Pesticide Residues and Stuck Fermentation in Wine: New Evidences Indicate the Urgent Need of Tailored Regulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strains and Growth Conditions
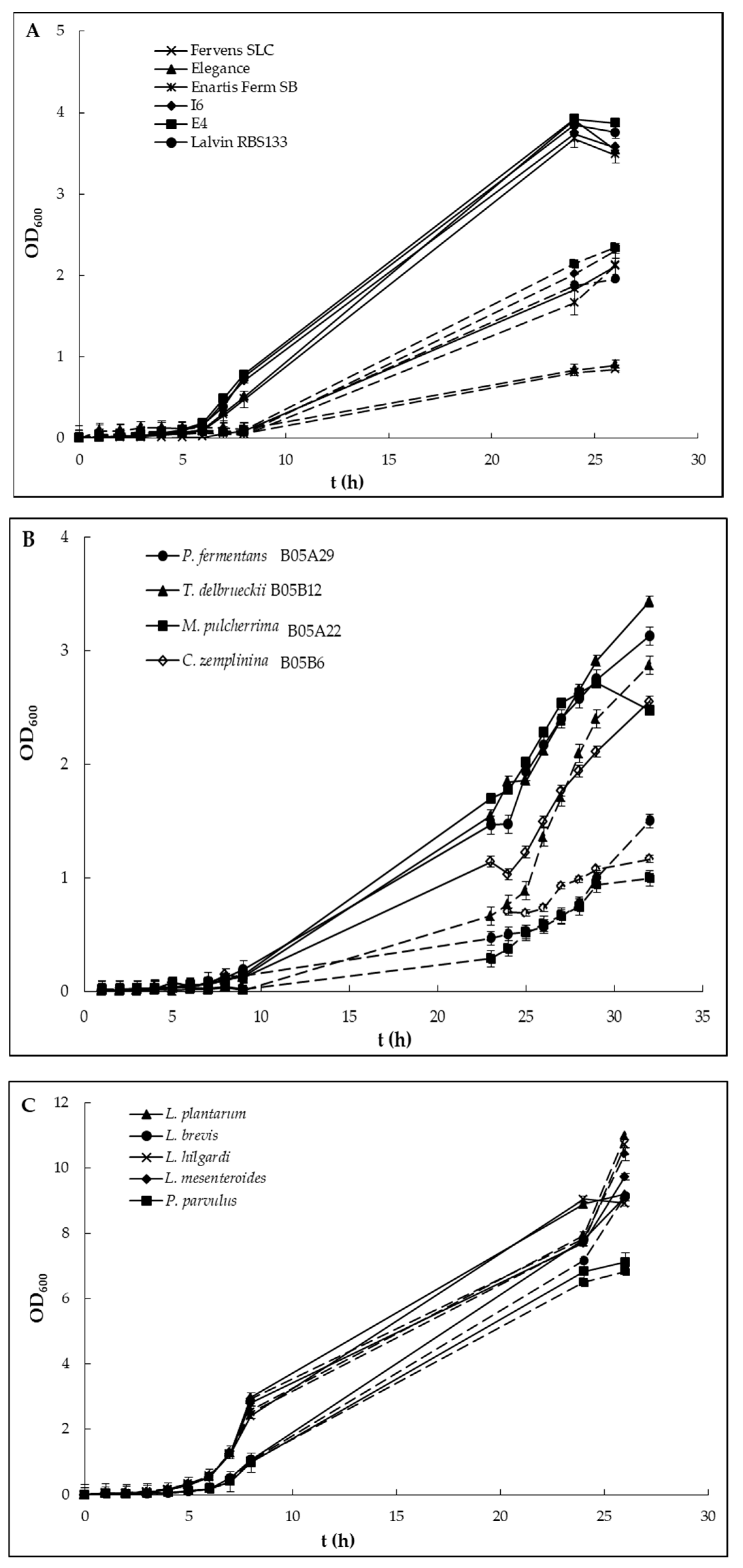
2.2. Growth Curves in Media Contaminated with the Pesticide
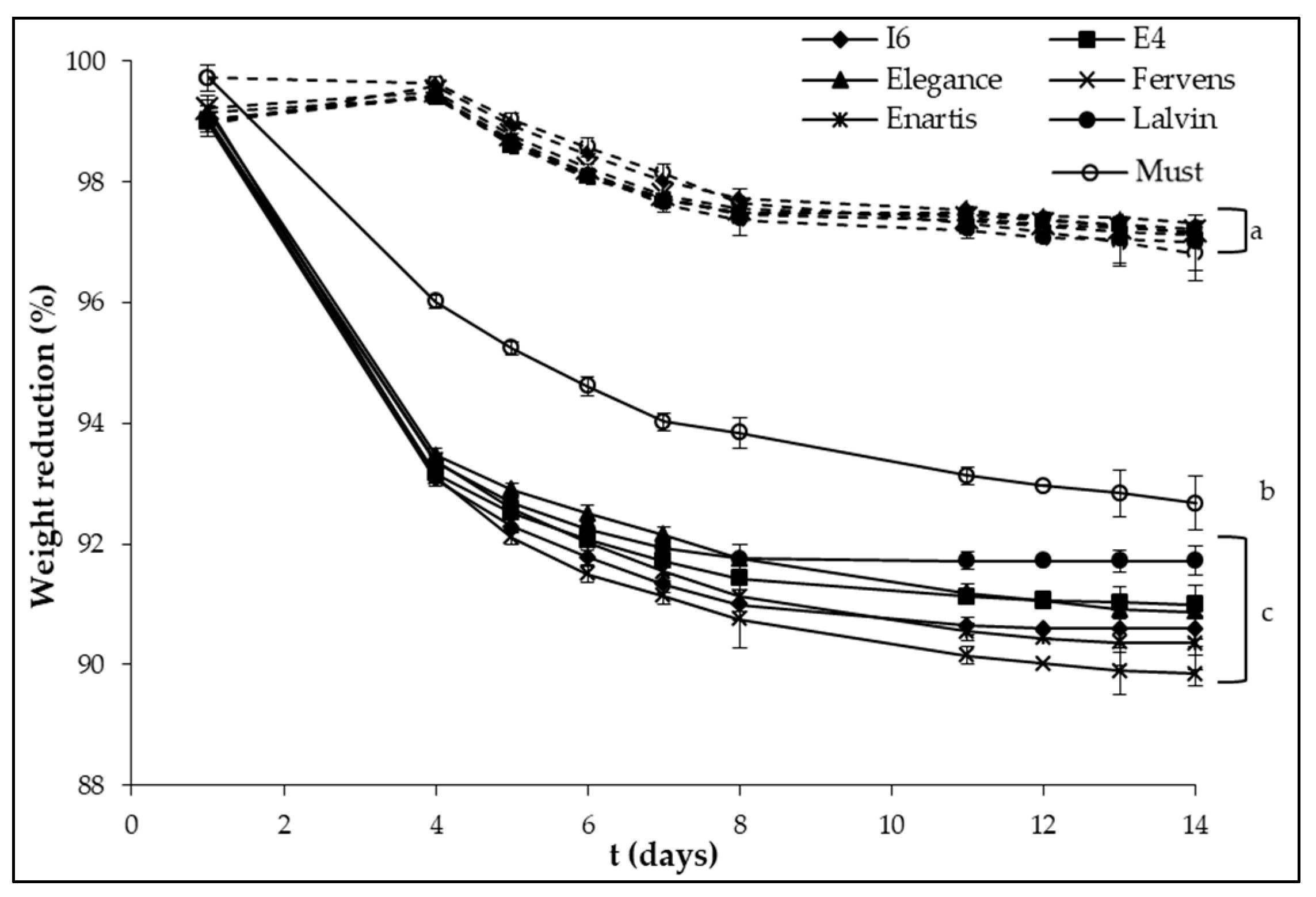
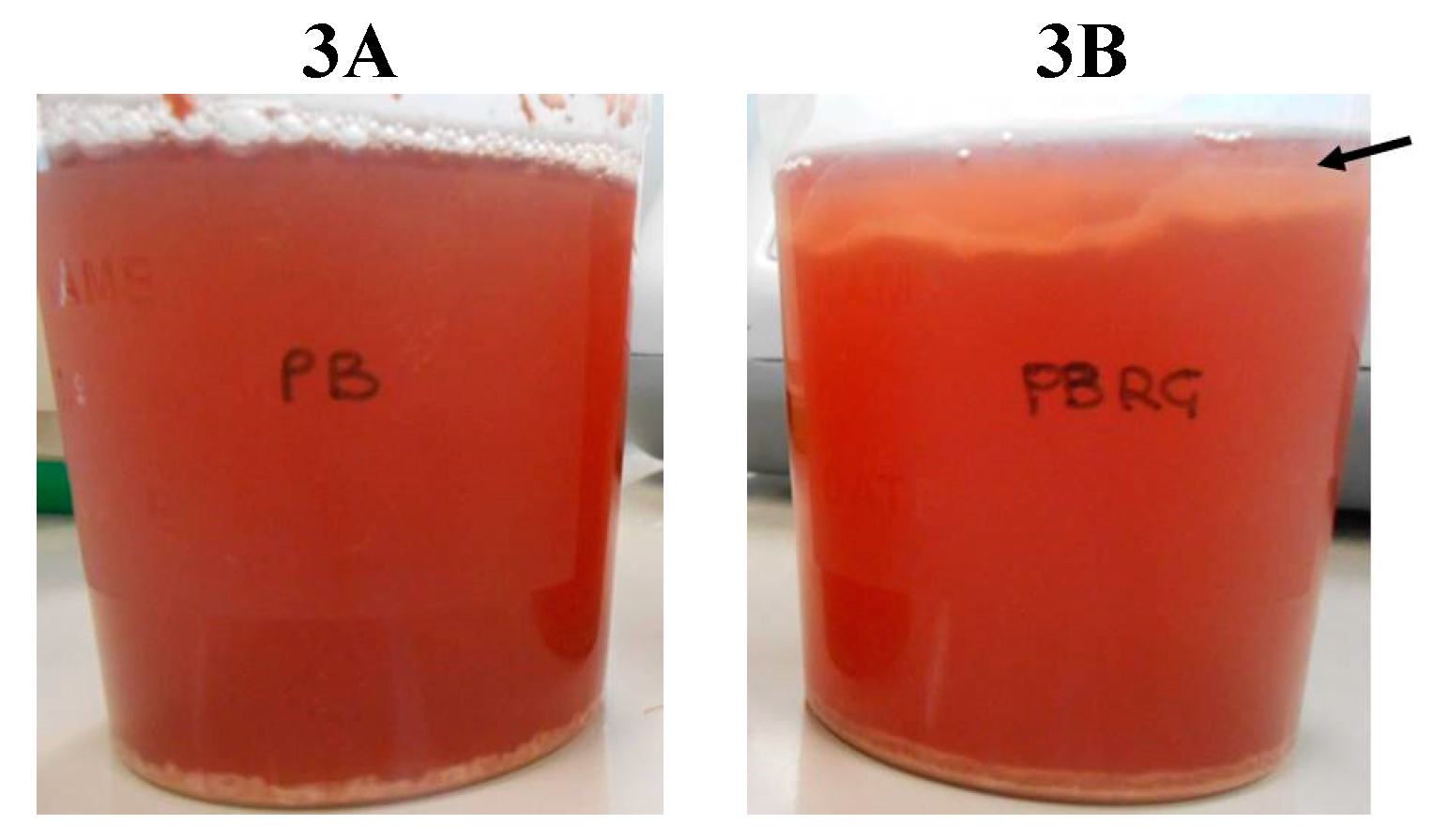
2.3. Laboratory-Scale Vinification Assay
2.4. Laboratory-Scale Vinification Assay with Analytical Grade Standards
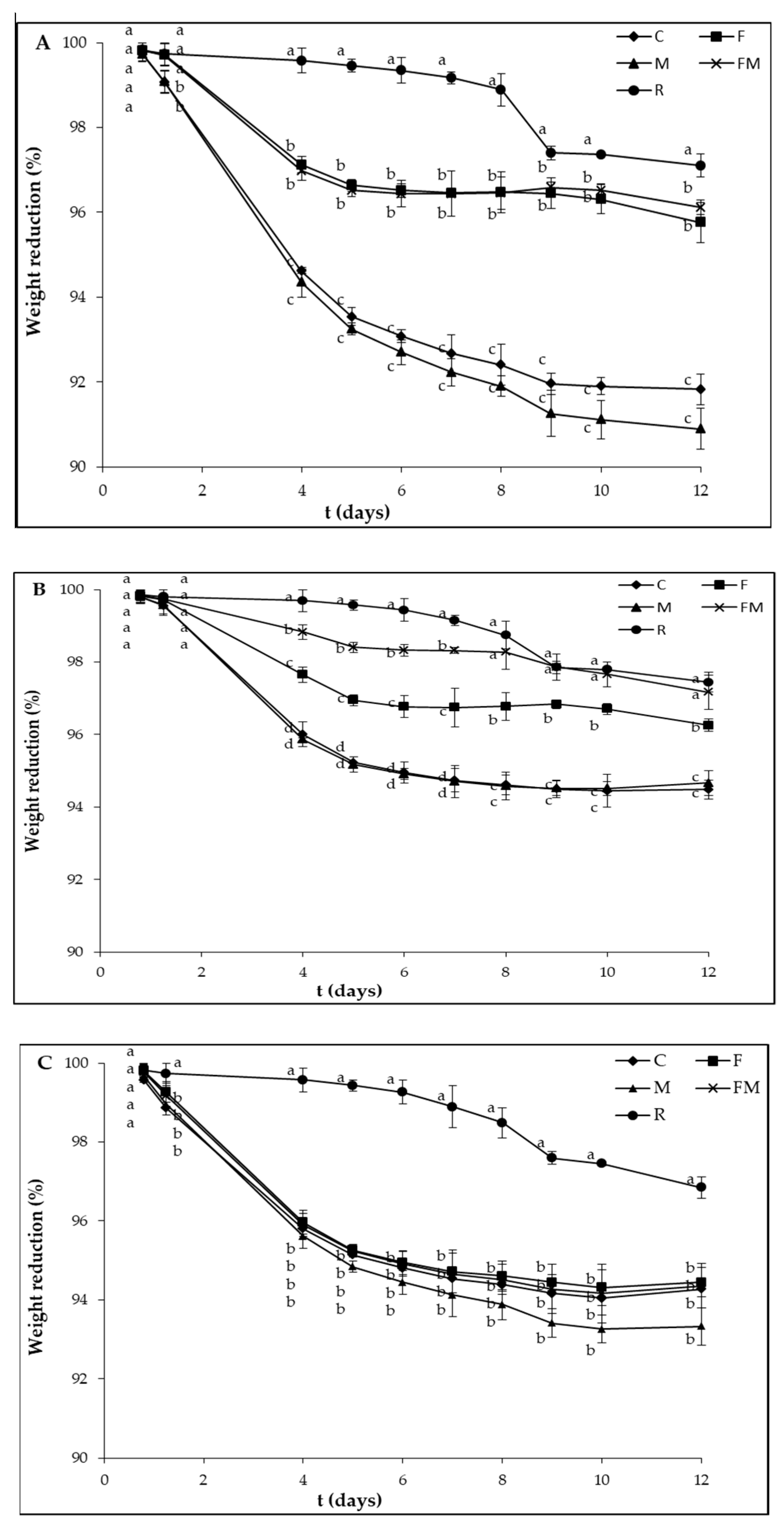
2.5. Laboratory-Scale Vinification Assay in Complex Microbial Ecosystem
2.6. Fungicide Residue Analysis
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Berbegal, C.; Spano, G.; Tristezza, M.; Grieco, F.; Capozzi, V. Microbial Resources and Innovation in the Wine Production Sector. S. Afr. J. Enol. Vitic. 2017, 38, 156–166. [Google Scholar] [CrossRef]
