Abstract
Sweet sorghum juice (SSJ) was evaluated as fermentation substrate for the production of l-lactic acid. A thermophilic Bacillus coagulans isolate was selected for batch fermentations without the use of additional nutrients. The first batch of SSJ (Batch A) resulted on higher lactic acid concentration, yield and productivity with values of 78.75 g∙L−1, 0.78 g∙g−1 and 1.77 g∙L−1 h−1, respectively. Similar results were obtained when the process was transferred into the pilot scale (50 L), with corresponding values of 73 g∙L−1, 0.70 g∙g−1 and 1.47 g∙L−1 h−1. A complete downstream process scheme was developed in order to separate lactic acid from the fermentation components. Coarse and ultra-filtration were employed as preliminary separation steps. Mono- and bipolar electrodialysis, followed by chromatography and vacuum evaporation were subsequently carried out leading to a solution containing 905.8 g∙L−1 lactic acid, with an optical purity of 98.9%. The results of this study highlight the importance of the downstream process with respect to using SSJ for lactic acid production. The proposed downstream process constitutes a more environmentally benign approach to conventional precipitation methods.
1. Introduction
Lactic acid (LA) is an organic acid which can be produced by lactic acid bacteria for many purposes, starting from food preservatives and finishing at medicines [1]. The increasing demand for poly-lactic acid (PLA), a biodegradable plastic, has also boosted lactic acid’s market [2]. Production of PLA requires high optical purity of the biotechnologically produced LA [3]. To this end, LA production requires many steps, starting from the pre-treatment of the specific feedstock, going through the hydrolysis, fermentation and finishing at the separation and purification, the so called downstream [4]. The downstream process during biological manufacturing of LA is still an important challenge, further complicated by the utilization of cheap and complex substrates. Efficient LA production has been reported from various alternative substrates such as sugarcane, food waste, coffee pulp, acid whey, molasses or avocado seeds to name a few [4,5,6,7,8,9]. Recently, there has been a lot of interest in lignocellulosic materials as fermentation feedstocks [9,10]; nevertheless, other easier accessible substrates can be taken into consideration for the production of LA.
Sweet sorghum is the most utilized crop for bio-based chemicals production in China, but it has been also recognized worldwide as an interesting feedstock [11]. It has already been used for the production of bioethanol or in two stage ethanol-methane production [12,13] and it is one of the most common feedstocks for bio-butanol production [14]. Sweet sorghum juice (SSJ) has also been used for the fermentative production of l-lactic acid. Wang et al. [15] used Bacillus coagulans for repeated batch fermentations of acid hydrolysate of SSJ and their results showed a maximum productivity of 2.90 g∙L−1 h−1 and a yield of 0.943 g∙g−1. On the other hand, Lactobacillus rhamnosus was used in batch fermentation coupled with a membrane separation in order to improve l-lactic acid productivity [16]. In this case, repeated batch fermentations in a 7 L bioreactor were performed, in which the yield and the productivity reached 0.954 g∙g−1 and 17.55 g∙L−1 h−1 respectively. B. coagulans is a very promising candidate for lactic acid production due to its temperature resistance, robustness and high LA productivity [17]. B. coagulans was also used as a platform organism in multi-substrate utilization [6].
Many methods have been proposed in the literature for the separation and purification of lactic acid from the fermentation broth [18]. The conventional process involves lactic acid precipitation using calcium hydroxide. The recovery of lactic acid is usually performed by using an excess of H2 SO4. This process generates high amounts of CaSO4, as waste stream [3]. Consequently, the purity of lactic acid decreases and together with chemicals used and waste streams produced, it is not an overall environmentally benign process. Research is currently focusing on alternative methods for the recovery of lactic acid from complex fermentation broths. Among the proposed methods, the most promising ones so far seem to be ultra- and nanofiltration, electrodialysis, ion-exchange/adsorption, reactive distillation and hybrid short path evaporation [3,19]. However, most of these methods have only been tested in model lactic acid solutions or in well-defined media. Only a few studies so far have performed downstream separation of lactic acid from complex fermentation media [6,13,14,20,21,22].
