Optimization of Diverse Carbon Sources and Cultivation Conditions for Enhanced Growth and Lipid and Medium-Chain Fatty Acid (MCFA) Production by Mucor circinelloides
Abstract
1. Introduction
2. Material and Methods
2.1. Microorganisms, Culture Conditions, and Experimental Design
2.2. Determination of Cell Dry Weight (CDW)
2.3. Total Lipid and Fatty Acid Profiling
2.4. Construction of Orthogonal Matrix Design (OMD)
2.5. Statistical Analysis
3. Results and Discussion
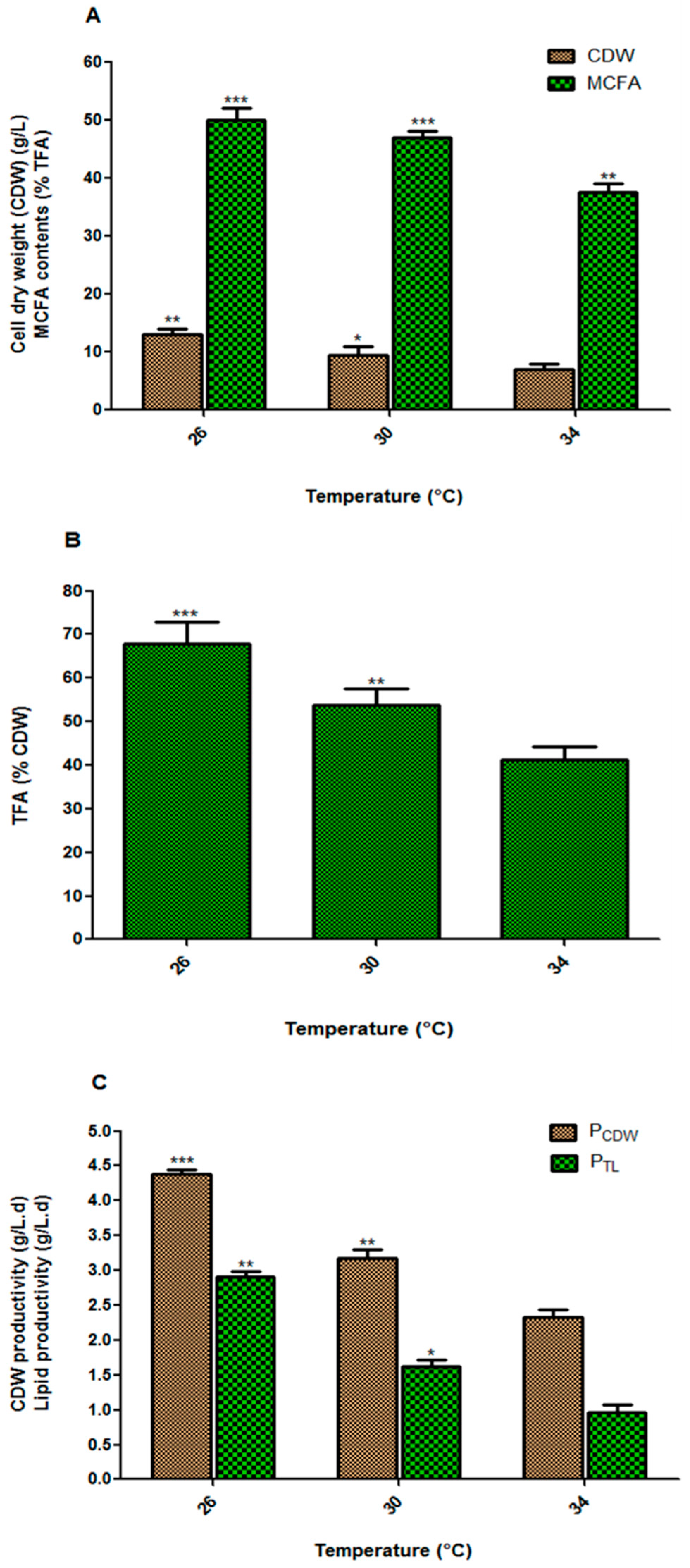
3.1. Effect of Incubation Temperature on Fungal Biomass and Lipid Accumulation
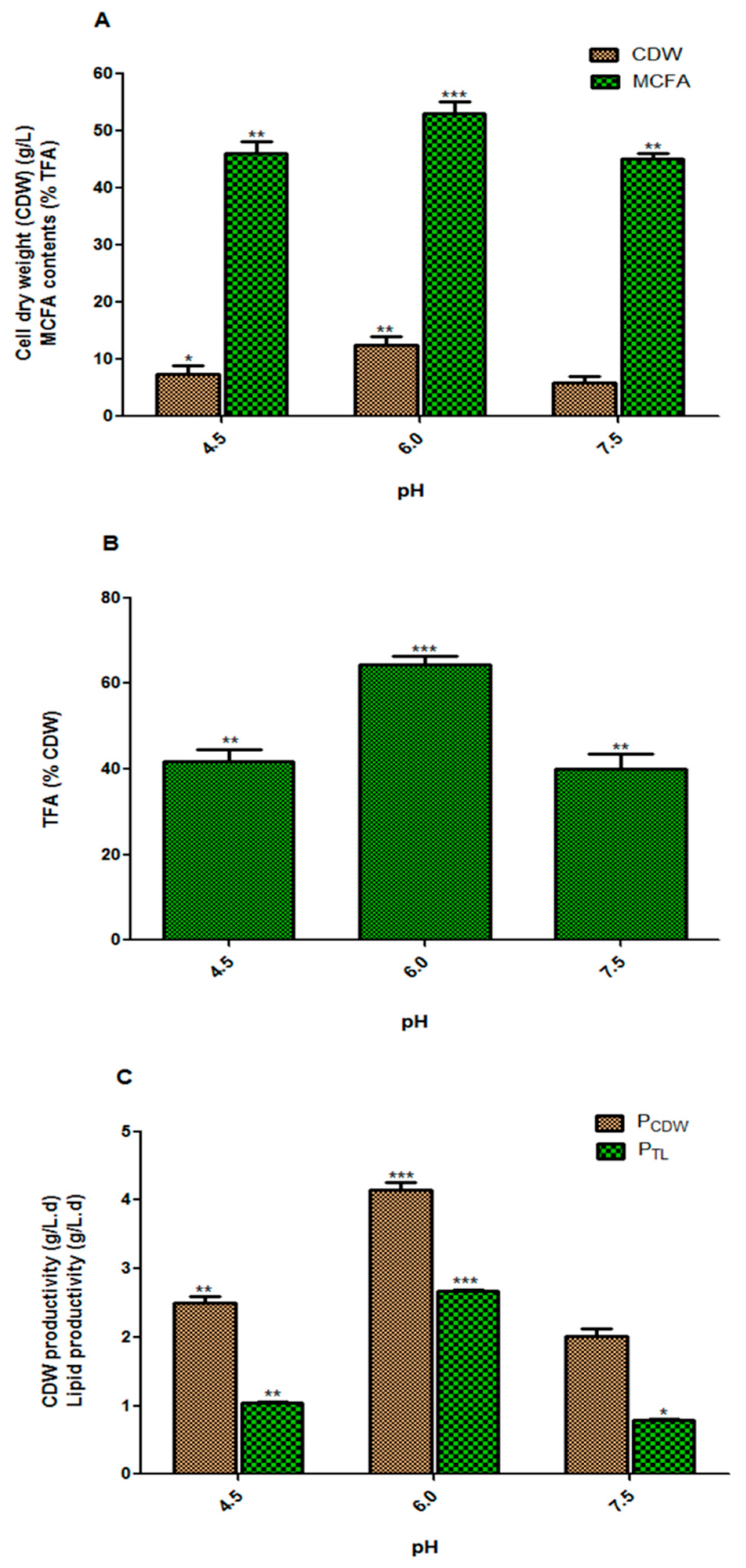
3.2. Effect of the Initial pH of the Culture Medium on Fungal Biomass and Lipid Accumulation
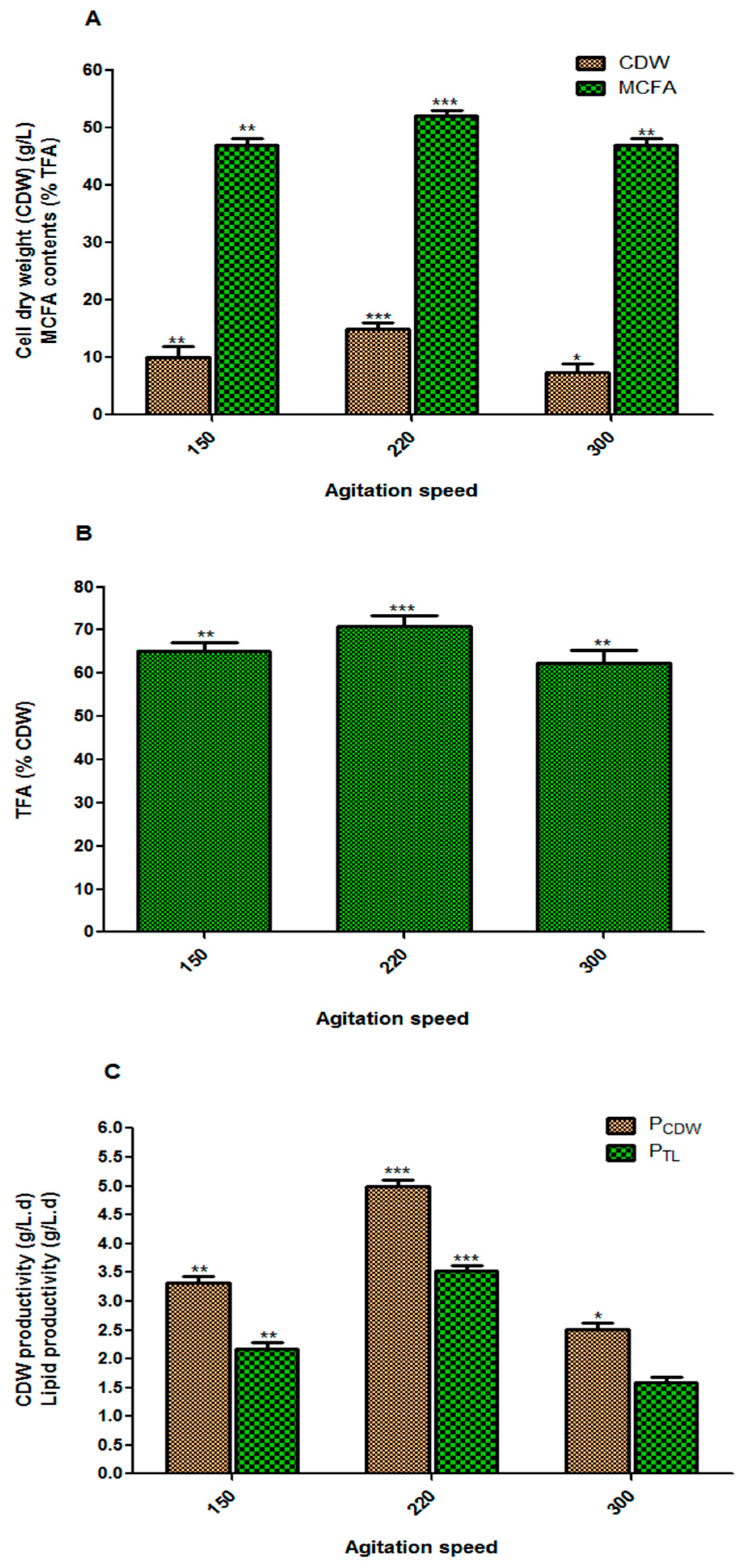
3.3. Effect of Agitation Speed on Fungal Biomass and Lipid Accumulation
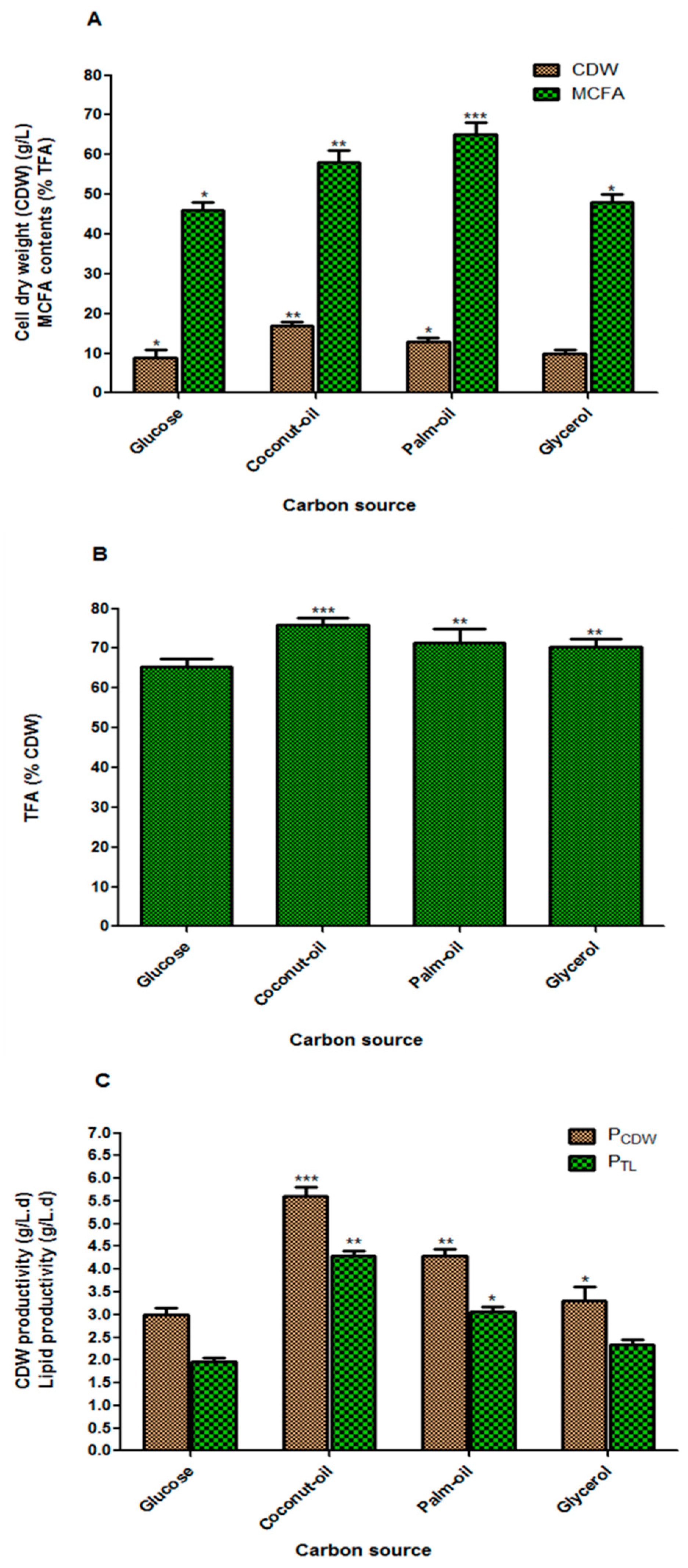
3.4. Effect of Carbon Sources on Fungal Biomass and Lipid Accumulation
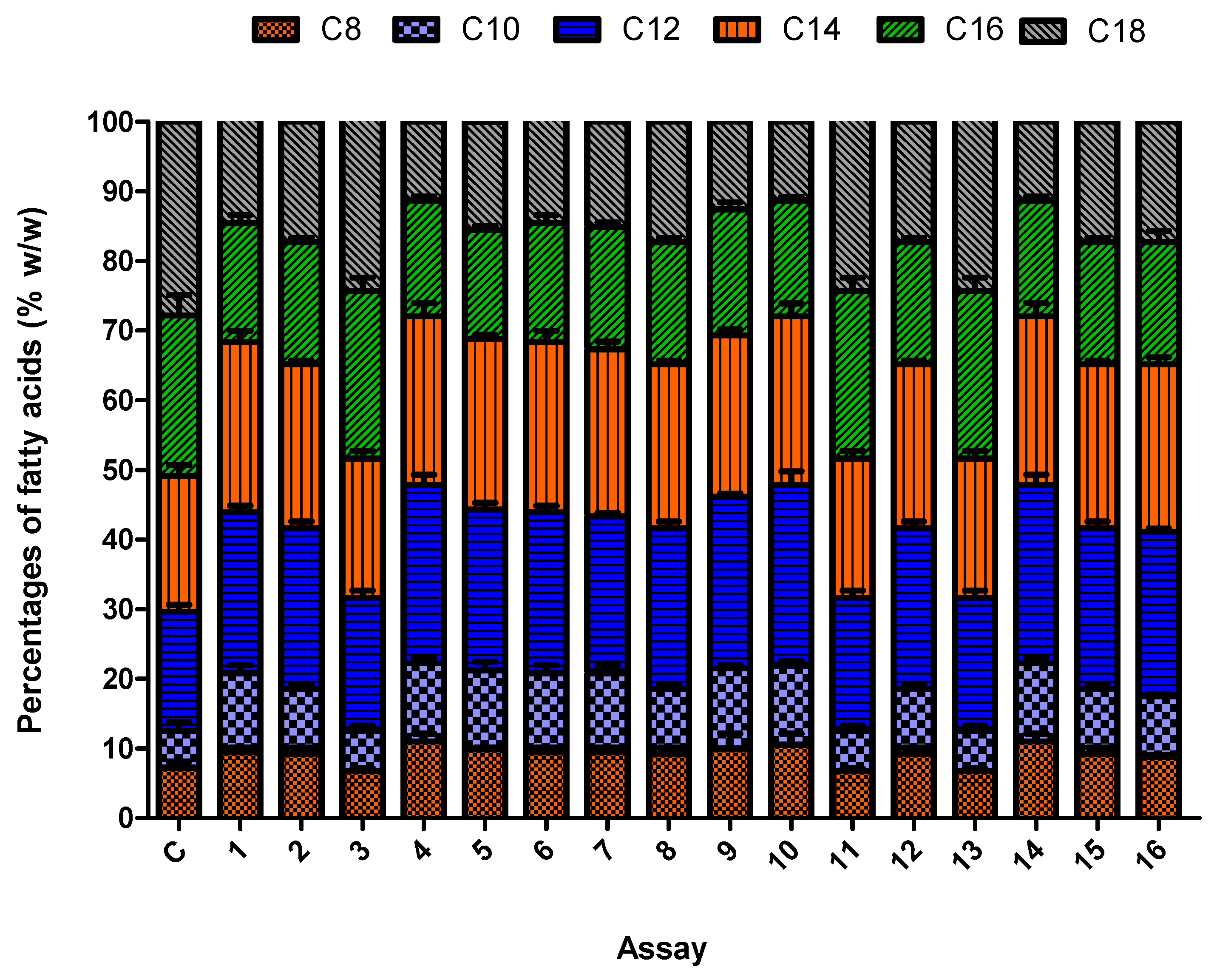
3.5. Synergistic Effect of the Culture Conditions on Fungal Biomass and Lipid Accumulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussain, S.A.; Hameed, A.; Khan, M.A.K.; Zhang, Y.; Zhang, H.; Garre, V.; Song, Y. Engineering of Fatty Acid Synthases (FASs) to Boost the Production of Medium-Chain Fatty Acids (MCFAs) in Mucor circinelloides. Int. J. Mol. Sci. 2019, 20, 786. [Google Scholar] [CrossRef] [PubMed]
- Sarria, S.; Kruyer, N.S.; Yahya, P.P. Microbial synthesis of medium-chain chemicals from renewable. Nat. Biotechnol. 2017, 35, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Nagao, K.; Yanagita, T. Medium-chain fatty acids: Functional lipids for the prevention and treatment of the metabolic syndrome. Pharm. Res. 2010, 61, 208–212. [Google Scholar] [CrossRef]