- Petruzzi, L.; Capozzi, V.; Berbegal, C.; Corbo, M.R.; Bevilacqua, A.; Spano, G.; Sinigaglia, M. Microbial Resources and Enological Significance: Opportunities and Benefits. Front. Microbiol. 2017, 8, 995. [Google Scholar] [CrossRef] [PubMed]
- Blateyron, L.; Sablayrolles, J.M. Stuck and slow fermentations in enology: statistical study of causes and effectiveness of combined additions of oxygen and diammonium phosphate. J. Biosci. Bioeng. 2001, 91, 184–189. [Google Scholar] [CrossRef]
- Bisson, L.F. Stuck and Sluggish Fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar]
- Capozzi, V.; Fragasso, M.; Romaniello, R.; Berbegal, C.; Russo, P.; Spano, G. Spontaneous Food Fermentations and Potential Risks for Human Health. Fermentation 2017, 3, 49. [Google Scholar] [CrossRef]
- Maisonnave, P.; Sanchez, I.; Moine, V.; Dequin, S.; Galeote, V. Stuck fermentation: development of a synthetic stuck wine and study of a restart procedure. Int. J. Food Microbiol. 2013, 163, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Szopinska, A.; Christ, E.; Planchon, S.; König, H.; Evers, D.; Renaut, J. Stuck at work? Quantitative proteomics of environmental wine yeast strains reveals the natural mechanism of overcoming stuck fermentation. Proteomics 2016, 16, 593–608. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.; Li, Z.-Q.; Liu, R.-Q.; Wang, L.; Wang, Y.