Combining these two approaches, the utilization of B. coagulans and SSJ created a perfect match for the development of a downstream process, especially because all of the above-mentioned studies showed only the utilization of SSJ for LA production, but none of those were performed at pilot scale followed by separation and purification of LA. In this study, lab scale experiments were initially carried out using SSJ as sole carbon and nutrient source. Subsequently, the process was transferred in pilot scales, aiming to investigate the scalability of the proposed scheme. Finally, a downstream process was applied in order to optimize purification steps with respect to SSJ. For this purpose, a set of filtration systems was used, equipped with ceramic membranes. Afterwards electrodialysis was performed, followed by decolorization and chromatography techniques in order to remove remaining ions. Finally, vacuum distillation was employed in order to obtain a high concentrated lactic acid solution. To our knowledge, besides the work published by the group of Wang et al. [14,15,23], SSJ has not been fully investigated as a fermentation feedstock, or in pilot scales. Moreover, complete lactic acid separation schemes from cheap renewable resources are scarce in the literature, so this work will contribute in the development of efficient downstream processes.
2. Materials and Methods
2.1. Substrate
Sweet sorghum juice was produced by using a mechanical screw press (Kufferath Akupress A500) for pressing fresh feedstock. Sweet sorghum feedstock consisted of biomass from two different species (Sweet Chopper and Sugar Grace). Feedstock was cultivated in 2009, harvested in the last week of September 2009 using a Class Jaguar harvester for chopping. Pressing of fresh feedstock was executed on the same day, only some hours after cutting. The disintegrated chopped feedstocks, containing approximately 20 mm or smaller solids, were continuously dosed into a mechanical screw press, at low rotation speed (12–14 rpm). The exit of the screw press was guided by pneumatically controlled counter cone in order to build up the necessary pressure (5 bar) to initiate the dewatering of the material. The fractionation of sweet sorghum was done in a single step pressing to recover a pure juice without adding any additional water. A course hydro sieve was used to separate fiber residues present in the juice. Directly after, the sieve fresh juice was recovered in two different batches. The fresh juice had a greenish color and it was slightly foaming. Fresh SSJ was immediately cooled after pressing and deep frozen (−20 °C) on the same day. The screw press was able to generate typical fresh juice samples from sweet sorghum, but sugar extraction was not optimized. The latter would include several fractionation steps to allow complete sugar extraction from feedstock. Deep frozen batches of juice were delivered to ATB for fermentation experiments in March and June of the year 2010. The composition of the two batches (Batch A and B) was analyzed in terms of sugar, nitrogen, phosphorus and ion content using methods described below.
2.2. Microorganism
The B. coagulans A-35 strain used for all the experiments was isolated from alfalfa press residue and characterized using matrix assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-ToF MS) method. The strain was also identified by the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH. The strain is available at the ATB collection and it is being stored as a cryo-stock at −80 °C. Inoculum preparation was carried out in shake flasks with De Man, Rogosa and Sharpe (MRS) broth (Merck, Darmstadt, Germany) with 0.67 g Everzit Dol (Evers e.K., Hopsten, Germany) dolomite as buffer. The strain was incubated at 52 °C for 14 h in an orbital shaker at 100 rpm.
2.3. Fermentation
2.3.1. Laboratory Scale Fermentations
Lab-scale fermentations using the different batches of sweet sorghum juice were carried out in 5 L BIOSTAT bioreactors (Sartorius AG, Goettingen, Germany), with 3 L working volume. Temperature and stirring were set at 52 °C and 200 rpm, while the pH was continuously adjusted to 6 using 20% (w/w) NaOH. Inoculum was 2% (v/v) in all the studied cases. Due to substrate limitation, the experiments were carried out only once.
2.3.2. Pilot Scale Fermentation
The pilot scale fermentation was carried out in a 72 L BIOSTAT UD bioreactor (B-Braun Biotech, Hessen, Germany), with 50 L working volume. The sweet sorghum juice was autoclaved at 121 °C for 15 min, before inoculation. The fermentation was performed at 52 °C and 200 rpm, and the pH was maintained at 6 by adding 20% (w/w) NaOH. Inoculum was grown in 1 L MRS broth for 14 h at 52 °C. At the end of the fermentation, the broth was inactivated at 90 °C for 30 min in order to be further processed.
