Physiological, Genetic, and Fermentative Traits of Oenococcus oeni Isolates from Spontaneous Malolactic Fermentation in Koshu Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain Isolation and Growth Conditions
2.2. Identification of Bacteria
2.2.1. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-TOF MS) Analysis
2.2.2. 16S rRNA Gene Sequencing
2.3. Preparation of Biomass
2.4. Fermentation in BM w/wo Reducing Sugars
2.5. Fermentation in Modified BM Without Reducing Sugars and Tomato Juice
2.6. Organic Acid Determination and Bacterial Population Count
2.7. Fermentation Test in Synthetic Koshu Wine Medium
2.8. Analysis of mleA and cfa Expression
2.9. Characterization of Strains API50
2.10. Statistical Analyses
3. Results
3.1. Changes in the Organic Acid Composition of Koshu Wines by MLF
3.2. Isolation and Identification of LAB Isolated from Koshu Wines and Grapes
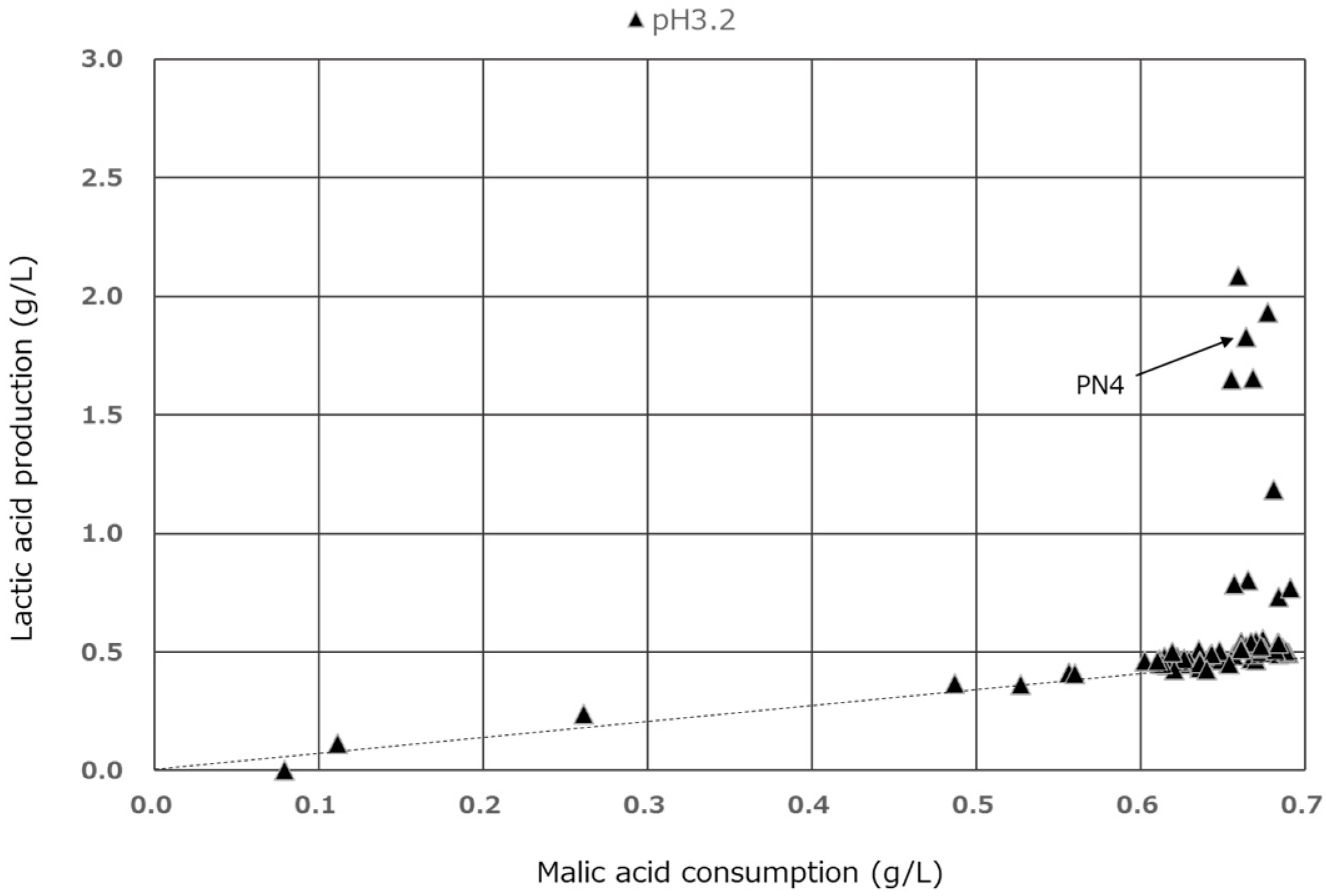
3.3. Comparison of Malic Acid Degradation and Lactic Acid Production on BM
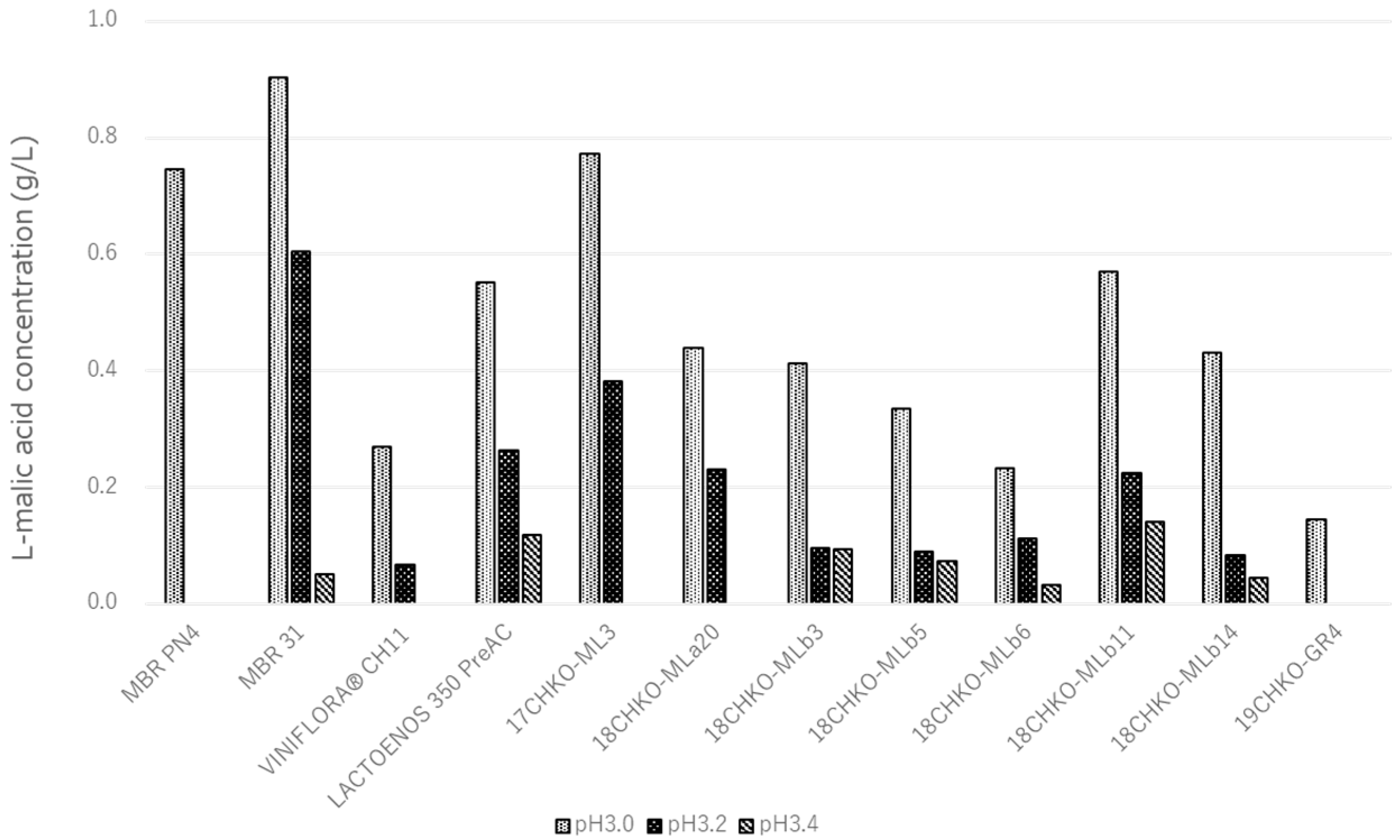
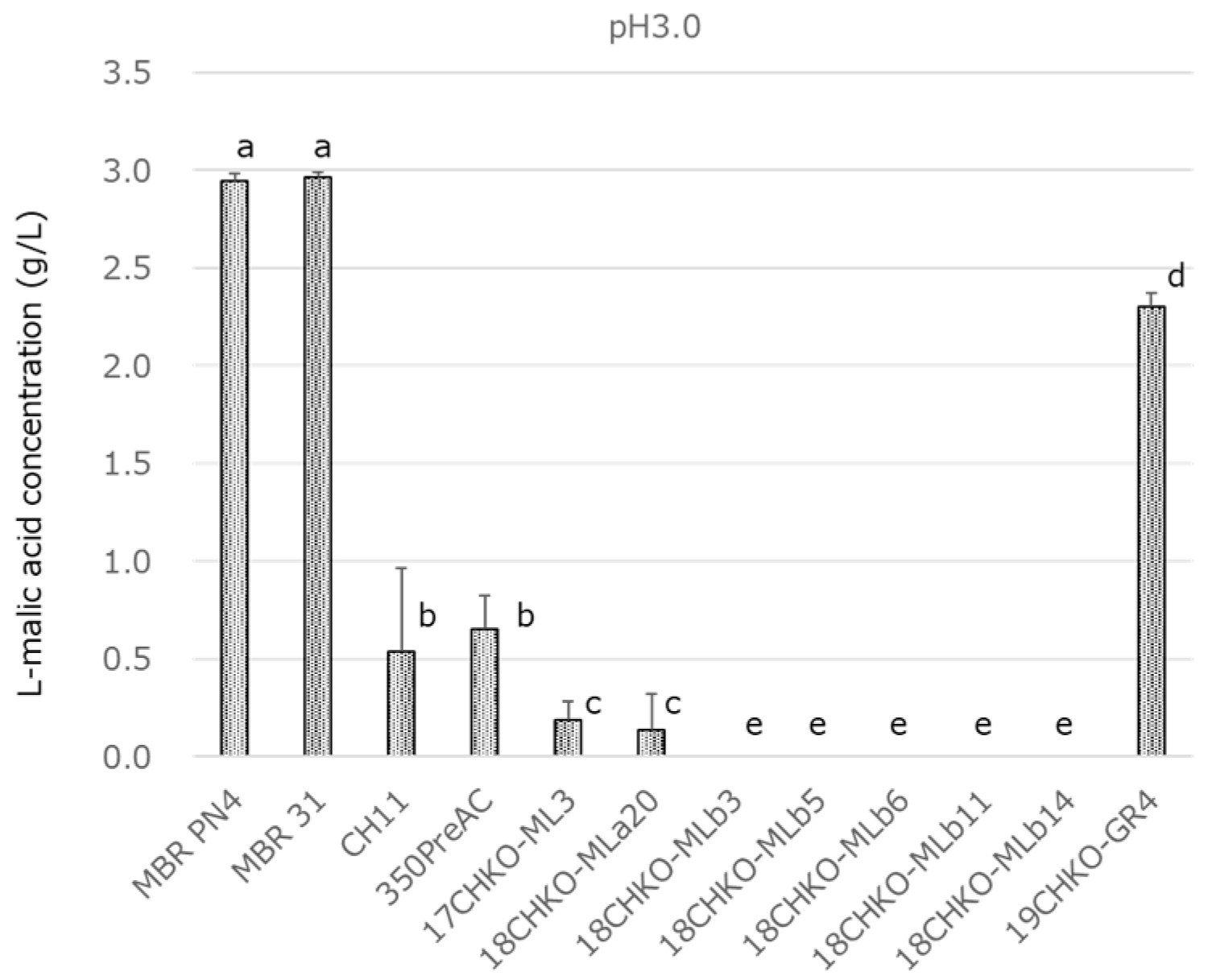
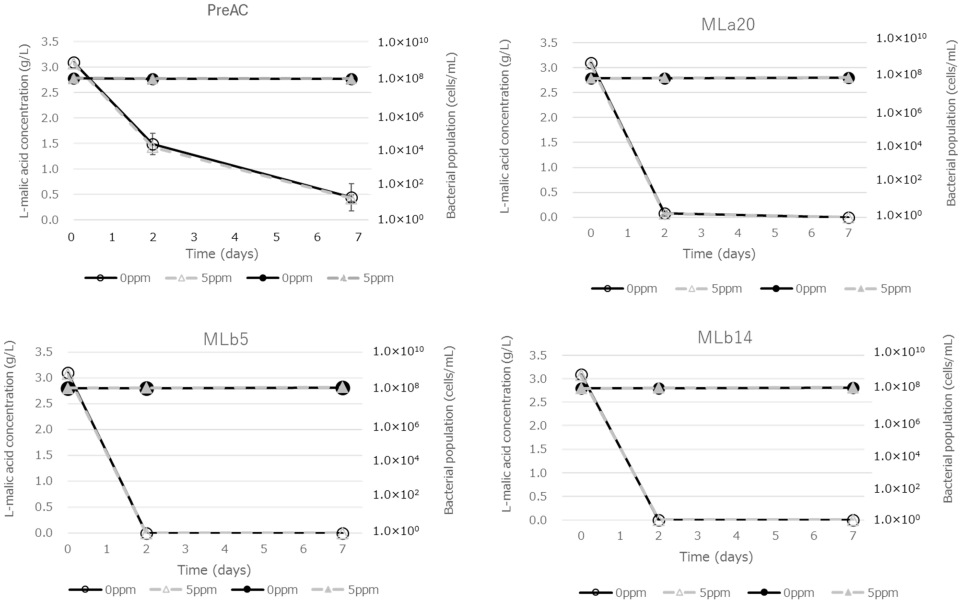
3.4. Effect of pH on MLF Activity in Modified BM
3.5. Effect of Total SO2 on MLF Activity in Modified BM
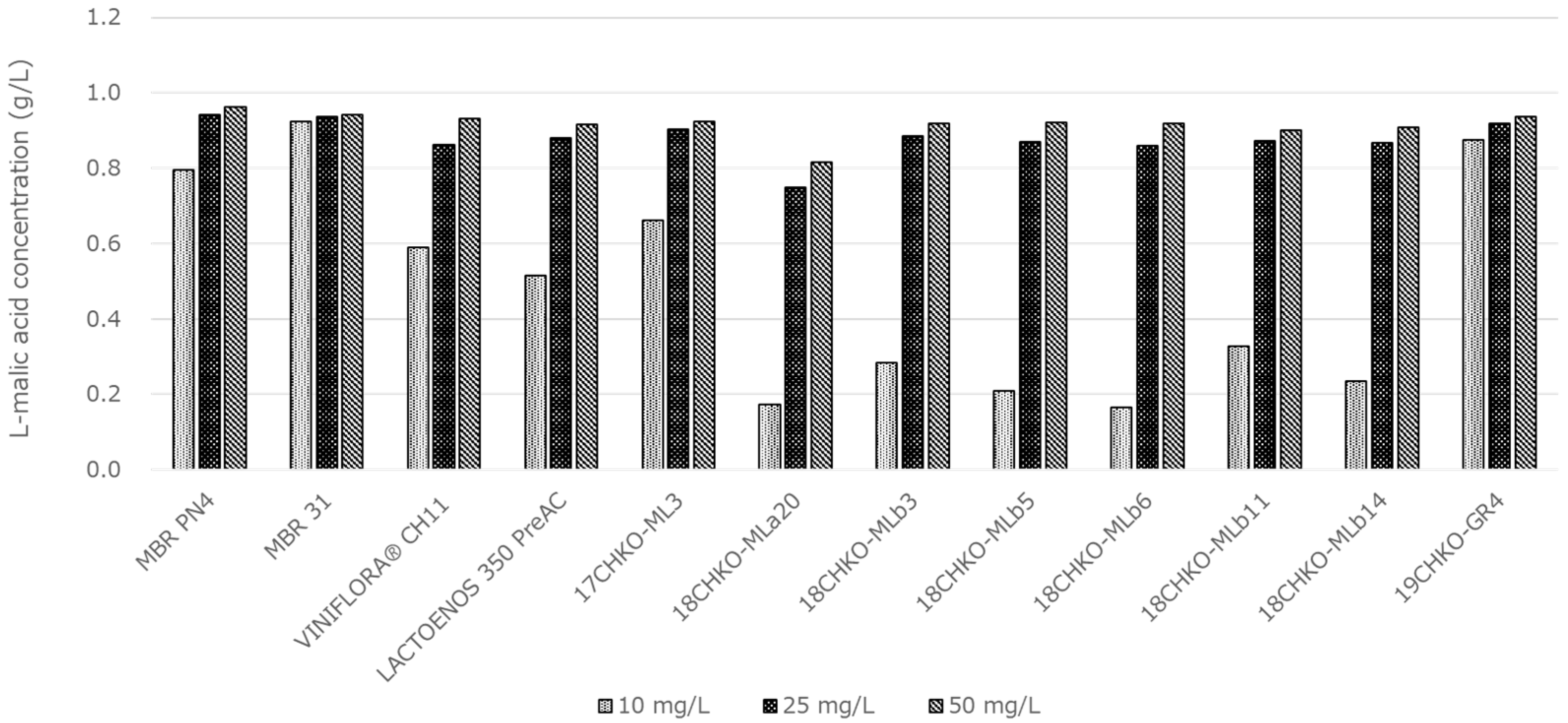
3.6. Effect of Ethanol on MLF Activity in Modified BM
3.7. Comparison of MLF Activity in Koshu Model Wine Medium
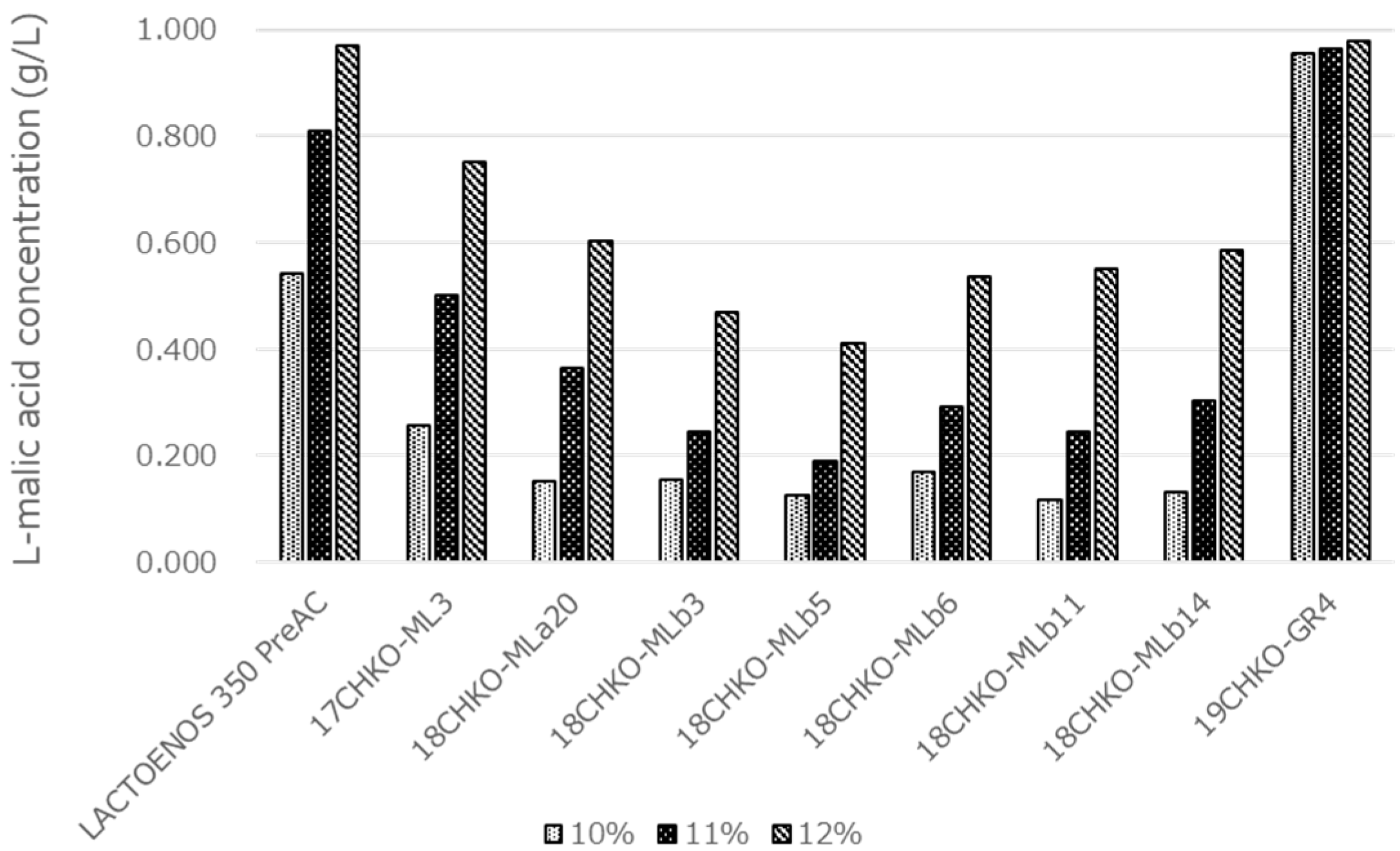
3.8. Effect of SO2 Concentration on MLF Activity in Koshu Model Wine Medium