-J.; Xu, Y. Transcriptome of Erysiphe necator-infected Vitis pseudoreticulata leaves provides insight into grapevine resistance to powdery mildew. Hortic. Res. 2014, 1, 14049. [Google Scholar] [CrossRef] [PubMed]
- Jacometti, M.A.; Wratten, S.D.; Walter, M. Review: Alternatives to synthetic fungicides for Botrytis cinerea management in vineyards. Aust. J. Grape Wine Res. 2010, 16, 154–172. [Google Scholar] [CrossRef]
- Edder, P.; Ortelli, D.; Viret, O.; Cognard, E.; De Montmollin, A.; Zali, O. Control strategies against grey mould (Botrytis cinerea Pers.: Fr) and corresponding fungicide residues in grapes and wines. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2009, 26, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Vaquero-Fernández, L.; Sanz-Asensio, J.; Fernández-Zurbano, P.; López-Alonso, M.; Martínez-Soria, M.-T. Determination of fungicide pyrimethanil in grapes, must, fermenting must and wine. J. Sci. Food Agric. 2013, 93, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Čuš, F.; Česnik, H.B.; Bolta, Š.V.; Gregorčič, A. Pesticide residues in grapes and during vinification process. Food Control 2010, 21, 1512–1518. [Google Scholar] [CrossRef]
- González-Rodríguez, R.M.; Cancho-Grande, B.; Simal-Gándara, J. Decay of fungicide residues during vinification of white grapes harvested after the application of some new active substances against downy mildew. Food Chem. 2011, 125, 549–560. [Google Scholar] [CrossRef]
- EU Pesticides Database—European Commission. Available online: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=homepage&language=EN (accessed on 22 December 2018).