2.4. Downstream Process
A schematic diagram describing the downstream process is shown in Figure 1.
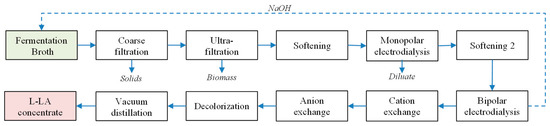
Figure 1.
Schematic diagram of the downstream process of lactic acid.
2.4.1. Filtration and Softening
A pre-filtration step (coarse filtration) was initially carried out using filter bags (Schwegmann Filtrations-Technik GmbH, Grafschaft-Ringen, Germany), with 150 µm pore size. The permeate stream was subsequently subjected to ultra-filtration at 1.5 bar using an UFI-TEC cross-flow filtration system (UFI-TEC, Oranienburg, Germany) equipped with four ABB ceramic membranes (UFI-TEC) with 0.1 µm pore size.
Cations removal (softening) was carried out using PUROLITE S950 acid chelating resin (Purolite, Ratingen, Germany) packed in an expanded bed setting. Initially the pH value of the permeate stream was adjusted to 10 by adding 20% NaOH. The permeate was introduced in the column from below at a flow of 6 bed volumes per h. Separation was complete when conductivity of the purified water was below 1 mS cm−1 [6].
2.4.2. Electrodialysis
The filtrate obtained from the softening column was afterwards concentrated via monopolar electrodialysis. A free lactic acid solution was achieved after bipolar electrodialysis, together with a NaOH solution. Both electrodialyses were carried out in batch mode, under constant polarity and at a temperature of 35 °C. Monopolar electrodialysis was composed by a sheet flow stack having 11 cation exchange membranes Type II (Fujifilm, Tilburg, the Netherlands) and 10 anion exchange membranes Type II (Fujifilm), operating at 20 V and 3 A. Conductivity values of the diluate below 0.5 mS cm−1 indicated the end of the process [6].
The concentrated stream produced via monopolar electrodialysis was then introduced to bipolar electrodialysis. Bipolar electrodialysis consisted also of 11 cation exchange membranes Type II (Fujifilm) operating at 30 V and 5 A and 10 anion exchange membranes Type II (Fujifilm) operating at 20 V and 3 A. The process was finished when the conductivity of the salt stream was approximately 5 mS∙cm−1. The acid stream was used for the following purification steps [6].
2.4.3. Decolorization and Chromatography
The removal of remaining cations and anions was carried out using cation and anion exchange chromatography. The strong cation exchange resin RELITE EXC 08 (Resindion S.R.L., Binasco, Italy) was initially applied followed by the weak anion exchange resin RELITE EXA 133 (Resindion S.R.L.). The packed columns had a 2 L volume and flow was set at 6 bed volumes per h. At the end of the process both columns were cleaned with water and regenerated. The strong acid resin PUROLITE MN-502 (Purolite) was employed in order to remove the color impurities. The filtrate was finally vacuum evaporated using a vacuum distillation plant (Büchi Labortechnik, Essen, Germany) at 55 °C, 0.05 bar and 200 rpm.
2.5. Analytical Assays
The determination of sugar content and lactic acid concentration was carried out by HPLC (DIONEX, Sunnyvale, CA, USA), coupled with a refractive index detector (RI-71, SHODEX, Yokohama, Japan) and equipped with a Eurokat H column (300 mm × 8 mm × 10 µm, Knauer, Berlin, Germany), eluted with 5 mM H2 SO4 at a flow rate of 0.8 mL∙min−1. An IonPac CS 16 column (250 mm × 4 µm, DIONEX, Sunnyvale, CA, USA) was used for the analysis of cations in the sweet sorghum juice and fermentation samples, operating at a flow rate of 1.0 mL∙min−1, at 40 °C, with 30 mM CH3 SO3 H as mobile phase. The analysis of anions was carried out using an IonPac AS9-HC column (250 mm × 4 µm, DIONEX, Sunnyvale, CA, USA), eluted with Na2 CO3 at a flow rate of 1.2 mL∙min−1, at room temperature.