- Torella, J.P.; Ford, T.J.; Kim, S.N.; Chen, A.M.; Way, J.C.; Silver, P.A. Tailored fatty acid synthesis via dynamic control of fatty acid elongation. Proc. Nat. Acad. Sci. USA. 2013, 110, 11290–11295. [Google Scholar] [CrossRef] [PubMed]
- Leber, C.; Da-Silva, N.A. Engineering of Saccharomyces cerevisiae for the synthesis of short chain fatty acids. Biotechnol. Bioeng. 2014, 111, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Qiao, K.; Ahn, W.S. Stephanopoulos, G. Engineering Yarrowia lipolytica as a platform for synthesis of drop-in transportation fuels and oleochemicals. Proc. Natl. Acad. Sci. USA. 2016, 113, 10848–10853. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, Y.J.; Krivoruchko, A.; Grininger, M.; Zhao, Z.K.; Nielsen, J. Expanding the product portfolio of fungal type I fatty acid synthases. Nat. Chem. Biol. 2017, 13, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hicks, W.M.; Silver, P.A.; Way, J.C. Engineering acyl carrier protein to enhance production of shortened fatty acids. Biotechnol. Biofuel 2016, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, J.; Pavlovic, R.; Fischer, M.; Boles, E.; Grininger, M. Engineering fungal de novo fatty acid synthesis for short chain fatty acid production. Nat. Commun. 2017, 8, 14650. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, T.; Xian, M.; Cao, Y.; Fang, F.; Zou, H. Fatty acid from the renewable sources: A promising feedstock for the production of biofuels and biobased chemicals. Biotechnol. Adv. 2014, 32, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Lynd, L.R.; Zyl, V.W.H.; Mc Bride, J.E.; Laser, M. Consolidated bioprocessing of cellulosic biomass: An update. Curr. Opin. Biotechnol. 