- Ochiai, N.; Fujimura, M.; Oshima, M.; Motoyama, T.; Ichiishi, A.; Yamada-Okabe, H.; Yamaguchi, I. Effects of iprodione and fludioxonil on glycerol synthesis and hyphal development in Candida albicans. Biosci. Biotechnol. Biochem. 2002, 66, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Calhelha, R.C.; Andrade, J.V.; Ferreira, I.C.; Estevinho, L.M. Toxicity effects of fungicide residues on the wine-producing process. Food Microbiol. 2006, 23, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; López-Fernández, O.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. A review on the fermentation of foods and the residues of pesticides-biotransformation of pesticides and effects on fermentation and food quality. Crit. Rev. Food Sci. Nutr. 2015, 55, 839–863. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, R.M.; González-Barreiro, C.; Rial-Otero, R.; Regueiro, J.; Torrado-Agrasar, A.; Martínez-Carballo, E.; Cancho-Grande, B. Influence of new fungicides—Metiram and pyraclostrobin—On Saccharomyces cerevisiae yeast growth and alcoholic fermentation course for wine production. CyTA J. Food 2011, 9, 329–334. [Google Scholar] [CrossRef]
- Kong, Z.; Li, M.; An, J.; Chen, J.; Bao, Y.; Francis, F.; Dai, X. The fungicide triadimefon affects beer flavor and composition by influencing Saccharomyces cerevisiae metabolism. Sci. Rep. 2016, 6, 33552. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; El Khoury, M.; Lucas, P.; Bely, M.; Russo, P.; Spano, G.; Capozzi, V. Autochthonous starter cultures and indigenous grape variety for regional wine production. J. Appl. Microbiol. 2015, 118, 1395–1408. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Tristezza, M.; Grieco, F.; Spano, G.; Capozzi, V. From grape berries to wine: population dynamics of cultivable yeasts associated to “Nero di Troia” autochthonous grape cultivar. World J. Microbiol. Biotechnol. 2016, 32, 59. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Fernandes, J.O.; Alves, A.; Oliveira, M.B.P.P. Fast low-pressure gas chromatography–mass spectrometry method for the determination of multiple pesticides in grapes, musts and wines. J. Chromatogr. A 2009, 1216, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Malherbe, S.; Bauer, F.F.; Du Toit, M. Understanding problem fermentations—A review. S. Afr. J. Enol. Vitic. 2007, 28, 169–186. [Google Scholar] [CrossRef]
- Russo, P.; Spano, G.; Capozzi, V. Microbiological and Chemical Integrative Experimental Data. Università di Foggia: Foggia, Italy, 2019; Material not intended for publication. [Google Scholar]
- Čuš, F.; Česnik, H.B.; Bolta, Š.V.; Gregorčič, A. Pesticide residues and microbiological quality of bottled wines. Food Control 2010, 21, 150–154. [Google Scholar] [CrossRef]
- Garofalo, C.; Russo, P.; Beneduce, L.; Massa, S.; Spano, G.; Capozzi, V. Non-Saccharomyces biodiversity in wine and the ‘microbial terroir’: A survey on Nero di Troia wine from the Apulian region, Italy. Ann. Microbiol. 2016, 66, 143–150. [Google Scholar] [CrossRef]
- Lonvaud-Funel, A. Lactic acid bacteria in winemaking: Influence on sensorial and hygienic quality. In Progress in Industrial Microbiology; Ved Pal, S., Raymond, D.S., Eds.; Biotransformations; Elsevier: Amsterdam, The Netherlands, 2002; Volume 36, pp. 231–262. [Google Scholar]
- Fatichenti, F.; Farris, G.A.; Deiana, P.; Cabras, P.; Meloni, M.; Pirisi, F.M. A preliminary investigation into the effect of Saccharomyces cerevisiae on pesticide concentration during fermentation. Eur. J. Appl. Microbiol. Biotechnol. 1983, 18, 323–325. [Google Scholar] [CrossRef]
- Di Toro, M.R.; Capozzi, V.; Beneduce, L.; Alexandre, H.; Tristezza, M.; Durante, M.; Tufariello, M.; Grieco, F.; Spano, G. Intraspecific biodiversity and ‘spoilage potential’ of Brettanomyces bruxellensis in Apulian wines. LWT Food Sci. Technol. 2015, 60, 102–108. [Google Scholar] [CrossRef]