Lactic acid optical purity analysis was carried out using HPLC (Knauer, Berlin Germany) coupled with a Chiralpak®MA(+) column (Daicel, Tokyo, Japan, 50 mm × 4.6 mm × 3 µm), using 2 mM CuSO4 as mobile phase at a flow rate of 0.8 mL∙min−1. Detection was carried out with an ultraviolet detector.
Protein content was measured following the [24] standard method. Flow injection analysis (FIA) was employed for the determination of total phosphorus (P) content, according to the international standard [25].
3. Results and Discussion
3.1. Optimization of the Fermentation Process
As shown in Table 1, the sweet sorghum juice mainly consisted of sucrose, fructose and glucose, with sucrose being the predominant sugar with a concentration of more than 60 g∙L−1, for both batches. The analytical composition of the substrate was very similar to the one presented by Di Cai et al. regarding sugar content [26]. The sweet sorghum juice also contained nitrogen and phosphorus, giving the possibility to be utilized as the sole nutrient source in lactic acid fermentations.

Table 1.
Chemical composition of two different batches of sweet sorghum juice.
3.2. Lab Scale Fermentations Using SSJ as Sole Nutrient Source
Lab scale batch fermentations were carried out using batches A and B of sweet sorghum juice. Initial sugar content did not differ significantly between the batches with approximately 105 g∙L−1 for A and 109 g∙L−1 for B (Table 1). Additionally, the distribution of sugars was similar in both batches with around 51–53% sucrose, 29–31% glucose and 16–17% fructose. Figure 2 shows the fermentation profiles for the two batches tested. As seen in the figure, both fermentations had a similar behavior with final LA concentrations of 78.75 and 72.71 g∙L−1 for batch A and batch B respectively. There was an apparent glucose repression over disaccharides and fructose, most noticeable in Figure 2A. Glucose consumption occurred rapidly with a complete depletion after only 10 h of fermentation. During the same period, the concentration of disaccharides did not show a significant reduction in batch A, while fructose concentration barely changed in both fermentations. Once glucose was depleted, the consumption of other sugars occurred at a faster rate and by 50 h, when the fermentations were stopped, no residual sugars were present in batch B (Figure 2B). Only 11 g∙L−1 of sugars remained in batch A at 50 h and most likely, since the concentration values had not reached a plateau, residual sugars concentration would have been lower if the fermentation had continued. In spite of that, the yield for batch A was higher with a value of 0.78 g∙g−1 compared to 0.68 g∙g−1 for batch B. LA production occurred faster during the first 10 h of fermentation, reaching about 30 g∙L−1 in both cases and a productivity of approximately 5 g∙L−1 h−1 from the time that the exponential phase started to the 10 h mark. The production rate slowed down after 10 h, possibly due to nutrients limitation. Nonetheless, overall productivities were 1.77 and 1.63 g∙L−1 h−1 for A and B which are promising considering that the fermentations were carried out without the addition of any extra nutrients. It was possible to overcome the limiting factors, such as lower sugar concentration when compared with other studies done by Wang et al. [27].
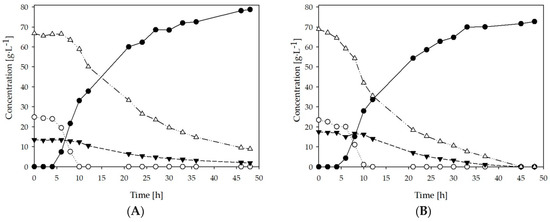
Figure 2.
Fermentative production of lactic acid (●) and consumption of glucose (○); fructose ▼; disaccharides (∆) by B.coagulans in two different batches (A,B) of SSJ.
3.3. Pilot Scale Fermentation using SSJ
Due to the higher LA yields and productivity values obtained in the previous fermentations, batch A was selected for pilot scale experiments. Fermentation was carried out using 50 L of batch A and the profile of the process is shown in Figure 3. As in the lab scale experiments, the glucose concentration decreased rapidly and was totally consumed after 13 h of fermentation. Together with glucose, the consumption of disaccharides started rapidly with an apparent decrease in its consumption rate after 13 h. As in the previous cases the consumption of fructose was noticeable only after glucose was depleted. Sugars were completely consumed after 60 h of fermentation. By the end of the fermentation, LA reached a maximum concentration of approximately 73 g∙L−1 with a yield of 0.70 g∙g−1 and a productivity of 1.47 g∙L−1 h−1.