2005, 16, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Runguphan, W.; Keasling, J.D. Metabolic engineering of Saccharomyces cerevisiae for production of fatty acid-derived biofuels and chemicals. Metab. Eng. 2014, 21, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Zhao, H. Reversal of the β-oxidation cycle in Saccharomyces cerevisiae for production of fuels and chemicals. ACS Synth. Biol. 2015, 4, 332–341. [Google Scholar] [CrossRef]
- Dellomonaco, C.; Clomburg, J.M.; Miller, E.N.; Gonzalez, R. Engineered reversal of the β-oxidation cycle for the synthesis of fuels and chemicals. Nature 2011, 476, 355–3559. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Clomburg, J.M.; Gonzalez, R. Synthesis of medium-chain length (C6-C10) fuels and chemicals via β-oxidation reversal in Escherichia coli. J. Ind. Microbiol. Biotechnol. 2015, 42, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Grisewood, M.J.; Grisewood, M.J.; Hernandez Lozada, N.J.; Thoden, J.B.; Gifford, N.P.; Mendez-Perez, D.; Schoenberger, H.A.; Allan, M.F.; Floy, M.E.; Lai, R.Y.; Holden, H.M.; et al. Computational redesign of acyl-ACP thioesterase with improved selectivity toward medium-chain-length fatty acids. ACS Catal. 2017, 7, 3837–3849. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef]
- Beopoulos, A.; Chardo, T.; Nicaud, J.M. Yarrowia lipolytica: A model and a tool to understand the mechanisms implicated in lipid accumulation. Biochimie 2009, 91, 692–696. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of oleaginous yeasts. Part II: Technology and potential applications. Euro. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Huan, L.; Zhao, L.; Zan, X.; Song, Y.; Ratledge, C. Boosting fatty acid synthesis in Rhodococcus opacus PD630 by overexpression of autologous thioesterases. Biotechnol. Lett. 2016, 38, 999–1008. [Google Scholar] [CrossRef]