3.9. Stress-Related Gene Expression Analysis
3.10. Utilization of Carbon Sources
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MLF | Malolactic fermentation |
| LAB | Lactic acid bacteria |
| BM | Basal medium |
| PCR | Polymerase chain reaction |
| API | Analytical Profile Index |
| RAPD | Random amplified polymorphic DNA |
| AF | Alcoholic fermentation |
References
- Goto-Yamamoto, N. Japan wine, its characteristics and research. Biosci. Biotechnol. Biochem. 2019, 83, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Ari’Izumi, K.; Suzuki, Y.; Kato, I.; Yagi, Y.; Otsuka, K.I.; Sato, M. Winemaking from Koshu Variety by the sur lie Method: Change in the Content of Nitrogen Compounds. Am. J. Enol. Vitic. 1994, 45, 312–315. [Google Scholar] [CrossRef]
- Sato, M.; Suzuki, Y.; Hanamure, K.I.; Katoh, I.; Yagi, Y.; Otsuka, K.I. Winemaking from Koshu variety by the sur lie method: Behavior of free amino acids and proteolytic activities in the wine. Am. J. Enol. Vitc. 1997, 48, 1–6. [Google Scholar] [CrossRef]
- Yamakawa, Y. Effects of espalier on must and wine trainings qualities cultivation of ‘Koshu’ and ‘Cabernet Sauvignon’ (Japanese). J. Brew. Soc. Jpn. 1995, 90, 65–67. [Google Scholar] [CrossRef]
- Misawa, A.; Nakano, H.; Amemiya, K.; Dobashi, M.; Shiogami, F.; Shimazu, Y.; Misawa, S. Quality characteristic of ‘Koshu’ grape cultivated under espalier training system and ‘Koshu’ wine. J. ASEV. Jpn. 2013, 24, 145–152. (In Japanese) [Google Scholar]