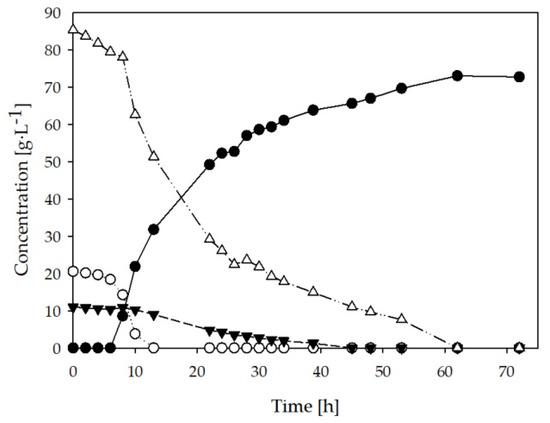
Figure 3.
B. coagulans fermentations carried out in pilot scale (50 L) with SSJ as sole carbon and nutrient source. Fermentative production of lactic acid (●) and consumption of glucose (○); fructose ▼; disaccharides (∆).
SSJ has been tested for the fermentative production of various products as shown in Table 2. Regarding l-lactic acid production, the highest yield and productivity have been achieved by employing a B. coagulans strain in repeated batch fermentations [15]. However, SSJ was supplemented with yeast extract and soya peptone, in contrast to this study that SSJ was utilized as sole carbon and nutrient source. To the authors’ knowledge, this is the first study in which SSJ is tested in pilot scales for l-lactic acid production, showing the industrial feasibility of the process.

Table 2.
Comparison between this work and previous studies on lactic acid and other fermentation products from SSJ.
3.4. Downstream Process of the Lactic Acid Produced in Pilot Scale
The utilization of lactic acid for high value applications requires high optical purity. Even though no residual sugars were left in the medium after fermentation, residual proteins, phosphorus and ions were still present in high concentrations (Table 3). Several steps were carried out in order to separate lactic acid from the rest of the fermentation’s components. After the fermentation, a coarse filtration step was employed in order to separate bigger particles that could possibly damage or block the ultra-filtration membranes. Low LA losses of <10% were observed in this step, as well as a slight decrease of all the other components of interest (total nitrogen and phosphorus, Cl−, SO42−, Na+, K+, Mg2+, Ca2+). Ultra-filtration was then carried out, mainly in order to separate the biomass. The majority of LA was found in the permeate stream (70.63 g∙L−1), corresponding to a recovery rate of 92.1%. This step also contributed to a removal of approximately 37.2% of total nitrogen.

Table 3.
Compositional analysis after every step of the downstream process (DSP) of the fermentation broth, obtained from the pilot scale fermentation, using SSJ as a feedstock.
Among the alternative technologies that have been proposed in the literature for the downstream separation and purification of lactic acid from complex fermentation broths, electrodialysis has been proven as a promising alternative [7,28,29]. In this study, monopolar electrodialysis (MED) has been investigated for the concentration of sodium lactate after ultra-filtration and bipolar electrodialysis (BED) was subsequently employed in order to convert lactate to lactic acid. Before electrodialysis, a softening step is necessary for the removal of divalent ions (mainly Mg2+ and Ca2+), which can cause fouling of the electrodialysis membranes. As can be seen from Table 3, the concentration of Mg2+ and Ca2+ after softening was 0.48 mg∙L−1 and 6.5 mg∙L−1, respectively, values corresponding to removals of 99.8% and 98.4%. This stream was initially treated with monopolar electrodialysis membranes, which generated two streams: the concentrate and the diluate. A volume of 20.6 L of concentrate stream containing 180.1 g∙L−1 of sodium lactate was obtained. Additionally, a considerable decrease of 69% in total nitrogen as well as in total phosphorus (22% removal) was achieved after this step. A second softening step was again required due to the high concentration of divalent anions in the concentrate stream. Bipolar electrodialysis was then carried out, resulting in three streams: acid (18.9 L), salt (8.5 L) and base (32 L). A recovery percentage of 85.6% of lactic acid was observed in the acid stream, whereas only 13.19 g∙L−1 was lost in the base stream.