- Rigouin, C.; Croux, C.; Borsenberger, V.; Khaled, M.B.; Chardot, T.; Marty, A.; Bordes, F. Increasing medium chain fatty acids production in Yarrowia lipolytica by metabolic engineering. Microb. Cell Fact. 2018, 17, 142. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, J.; Chen, W.N. Engineering the Saccharomyces cerevisiae β-Oxidation Pathway to Increase Medium Chain Fatty Acid Production as Potential Biofuel. PLoS ONE 2014, 9, e84853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aggelis, G. Two alternative pathways for substrate assimilation by Mucor circinelloides. Folia Microbiol. 1996, 41, 254–256. [Google Scholar] [CrossRef]
- Chen, H.C.; Liu, T.M. Inoculum effects on the production of γ-linolenic acid by the shake culture of Cunninghamella echinulata CCRC 31840. Enz. Microb. Technol. 1997, 21, 137–142. [Google Scholar] [CrossRef]
- Conti, E.; Stredansky, M.; Stredanska, S.; Zanetti, F. γ-Linolenic acid production by solid-state fermentation of Mucorales strains on cereals. Bioresour. Technol. 2001, 76, 283–286. [Google Scholar] [CrossRef]
- Emelyanova, E.V. Lipid and γ-linolenic acid production by Mucor inaquisporus. Proc. Biochem. 1997, 32, 173–177. [Google Scholar] [CrossRef]
- Roux, M.P.; Kock, J.L.F.; Du Preez, J.C.; Botha, A. The influence of dissolved oxygen tension on the production of cocoa butter equivalents and gamma-linolenic acid by Mucor circinelloides. System. Appl. Microbiol. 1995, 18, 329–334. [Google Scholar] [CrossRef]
- Khan, M.A.K.; Yang, J.; Hussain, S.A.; Zhang, H.; Garre, V.; Song, Y. Genetic modification of Mucor circinelloides to construct stearidonic acid producing cell factory. Int. J. Mol. Sci. 2019, 20, 1683. [Google Scholar] [CrossRef]
