0.80 ± 0.06 a |
| Pyrzine (1) | |||||
| Pyrazine, 2-methoxy-3-(2-methylpropyl) | Roasted almond hazelnut peanut | 0.92 ± 0.04 a | ND | 0.93 ± 0.08 a | ND |
| Furan (1) | |||||
| Furan, 2-pentyl | Waxy, with musty, cooked caramellic nuances | 1.16 ± 0.04 | ND | ND | ND |
| Compounds | Aroma Perception | Fermented Immature Coffee Beans | |||
|---|---|---|---|---|---|
| Spontaneous | P. fermentans | P. acidilactici | P. fermentans + P. acidilactici | ||
| Organic acids (3) | |||||
| Butanoic acid, 3-methyl | - | 7.77 ± 1.05 a | 5.99 ± 2.31 a | 8.56 ± 1.57 a,b | 5.15 ± 0.98 a,c |
| Butanoic acid, 2-methyl | Fruity, acidic with a dairy buttery | 1.32 ± 0.36 a | 1.24 ± 0.86 a | 1.65 ± 0.13 a,b | 0.69 ± 0.15 a,c |
| Hexanoic acid | Sour, fatty, sweat, cheesy | ND | 0.63 ± 0.15 a | 0.50 ± 0.11 a | ND |
| Higher alcohols (4) | |||||
| 1-Hexanol | Green, fruity, apple-skin and oily | 10.02 ± 0.26 a | 10.57 ± 1.33 a | 9.14 ± 1.51 a | 9.85 ± 1.17 a |
| 2-Hexanol | - | 0.36 ± 0.05 a | 0.75 ± 0.01 b | ND | 0.45 ± 0.10 a |
| Benzylalcohol | Sweet, fruity with balsamic nuances | 0.54 ± 0.15 a | 0.62 ± 0.08 a | 0.51 ± 0.20 a | 0.61 ± 0.04 a |
| Phenylethyl alcohol | Floral, sweet and bready | 2.54 ± 0.38 a | 2.78 ± 0.17 b | 2.24 ± 0.12 a | 2.20 ± 0.18 a |
| Aldehydes (3) | |||||
| Nonanal | Citrus, with a fresh green lemon peel-like nuance | 0.30 ± 0.08 a | 0.74 ± 0.04 b | 0.46 ± 0.07 c,d | 0.48 ± 0.05 d |
| Benzeneacetaldehyde | Almond, fruity, powdery, nutty | 0.21 ± 0.00 a | 0.28 ± 0.01 a | 0.27 ± 0.13 a | 0.38 ± 0.00 a |
| Decanal | Sweet, orange, waxy and citrus rind | 0.24 ± 0.09 a | 0.43 ± 0.06 a | 0.30 ± 0.10a | 0.42 ± 0.08 a |
| Pyridines (2) | |||||
| Pyridine, 2,3-dimethyl | - | ND | 1.45 ± 0.34 a | 0.62 ± 0.14 a | 1.10 ± 0.36 a |
| Pyridine, 2,6-Lutidine | Nutty, woody, bready and vegetable-like | 0.95 ± 0.35 | ND | ND | ND |
| Lactone (1) | |||||
| Butyrolactone | Creamy, oily, fatty, caramellic | 0.92 ± 0.07 a | 0.46 ± 0.20 a | 0.66 ± 0.26 a | 0.49 ± 0.17 a |
| Terpenes (1) | |||||
| D-Limonene | Sweet, orange, citrus | 0.36 ± 0.06 a | 0.72 ± 0.16 b | 0.16 ± 0.09 c | 0.41 ± 0.19 a |
| Furans (2) | |||||
| Furan-2-pentyl | Waxy, cooked caramellic nuances | 0.57 ± 0.17 a | 0.74 ± 0.12 a | 0.58 ± 0.15 a | 0.69 ± 0.13 a |
| 2(3)-Furanone, dihydro-5-methyl | Creamy, waxy with a citrus fruity nuance | 0.55 ± 0.14 a | 0.38 ± 0.16 a | ND | 0.45 ± 0.15 a |
| Hydrocarbons (2) | |||||
| Hexadecane | - | ND | 0.55 ± 0.01 | ND | ND |
| Tetradecane | Waxy | 0.62 ± 0.02 a | 0.24 ± 0.07 b | 0.30 ± 0.06 c | 0.76 ± 0.10 a,c |
| Pyrzine (1) | |||||
| Pyrazine, 2-methoxy-3-(2-methylpropyl) | Roasted almond hazelnut peanut | 0.78 ± 0.07 a | 0.80 ± 0.01 a | 0.85 ± 0.12 a | 0.92 ± 0.08 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Vale, A.; de Melo Pereira, G.V.; de Carvalho Neto, D.P.; Rodrigues, C.; Pagnoncelli, M.G.B.; Soccol, C.R. Effect of Co-Inoculation with Pichia fermentans and Pediococcus acidilactici on Metabolite Produced During Fermentation and Volatile Composition of Coffee Beans. Fermentation 2019, 5, 67. https://doi.org/10.3390/fermentation5030067
da Silva Vale A, de Melo Pereira GV, de Carvalho Neto DP, Rodrigues C, Pagnoncelli MGB, Soccol CR. Effect of Co-Inoculation with Pichia fermentans and Pediococcus acidilactici on Metabolite Produced During Fermentation and Volatile Composition of Coffee Beans. Fermentation. 2019; 5(3):67. https://doi.org/10.3390/fermentation5030067
Chicago/Turabian Styleda Silva Vale, Alexander, Gilberto Vinícius de Melo Pereira, Dão Pedro de Carvalho Neto, Cristine Rodrigues, Maria Giovana B. Pagnoncelli, and Carlos Ricardo Soccol. 2019. "Effect of Co-Inoculation with Pichia fermentans and Pediococcus acidilactici on Metabolite Produced During Fermentation and Volatile Composition of Coffee Beans" Fermentation 5, no. 3: 67. https://doi.org/10.3390/fermentation5030067
APA Styleda Silva Vale, A., de Melo Pereira, G. V., de Carvalho Neto, D. P., Rodrigues, C., Pagnoncelli, M. G. B., & Soccol, C. R. (2019). Effect of Co-Inoculation with Pichia fermentans and Pediococcus acidilactici on Metabolite Produced During Fermentation and Volatile Composition of Coffee Beans. Fermentation, 5(3), 67. https://doi.org/10.3390/fermentation5030067






