Preliminary Screening of Growth and Viability of 10 Strains of Bifidobacterium spp.: Effect of Media Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Substrate Inoculation and Conditions of Cultivation
2.3. Enumeration of Bifidobacteria and Determination of Active Acidity
2.4. Data Modeling
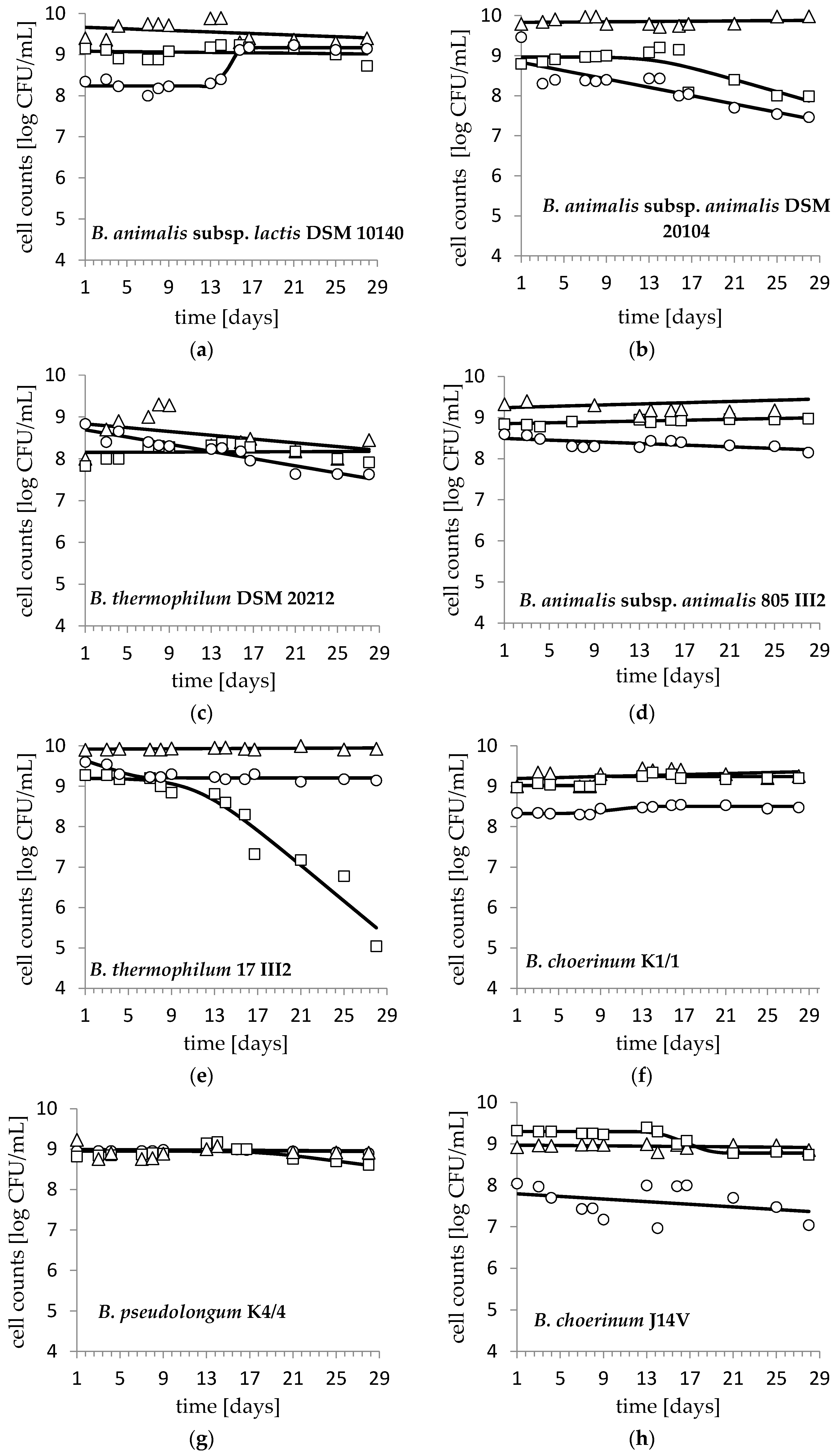
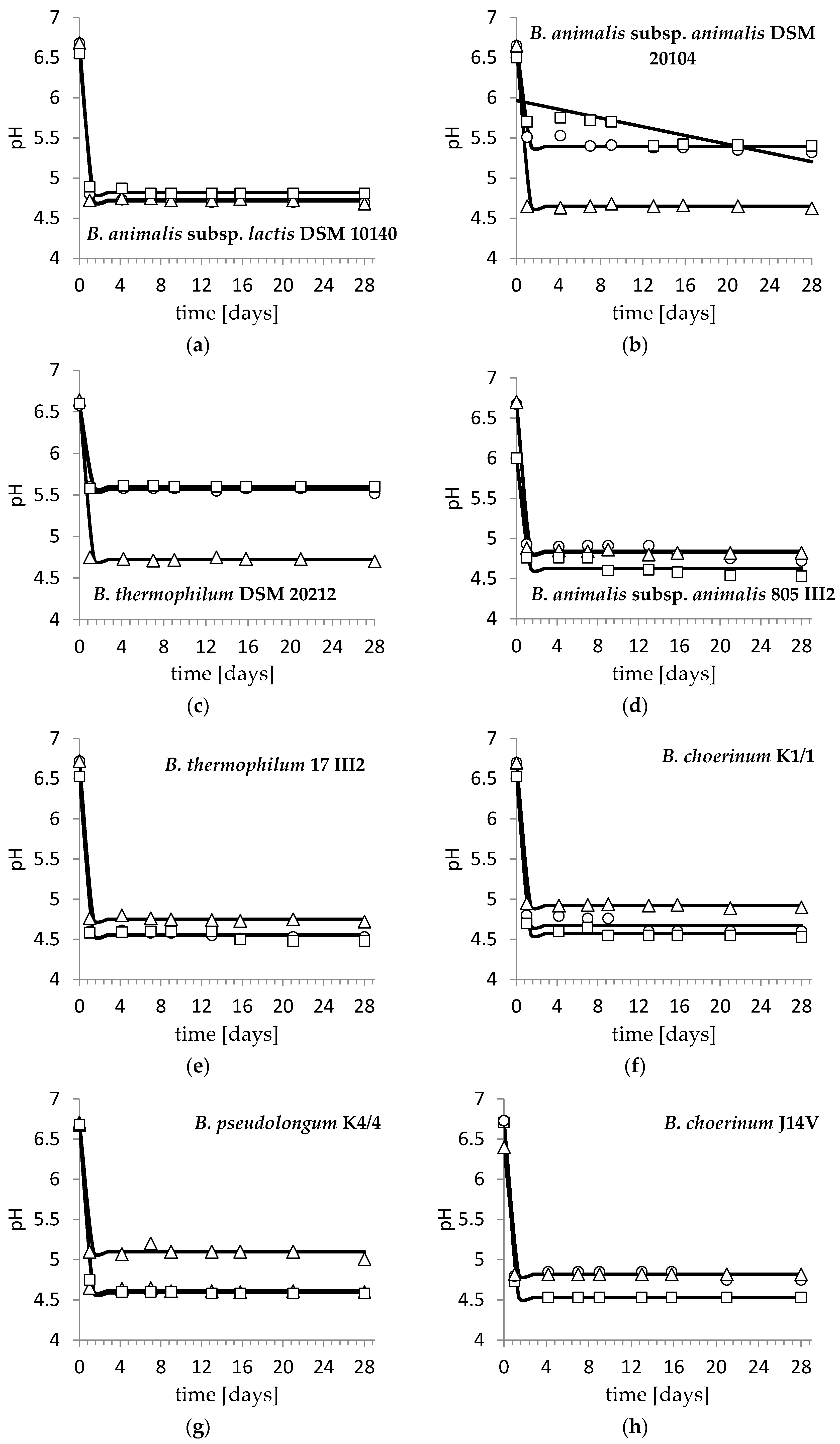
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Awasti, N.; Tomar, S.K.; Pophaly, S.D.; Poonam, V.K.; Singh, T.P.; Anand, S. Probiotic and functional characterization of bifidobacteria of Indian human origin. J. Appl. Microbiol. 2015, 120, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Bunešová, V.; Vlková, E.; Rada, V.; Killer, J.; Musilova, S. Bifidobacteria from the gastrointestinal tract of animals: Differences and similarities. Benefic. Microbes 2014, 5, 377–388. [Google Scholar] [CrossRef]
- Delcenserie, V.; Gavini, F.; Beerens, H.; Tresse, O.; Franssen, C.; Daube, G. Description of a new species, Bifidobacterium crudilactis sp. Nov., isolated from raw milk and raw milk cheeses. Syst. Appl. Microbiol. 2007, 30, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Z.; Xin, Y.H.; Jian, W.Y.; Liu, X.L.; Ling, D.W. Bifidoabcterium thermacidophilum sp. nov., isolated from an anaerobic digester. Int. J. Syst. Evol. Microbiol. 2000, 50, 119–125. [Google Scholar] [CrossRef]
- Simpson, J.M.; Martineau, B.; Jones, W.E.; Ballam, J.M.; Mackie, R.I. Characterization of fecal bacterial populations in canines: Effects of age, breed and dietary fibre. Microb. Ecol. 2002, 44, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Jakubczak, A.; Stachelska, M.A.; Świstocka, R.; Lewandowski, W. The application of probiotic bacteria in the fermented vegetable cereal and meat products. Polish J. Nat. Sci. 2012, 27, 81–92. [Google Scholar]
- Leahy, S.C.; Higgins, D.G.; Fitzgerald, G.F.; Sinderen, D. Getting better with bifidobacteria. J Appl Microbiol 2005, 98, 1303–1315. [Google Scholar] [PubMed]
- Cronin, M.; Ventura, M.; Fitzgerald, G.F.; Sinderen, D. Progress in genomics, metabolism and biotechnology of bifidobacteria. Int. J. Food Microbiol. 2011, 149, 4–18. [Google Scholar] [CrossRef]