- Stredansky, M.; Conti, E.; Stredanska, S.; Zanetti, F. γ-linolenic acid production with Thamnidium elegans by solid state fermentation on apple pomace. Bioresour. Technol. 2000, 73, 41–45. [Google Scholar] [CrossRef]
- Tauk-Tornisielo, S.M.; Vieira, J.M.; Carneiro, M.C.V.S.; Govone, J.S. Fatty acid production by four strains of Mucor hiemalis grown in plant oil and soluble carbohydrates. Afr. J. Biotechnol. 2007, 6, 1840–1847. [Google Scholar]
- Torlanova, B.O.; Funtikova, N.S.; Konova, I.V.; Babanova, N.K. Synthesis of the lipid complex containing gamma-linolenic acid and carotenoids by a mucorous fungus under various cultivation conditions. Microbiology 1995, 64, 492–496. [Google Scholar]
- Vaughn, D.M.; Reinhart, G.A. Influence dietary fatty acid ratios on tissue eicosanoid production and blood coagulation parameters in dog. In Recent Advances in Canine and Feline Nutritional Research—Ians International Nutrition Symposium; Orange Frazer Press: Wilmington, OH, USA, 1996; pp. 243–255. [Google Scholar]
- Khan, M.A.K.; Yang, J.; Luan, X.; Hussain, S.A.; Zhang, H.; Liang, L.; Garre, V.; Song, Y. Construction of DGLA producing cell factory by genetic modification of Mucor circinelloides. Microb. Cell Fact. 2019, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Zhang, J.; Zhang, W.; Hu, B. A new cultivation method for microbial oil production: cell pelletization and lipid accumulation by Mucor circinelloides. Biotechnol. Biofuels 2011, 4, 15. [Google Scholar] [CrossRef]
- Morin-Sardin, S.K.; Coroller, L.; Jany, J.L.; Coton, E. Effect of temperature, pH, and water activity on Mucor spp. growth on synthetic medium, cheese analog and cheese. Food Microbiol. 2016, 56, 69–79. [Google Scholar] [PubMed]