The acid stream was further treated by cation and anion exchange resins for further removal of residual ions. By applying chromatography, more than 90% of the monovalent ions were successfully removed. Before vacuum evaporation, a decolorization step was employed for the removal of residual compounds contributing in the yellowish color of the stream. Finally a 2.9 L solution containing 905.8 g∙L−1 of lactic acid was obtained, whereas the concentration of residual impurities was <1.5 g∙L−1, corresponding to an overall lactic acid purity of approximately 99.8% (w/w). Optical purity was slightly lower in comparison to the end of the fermentation (99.8%), with a value of 98.9%. High lactic acid purities are of major importance, as already highlighted, especially when the production of PLA is the final goal. These processing steps resulted at high lactic acid purity; however, the overall lactic acid yield (from the end of the fermentation until the final distillated solution) was 62.4% meaning that a considerable amount is lost during the different treatments.
Even though the production of lactic acid from renewable resources has been studied extensively, its separation and purification from complex fermentation broths has been seldom investigated. Among the separation steps used in this study, filtration and chromatography are the most studied processes so far in the literature. The weak base resin Amberlite IRA-67 was tested by Moldes et al. [30] for the recovery of lactic acid from Eucalyptus wood hydrolysate. The same resin was evaluated by Garrett et al. [31] in an extractive fermentation using B. coagulans in corn stover hydrolysate. Their results showed that the resin was able to maintain the pH of the fermentation for more than 108 days. More than 99% of lactic acid was recovered, but the authors provided no data on the resulting purity. The resin Amberlite IRA-92 has been investigated for lactic acid recovery from paper sludge [23]. Flow rate and sample volume load were optimized resulting at lactic acid recovery yield and productivity of 82.6% and 96.2%, respectively. However, there were not insights on the individual ions that might be still present in the fermentation broth.
Nanofiltration has been employed as the primary separation step of lactic acid from residual sugars and other fermentation components from different broths such as coffee mucilage, sugarcane bagasse hydrolysate, food waste, acid whey as well as sugar bread and crust bread hydrolysates [5,13,14,18]. The optimization of each processing step could enhance the recovery yields of the biotechnologically produced lactic acid. Nanofiltration and tailor-made resins could be some options for a more cost-effective and economically viable downstream of l-lactic acid.
4. Conclusions
The results of this study indicate that sweet sorghum juice is a promising substrate for the l-lactic acid production when a thermophilic B. coagulans strain is employed. However, the lower concentration of other important nutrients for growth, such as proteins and phosphorus, could be responsible for the decreased lactic acid yield and productivity when batch B was used (0.68 g∙g−1 and 1.63 g∙L−1 h−1 respectively). Since batch A resulted in the highest lactic acid production, the same substrate was tested at a pilot scale fermentation, leading to similar results as in the lab-scale. The effective lactic acid downstream enabled to reach 99.8% (w/w) product purity, what indicates that the purification process based on ultra-filtration, electrodialysis, chromatography and distillation was effective.
Author Contributions
J.V. conceived of idea and the concept. Data curation A.O.-W. and R.S.; Supervision J.V.; Writing-original draft, A.O.-W., M.A., J.P.L.-G. and M.M.; J.V. supervised the development of the work. All authors discussed the results of the experiments and contributed to the final manuscript.