- Shah, N.P. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Talwalkar, A.; Kailasapathy, K. The role of oxygen in the viability of probiotic bacteria with reference to L. acidophilus and Bifidobacterium spp. Current Issues Int. Microbiol. 2004, 5, 1–8. [Google Scholar]
- Kawasaki, S.; Mimura, T.; Satoh, T.; Takeda, K.; Niimura, Y. Response of the microaerophilic Bifidobacterium species, B. boum and B. thermophilum, to oxygen. Appl. Environ. Microbiol. 2006, 72, 6854–6858. [Google Scholar] [CrossRef]
- Lamoureux, L.; Roy, D.Y.; Gauthier, S.F. Production of oligosachcarides in yogurt containing bifidobacteria and yogurt cultures. J. Dairy Sci. 2002, 85, 1058–1069. [Google Scholar] [CrossRef]
- Havas, P.; Kun, S.; Perger-Mészáros, I.; Rezseey-Szabó, J.M.; Nguyen, Q.D. Performances of new isolates of Bifidobacterium on fermentation of soymilk. Acta Microbiol. et Imunol. Hung. 2015, 62, 463–475. [Google Scholar] [CrossRef]
- Rada, V.; Petr, J. A new selective medium for the isolation of glusoce non-fermenting bifidobacteria from hen caeca. J. Microbiol. Methods 2000, 43, 127–132. [Google Scholar] [CrossRef]
- Vlková, E.; Salmonová, H.; Bunešová, V.; Geigerová, M.; Rada, V.; Musilová, Š. A new medium containing mupirocin, acetic acid, and norfloxacin for the selective cultivation of bifidobacteria. Anaerobe 2015, 34, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Baranyi, J.; Roberts, A.T. Mathematics of predictive food microbiology. Int. J. Food Microbiol. 1995, 26, 199–218. [Google Scholar] [CrossRef]
- Davey, K.R. Modelling the combined effect of temperature and pH on the rate coefficient for bacterial growth. Int. J. Food Microbiol. 1994, 23, 295–303. [Google Scholar] [CrossRef]
- Shimamura, S.; Abe, F.; Ishibashi, N.; Miyakawa, H.; Yaeshima, T.A.; Tomita, M. Relationship between oxygen sensitivity and oxygen metabolism of Bifidobacterium species. J. Dairy Sci. 1992, 75, 3296–3306. [Google Scholar] [CrossRef]
- Dave, R.I.; Shah, N.P. Viability of yoghurt and probiotic bacteria in yoghurts made from commercial starter cultures. Int. Dairy J. 1997, 7, 31–41. [Google Scholar] [CrossRef]