- Suutari, M. Effect of growth temperature on lipid fatty acids of four fungi (Aspergillus niger, Nurosporacrassa, Penicillium chrysogenum and Trichoderma reesei. Arc. Micro. 1995, 164, 212–216. [Google Scholar] [CrossRef]
- Vylkova, S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 2017, 13, e1006149. [Google Scholar] [CrossRef] [PubMed]
- Trzaska, W.J.; Correia, J.N.; Villegas, M.T.; May, R.C.; Voelz, K. pH Manipulation as a Novel Strategy for Treating Mucormycosis. Anti Age. Chemother. 2015, 59, 6968–6974. [Google Scholar] [CrossRef] [PubMed]
- Azmi, W.; Thakur, M.; Javed, A.; Thakur, N. Interactive Effect of Agitation Speed and Aeration Rate on Heat Stable β-carotene Production From Mucor azygosporus Using Deprotenized Waste Whey Filtrate in Stirred Tank Reactor. Curr. Biochem. Engin. 2015, 2, 65–72. [Google Scholar] [CrossRef]
- Ibrahim, D.; Weloosamy, H.; Lim, S.H. Effect of agitation speed on the morphology of Aspergillus niger HFD5A-1 hyphae and its pectinase production in submerged fermentation. World J. Biol. Chem. 2015, 6, 265–271. [Google Scholar] [CrossRef]
- Saad, N.; Abdeshahian, P.; Kalil, M.S.; Yusoff, W.M.W.; Hamid, A.A. Optimization of Aeration and Agitation Rate for Lipid and Gamma Linolenic Acid Productio by Cunningha mellabainieri 2A1 in Submerged Fermentation Using Response Surface Methodology. Sci. World J. 2014, 2014, 280146. [Google Scholar] [CrossRef]
- Zan, X.; Tang, X.; Chu, L.; Song, Y. Characteristics of cell growth and lipid accumulation of high and low lipid-producing strains of Mucor circinelloides grown on different glucose-oil mixed media. Pro. Biochem. 2018, 72, 31–40. [Google Scholar] [CrossRef]