Acknowledgments
We kindly acknowledge support provided by Ökoenergie Utzenaich GmbH for the production and harvest of sweet sorghum.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodrigues, C.; Vandenberghe, L.P.S.; Woiciechowski, A.L.; de Oliveira, J.; Letti, L.A.J.; Soccol, C.R. Production and application of lactic Acid. Curr. Dev. Biotechnol. Bioeng. Prod. Isol. Purif. Ind. Prod. 2016, 543–556. [Google Scholar] [CrossRef]
- Ghaffar, T.; Irshad, M.; Anwar, Z.; Aqil, T.; Zulifqar, Z.; Tariq, A.; Kamran, M.; Ehsan, N.; Mehmood, S. Recent trends in lactic acid biotechnology: A brief review on production to purification. J. Radiat. Res. Appl. Sci. 2014, 7, 222–229. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Komesu, A.; Vaz Rossell, C.E.; Maciel Filho, R. Challenges and opportunities in lactic acid bioprocess design—From economic to production aspects. Biochem. Eng. J. 2018, 133, 219–239. [Google Scholar] [CrossRef]
- Brock, S.; Kuenz, A.; Prüße, U. Impact of hydrolysis methods on the utilization of agricultural residues as nutrient source for D-lactic acid production by Sporolactobacillus inulinus. Fermentation 2019, 5, 12. [Google Scholar] [CrossRef]
- Alves de Oliveira, R.; Vaz Rossell, C.E.; Venus, J.; Cândida Rabelo, S.; Maciel Filho, R. Detoxification of sugarcane-derived hemicellulosic hydrolysate using a lactic acid producing strain. J. Biotechnol. 2018, 278, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pleissner, D.; Lau, K.Y.; Schneider, R.; Venus, J.; Lin, C.S.K. Fatty acid feedstock preparation and lactic acid production as integrated processes in mixed restaurant food and bakery wastes treatment. Food Res. Int. 2015, 73, 52–61. [Google Scholar] [CrossRef]
- Neu, A.K.; Pleissner, D.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative utilization of coffee mucilage using Bacillus coagulans and investigation of down-stream processing of fermentation broth for optically pure l(+)-lactic acid production. Bioresour. Technol. 2016, 211, 398–405. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Alexandri, M.; Schneider, R.; Venus, J. A review on the current developments in continuous lactic acid fermentations and case studies utilising inexpensive raw materials. Process Biochem. 2019, 79, 1–10. [Google Scholar] [CrossRef]
- Tarraran, L.; Mazzoli, R. Alternative strategies for lignocellulose fermentation through lactic acid bacteria: the state of the art and perspectives. FEMS Microbiol. Lett. 2018, 365. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernandez, C.; Bellesteros, M.; Tomas-Pejo, E. Biotechnological advances in lactic acid production by lactic acid bacteria: lignocellulose as novel substrate. Biofuels Bioprod. Biorefining 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Johnston, D.; Nghiem, N. Evaluation of sweet sorghum juice for the production of lysine using Corynebacterium glutamicum. Fermentation 2018, 4, 29. [Google Scholar] [CrossRef]
- Zhang, C.; Wen, H.; Zheng, J.; Fu, C.; Chen, C.; Qin, P.; Wang, Z. A combination of evaporation and chemical preservation for long-term storage of fresh sweet sorghum juice and subsequent bioethanol production. J. Food Process. Preserv. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Takaki, M.; Tan, L.; Murakami, T.; Tang, Y.Q.; Sun, Z.Y.; Morimura, S.; Kida, K. Production of biofuels from sweet sorghum juice via ethanol-methane two-stage fermentation. Ind. Crops Prod. 2015, 63, 329–336. [Google Scholar] [CrossRef]
- Ndaba, B.; Chiyanzu, I.; Marx, S. Direct fermentation of sweet sorghum juice by Clostridium acetobutylicum and Clostridium tetanomorphum to produce bio-butanol and organic acids. Biofuel Res. J. 2015, 6, 248–252. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, J.; Cai, D.; Wang, Z.; Qin, P.; Tan, T. Repeated-batch fermentation of L-lactic acid from acid hydrolysate of sweet sorghum juice using mixed neutralizing agent under unsterilized conditions. J. Chem. Technol. Biotechnol. 2011, 61, 66–68. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, H.; Cai, D.; Wang, B.; Qin, P.; Wang, Z.; Tan, T. Improvement of L-lactic acid productivity from sweet sorghum juice by repeated batch fermentation coupled with membrane separation. Bioresour. Technol. 2016, 211, 291–297. [Google Scholar] [CrossRef]