- Shimakama, Y.; Matsubara, S.; Yuki, N.; Ikeda, M.; Ishikawa, F. Evaluation of Bifidobacterium breve strain Yakult-fermented soymilk as a probiotic food. Int. J. Food Microbiol. 2003, 81, 131–136. [Google Scholar] [CrossRef]
- Kamaly, K.M. Bifidobacteria fermentation of soybean milk. Food Res. Int. 1997, 30, 675–682. [Google Scholar] [CrossRef]
- Matejčeková, Z.; Liptáková, D.; Valík, Ľ. Functional probiotic products based on fermented buckwheat with Lactobacillus rhamnosus. LWT- Food Sci. Technol. 2017, 81, 35–41. [Google Scholar] [CrossRef]
- Wu, Q.Q.; You, H.J.; Ahn, H.J.; Kwon, B.; Ji, G.E. Changes in growth and survival of Bifidobacterium by coculture with Propionibacterium in soy milk, cow`s milk, and modified MRS medium. Int. J. Food Microbiol. 2012, 157, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Maganha, L.C.; Rosim, R.; Corassin, C.H.; Cruz, A.G.; Faria, J.; Oliviera, C. Viability of probiotic bacteria in fermented skim milk produced with different levels of milk powder and sugar. Int. J. Dairy Technol. 2014, 67, 89–94. [Google Scholar] [CrossRef]
- Matejčeková, Z.; Soltészová, F.; Ačai, P.; Liptáková, D.; Valík, Ľ. Application of Lactobacillus plantarum in functional products based on fermented buckwheat. J. Food Sci. 2018, 83, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Gueimonde, M.; Delgado, S.; Mayo, B.; Ruas-Madiedo, P.; Margolles, A.; Reyes-Gavilán, C.G. Viability and diversity of probiotic Lactobacillus and Bifidobacterium populations included in commercial fermented milks. Food Res. Int. 2004, 37, 839–850. [Google Scholar] [CrossRef]
- Shaffie, G.; Mortazavian, A.M.; Mohammadifar, M.A.; Koushki, M.R.; Mohammadi, A.R.; Mohammadi, R. Combined effects of dry matter content, incubation temperature and final pH of fermentation on biochemical and microbiological characteristics of probiotic fermented milk. Afr. J. Microbiol. Res. 2010, 4, 1265–1274. [Google Scholar]
- Barona, M.; Roy, D.; Vuillemard, J. Biochemical characteristics of fermented milk produced by mixed-cultures of lactic starters and bifidobacteria. Lait 2000, 80, 465–478. [Google Scholar] [CrossRef][Green Version]
- Zielińska, D.; Kolozyn-Krajewska, D. Food-origin lactic acid bacteria may exhibit probiotic properties: Review. BioMed Res. Int. 2018, 3, 1–15. [Google Scholar] [CrossRef]