- Tauk-Tornisielo, S.M.; Arasato, L.S.; de-Almeida, A.F.; Govone, J.S.; Malagutti, E.N. Lipid formation and γ-linolenic acid production by Mucor circinelloides and Rhizopus sp., grown on vegetable oil. Braz. J. Microbiol. 2009, 40, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, J.C.; Immelman, M.; Kock, J.L.F.; Kilian, S.G. Production of γ-linolenic acid by Mucor circinelloides and Mucor rouxii with acetic acid as carbon substrate. Biotechnol. Lett. 1995, 17, 933–938. [Google Scholar] [CrossRef]
| Factors | Code 1 | Code 2 | Code 3 | Code 4 |
|---|---|---|---|---|
| Temperature | 24 °C | 26 °C | 30 °C | 34 °C |
| Agitation | 150rpm | 220rpm | 300rpm | -- |
| pH | 4.5 | 6 | 7.5 | -- |
| Carbon Source | Glucose | Palm-oil + glucose | Coconut-oil + glucose | Glycerol + glucose |
| Assay | pH | Agitation speed | Temperature | Carbon-Source | CDW | TFA | MCFA | CTL | CMCFA | PTL | PMCFA | Yield |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 6.4 | 45.5 | 44.72 | 2.9 | 1.3 | 967 | 435 | 0.016 |
| 2 | 3 | 3 | 1 | 3 | 3.3 | 55.45 | 48.25 | 1.8 | 0.86 | 600 | 286 | 0.010 |
| 3 | 1 | 1 | 1 | 4 | 4.3 | 32.2 | 32.48 | 1.3 | 0.42 | 434 | 140 | 0.005 |
| 4 | 2 | 2 | 1 | 2 | 10.7 | 69.2 | 55.25 | 7.4 | 4.01 | 2467 | 1337 | 0.051 |
| 5 | 2 | 1 | 3 | 1 | 8.2 | 56.7 | 46.45 | 4.6 | 2.1 | 1534 | 700 | 0.026 |
| 6 | 1 | 3 | 2 | 1 | 4.5 | 26.7 | 39.25 | 1.7 | 0.66 | 567 | 220 | 0.008 |
| 7 | 3 | 2 | 4 | 1 | 7.8 | 18.2 | 52.3 | 1.4 | 0.73 | 467 | 244 | 0.009 |
| 8 | 1 | 1 | 4 | 3 | 5.4 | 13.5 | 35.23 | 0.72 | 0.25 | 240 | 84 | 0.003 |
| 9 | 1 | 1 | 4 | 2 | 4.5 | 16.9 | 45.26 | 0.76 | 0.34 | 254 | 114 | 0.004 |
| 10 | 1 | 3 | 3 | 2 | 6.5 | 22.5 | 40.29 | 1.4 | 1.61 | 467 | 538 | 0.020 |
| 11 | 2 | 3 | 4 | 4 | 9.7 | 40.3 | 42.49 | 3.9 | 1.65 | 1300 | 550 | 0.020 |