- Capozzi, V.; Di Toro, M.R.; Grieco, F.; Michelotti, V.; Salma, M.; Lamontanara, A.; Russo, P.; Orrù, L.; Alexandre, H.; Spano, G. Viable But Not Culturable (VBNC) state of Brettanomyces bruxellensis in wine: New insights on molecular basis of VBNC behaviour using a transcriptomic approach. Food Microbiol. 2016, 59, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Berbegal, C.; Garofalo, C.; Russo, P.; Pati, S.; Capozzi, V.; Spano, G. Use of Autochthonous Yeasts and Bacteria in Order to Control Brettanomyces bruxellensis in Wine. Fermentation 2017, 3, 65. [Google Scholar] [CrossRef]
- Tristezza, M.; di Feo, L.; Tufariello, M.; Grieco, F.; Capozzi, V.; Spano, G.; Mita, G.; Grieco, F. Simultaneous inoculation of yeasts and lactic acid bacteria: Effects on fermentation dynamics and chemical composition of Negroamaro wine. LWT Food Sci. Technol. 2016, 66, 406–412. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Melis, M.; Pirisi, F.M.; Minelli, E.V.; Cabitza, F.; Cubeddu, M. Fate of Some New Fungicides (Cyprodinil, Fludioxonil, Pyrimethanil, and Tebuconazole) from Vine to Wine. J. Agric. Food Chem. 1997, 45, 2708–2710. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A. Pesticide residues in grapes, wine, and their processing products. J. Agric. Food Chem. 2000, 48, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Cayuela, M.; Paya, P.; Martinez-Cacha, A.; Cámara, M.A.; Barba, A. Influence of fungicides on grape yeast content and its evolution in the fermentation. Commun. Agric. Appl. Biol. Sci. 2007, 72, 181–189. [Google Scholar] [PubMed]
- Girond, S.; Blazy-Maugen, F.; Michel, G.; Bosch, M. Influence of some grapevine pesticides on yeasts and fermentation. Revue Francaise d’Oenologie 1989, 29, 14–22. [Google Scholar]
- Sapis-domercq, S.; Bertrand, A.; Joyeux, A.; Lucmaret, V.; Sarre, C. Etude de l’influence des produits de traitement de la vigne sur la microflore des raisins et des vins. Experimentation 1977. Comparaison avec les résultats de 1976 et 1975. OENO One 1978, 12, 245–275. [Google Scholar] [CrossRef]
- Đorđević, T.M.; Đurović-Pejčev, R.D. Dissipation of chlorpyrifos-methyl by Saccharomyces cerevisiae during wheat fermentation. LWT Food Sci. Technol. 2015, 61, 516–523. [Google Scholar] [CrossRef]

| Non-Saccharomyces | Lactic Acid Bacteria | |
|---|---|---|
| Panel S | Panel R | |
| I. orientalis B05B2 | P. fermentans B05A29 | L. brevis IOEB9809 |
| M. pulcherrima B05A22 | P. fermentans M105A3 | L. hilgardii CECT4786 |
| H. guillermondi M105A31 | C. zemplinina B05B6 | L. plantarum UFG44 |
| H. uvarum B05B29 | T. delbrueckii B05B12 | L. mesenteroides OT54 |
| M. pulcherrima B05A36 | I. terricola B05B8 | P. parvulus UFG126 |
| K. thermotolerans B05B32 | Flavia® | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, P.; Berbegal, C.; De Ceglie, C.; Grieco, F.; Spano, G.; Capozzi, V. Pesticide Residues and Stuck Fermentation in Wine: New Evidences Indicate the Urgent Need of Tailored Regulations. Fermentation 2019, 5, 23. https://doi.org/10.3390/fermentation5010023
Russo P, Berbegal C, De Ceglie C, Grieco F, Spano G, Capozzi V. Pesticide Residues and Stuck Fermentation in Wine: New Evidences Indicate the Urgent Need of Tailored Regulations. Fermentation. 2019; 5(1):23. https://doi.org/10.3390/fermentation5010023
Chicago/Turabian StyleRusso, Pasquale, Carmen Berbegal, Cristina De Ceglie, Francesco Grieco, Giuseppe Spano, and Vittorio Capozzi. 2019. "Pesticide Residues and Stuck Fermentation in Wine: New Evidences Indicate the Urgent Need of Tailored Regulations" Fermentation 5, no. 1: 23. https://doi.org/10.3390/fermentation5010023
APA StyleRusso, P., Berbegal, C., De Ceglie, C., Grieco, F., Spano, G., & Capozzi, V. (2019). Pesticide Residues and Stuck Fermentation in Wine: New Evidences Indicate the Urgent Need of Tailored Regulations. Fermentation, 5(1), 23. https://doi.org/10.3390/fermentation5010023