- Rathore, S.; Salmerón, I.; Pandiella, S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef]
- Matejčeková, Z.; Liptáková, D.; Spodniaková, S.; Valík, Ľ. Characterization of the growth of Lactobacillus plantarum in milk in dependence on temperature. Acta. Chim. Slovaca. 2016, 9, 104–108. [Google Scholar] [CrossRef]
- Valík, Ľ.; Medveďová, A.; Liptáková, D. Characterization of the growth of Lactobacillus rhamnosus GG in milk at suboptimal temperature. J. Food Nutr. Res. 2008, 47, 60–67. [Google Scholar]
- Liptáková, D.; Matejčeková, Z.; Valík, Ľ. Lactic acid bacteria and fermentation of cereals and pseudocereals. In Fermentation Processes; Jozala, A.F., Ed.; InTech: Rijeka, Croatia, 2017; Volume 3, pp. 223–254. [Google Scholar]
- Krausova, G.; Rada, V.; Marsik, P.; Musilova, S.; Svejstil, R.; Drab, V.; Hyrslova, I.; Vlková, E. Impact of purified human milk oligosachcarides as a sole carbon source on the growth of lactobacilli in in vitro model. Afr. J. Microbiol. Res. 2015, 9, 565–571. [Google Scholar]
- Campos, D.C.D.S.; Neves, L.T.B.C.; Flach, A.; Costa, L.A.M.A.; De Sousa, B.O. Post-acidification and evaluation of anthocyanins stability and antioxidant activity in acai fermented milk and yoghurts. Rev. Bras. Frutic. 2017, 39, 1–13. [Google Scholar] [CrossRef]


| Bifidobacterium spp. | Origin |
|---|---|
| B. animalis subsp. lactis DSM 10140 | Collection strain DSMZ |
| B. animalis subsp. animalis DSM 20104 | Collection strain DSMZ |
| B. thermophilum DSM 20212 | Collection strain DSMZ |
| B. animalis subsp. animalis 805 III2 | Feces of calf |
| B. thermophilum 17 III2 | Feces of calf |
| B. choerinum K1/1 | Feces of goat |
| B. pseudolongum K4/4 | Feces of goat |
| B. choerinum J14V | Feces of lamb |
| B. animalis subsp. animalis J5IIA | Feces of lamb |
| B. animalis subsp. animalis J3II | Feces of lamb |
| Microorganism | Reconstituted Milk | Ultra-high-temperature Milk | Wilkins–Chalgren Broth | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N24 | Nend | kd | N24 | Nend | kd | N24 | Nend | kd | |
| B. animalis subsp. lactis DSM 10140 | 9.15 * | 8.72 * | −0.001 * | 8.34 * | 9.15 * | 0.019 * | 9.40 * | 9.40 * | - |