| 12 | 2 | 1 | 2 | 3 | 16.3 | 67.1 | 49.47 | 10.1 | 5.01 | 3368 | 1670 | 0.062 |
| 13 | 3 | 1 | 3 | 4 | 8.8 | 30.45 | 42.29 | 2.6 | 1.1 | 867 | 367 | 0.013 |
| 14 | 3 | 1 | 2 | 2 | 11.5 | 38.22 | 39.21 | 4.39 | 1.7 | 1464 | 569 | 0.021 |
| 15 | 1 | 2 | 3 | 3 | 3.6 | 36.2 | 48.45 | 1.3 | 0.62 | 434 | 207 | 0.007 |
| 16 | 2 | 3 | 2 | 3 | 19.4 | 74.1 | 54.2 | 14.3 | 4.71 | 4767 | 2570 | 0.058 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.A.; Nazir, Y.; Hameed, A.; Yang, W.; Mustafa, K.; Song, Y. Optimization of Diverse Carbon Sources and Cultivation Conditions for Enhanced Growth and Lipid and Medium-Chain Fatty Acid (MCFA) Production by Mucor circinelloides. Fermentation 2019, 5, 35. https://doi.org/10.3390/fermentation5020035
Hussain SA, Nazir Y, Hameed A, Yang W, Mustafa K, Song Y. Optimization of Diverse Carbon Sources and Cultivation Conditions for Enhanced Growth and Lipid and Medium-Chain Fatty Acid (MCFA) Production by Mucor circinelloides. Fermentation. 2019; 5(2):35. https://doi.org/10.3390/fermentation5020035
Chicago/Turabian StyleHussain, Syed Ammar, Yusuf Nazir, Ahsan Hameed, Wu Yang, Kiren Mustafa, and Yuanda Song. 2019. "Optimization of Diverse Carbon Sources and Cultivation Conditions for Enhanced Growth and Lipid and Medium-Chain Fatty Acid (MCFA) Production by Mucor circinelloides" Fermentation 5, no. 2: 35. https://doi.org/10.3390/fermentation5020035
APA StyleHussain, S. A., Nazir, Y., Hameed, A., Yang, W., Mustafa, K., & Song, Y. (2019). Optimization of Diverse Carbon Sources and Cultivation Conditions for Enhanced Growth and Lipid and Medium-Chain Fatty Acid (MCFA) Production by Mucor circinelloides. Fermentation, 5(2), 35. https://doi.org/10.3390/fermentation5020035