- Glaser, R.; Venus, J. Co-fermentation of the main sugar types from a beechwood organosolv hydrolysate by several strains of Bacillus coagulans results in effective lactic acid production. Biotechnol. Rep. 2018, 18, 22–27. [Google Scholar] [CrossRef]
- Joglekar, H.G.; Rahman, I.; Babu, S.; Kulkarni, B.D.; Joshi, A. Comparative assessment of downstream processing options for lactic acid. Sep. Purif. Technol. 2006, 52, 1–17. [Google Scholar] [CrossRef]
- Sasiradee, J.; Kienberger, M.; Nuttakul, M.; Siebenhofer, M. Potential and assessment of lactic acid production and isolation—A review. J. Chem. Technol. Biotechnol. 2017, 92, 2885–2893. [Google Scholar]
- Alexandri, M.; Schneider, R.; Venus, J. Membrane technologies for lactic acid separation from fermentation broths derived from renewable resources. Membranes (Basel) 2018, 8, 94. [Google Scholar] [CrossRef]
- Oonkhanond, B.; Jonglertjunya, W.; Srimarut, N.; Bunpachart, P.; Tantinukul, S.; Nasongkla, N.; Sakdaronnarong, C. Lactic acid production from sugarcane bagasse by an integrated system of lignocellulose fractionation, saccharification, fermentation, and ex-situ nanofiltration. J.Environ. Chem. Eng. 2017, 5, 2533–2541. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Ngo, H.H.; Li, Y. Dynamic membrane-assisted fermentation of food wastes for enhancing lactic acid production. Bioresour. Technol. 2017, 234, 40–47. [Google Scholar] [CrossRef]
- Tong, W.Y.; Fu, X.Y.; Lee, S.M.; Yu, J.; Liu, J.W.; Wei, D.Z.; Koo, Y.M. Purification of L(+)-lactic acid from fermentation broth with paper sludge as a cellulosic feedstock using weak anion exchanger Amberlite IRA-92. Biochem. Eng. J. 2004, 18, 89–96. [Google Scholar] [CrossRef]
- DIN-EN-25663 Total Kjeldahl Nitrogen in Water and Biosolids by Automated Colorimetry with Preliminary Distillation/Digestion. Available online: https://www.epa.gov/sites/production/files/2015-10/documents/method_1687_draft_2001.pdf (accessed on 1 January 2019).
- ISO 15681-1 Water quality—Determination of orthophosphate and total phosphorus contents by flow analysis (FIA and CFA)—Part 1: Method by flow injection analysis (FIA) Qualité. 2003. Available online: https://www.sis.se/api/document/preview/904242/ (accessed on 1 January 2019).
- Cai, D.; Wang, Y.; Chen, C.; Qin, P.; Miao, Q.; Zhang, C.; Li, P.; Tan, T. Acetone-butanol-ethanol from sweet sorghum juice by an immobilized fermentation-gas stripping integration process. Bioresour. Technol. 2016, 211, 704–710. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Cai, D.; Wang, Z.; Qin, P.; Tan, T. The optimization of L-lactic acid production from sweet sorghum juice by mixed fermentation of Bacillus coagulans and Lactobacillus rhamnosus under unsterile conditions. Bioresour. Technol. 2016, 218, 1098–1105. [Google Scholar] [CrossRef]
- Hoffmann, E.; Ye, J.; Hahn, H.H. Recent advances in application of electrodialysis with bipolar membranes for organic acid recovery from fermentation broth. Curr. Org. Chem. 2016, 20, 2753–2761. [Google Scholar] [CrossRef]
- Lech, M.; Trusek, A. Batch Electrodialysis of Lactic Acid Obtained from Lab Fermentation. Polish J. Chem. Technol. 2018, 20, 81–86. [Google Scholar] [CrossRef]
- Moldes, A.B.; Alonso, J.L.; Parajó, J.C. Recovery of lactic acid from simultaneous saccharification and fermentation media using anion exchange resins. Bioprocess Biosyst. Eng. 2003, 25, 357–363. [Google Scholar] [CrossRef]
- Garrett, B.G.; Srinivas, K.; Ahring, B.K. Performance and stability of AmberliteTM IRA-67 ion exchange resin for product extraction and pH control during homolactic fermentation of corn stover sugars. Biochem. Eng. J. 2015, 94, 1–8. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).