| B. animalis subsp. lactis DSM 20104 | 8.79 * | 7.98 * | −0.003 * | 9.46 * | 7.46 * | −0.002 * | 9.79 * | 9.98 * | 0.001 * |
| B. thermophilum DSM 20212 | 8.01 * | 7.91 * | −0.003 * | 8.83 * | 7.62 * | −0.002 * | 8.00 * | 8.45 * | 0.001 * |
| B. animalis subsp. animalis 805 III2 | 9.32 * | 9.18 * | 0.001 * | 8.59 * | 8.15 * | −0.001 * | 8.83 * | 8.97 * | 0.00 * |
| B. thermophilum 17 III2 | 9.28 * | 5.04 * | −0.009 * | 9.63 * | 9.15 * | −0.004 * | 9.90 * | 9.93 * | - |
| B. choerinum K1/1 | 8.97 * | 9.20 * | 0.034 * | 8.35 * | 8.50 * | 0.001 * | 9.00 * | 9.26 * | 0.001 * |
| B. pseudolongum K4/4 | 8.82 * | 8.61 * | −0.002 * | 9.23 * | 8.91 * | 0.000 * | 8.95 * | 8.90 * | −0.001 * |
| B. choerinum J14V | 9.32 * | 8.74 * | −0.005 * | 8.04 * | 7.04 * | −0.001 * | 8.92 * | 8.85 * | −0.001 * |
| B. animalis subsp. animalis J5IIA | 9.35 * | 9.20 * | −0.001 * | 8.76 * | 9.34 * | 0.012 * | 8.43 * | 8.26 * | −0.038 * |
| B. animalis subsp. animalis J3II | 8.85 * | 8.69 * | - | 9.48 * | 5.78 * | −0.026 * | 8.47 * | 8.98 * | 0.004 * |
| Medians for all bifidobacteria | 9.06 ** | 8.71 ** | 0.002 ** | 8.80 ** | 8.33 ** | 0.001 ** | 8.94 ** | 8.98 ** | 0.001 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matejčeková, Z.; Vlková, E.; Liptáková, D.; Valík, Ľ. Preliminary Screening of Growth and Viability of 10 Strains of Bifidobacterium spp.: Effect of Media Composition. Fermentation 2019, 5, 38. https://doi.org/10.3390/fermentation5020038
Matejčeková Z, Vlková E, Liptáková D, Valík Ľ. Preliminary Screening of Growth and Viability of 10 Strains of Bifidobacterium spp.: Effect of Media Composition. Fermentation. 2019; 5(2):38. https://doi.org/10.3390/fermentation5020038
Chicago/Turabian StyleMatejčeková, Zuzana, Eva Vlková, Denisa Liptáková, and Ľubomír Valík. 2019. "Preliminary Screening of Growth and Viability of 10 Strains of Bifidobacterium spp.: Effect of Media Composition" Fermentation 5, no. 2: 38. https://doi.org/10.3390/fermentation5020038
APA StyleMatejčeková, Z., Vlková, E., Liptáková, D., & Valík, Ľ. (2019). Preliminary Screening of Growth and Viability of 10 Strains of Bifidobacterium spp.: Effect of Media Composition. Fermentation, 5(2), 38. https://doi.org/10.3390/fermentation5020038