- Lerm, E.; Engelbrecht, L.; Du Toit, M. Malolactic fermentation: The ABC’s of MLF. S. Afr. J. Enol. Vitic. 2010, 31, 186–212. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Implications of new research and technologies for malolactic fermentation in wine. Appl. Microbiol. Biotechnol. 2014, 98, 8111–8132. [Google Scholar] [CrossRef]
- Bech-Terkilsen, S.; Westman, J.O.; Swiegers, J.H.; Siegumfeldt, H. Oenococcus oeni, a species born and moulded in wine: A critical review of the stress impacts of wine and the physiological responses. Aust. J. Grape Wine Res. 2020, 26, 188–206. [Google Scholar] [CrossRef]
- Onda, T.; Komatsu, M.; Nakayama, T. Comparison of compositions of fractionated grape juice prepared from Koshu and Chardonnay grapes by traditional pressing method. Food Preserv. Sci. 2021, 47, 145–152. [Google Scholar] [CrossRef]
- Nonomura, H.; Obara, I. Studies on malolactic fermentations (I) Effects of various factors affecting the malolactic fermentation by malic-adapted cells. J. Brew. Soc. Jpn. 1963, 58, 491–492. [Google Scholar] [CrossRef]
- Yanagida, F.; Srionnual, S.; Chen, Y.S. Isolation and characteristics of lactic acid bacteria from koshu vineyards in Japan. Lett. Appl. Microbiol. 2008, 47, 134–139. [Google Scholar] [CrossRef]
- Shibayama, K.; Kondo, K.; Otoguro, M. Yeast Diversity in Wine Grapes from Japanese Vineyards and Enological Traits of Indigenous Saccharomyces cerevisiae Strains. Microorganisms 2024, 12, 1769. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Otoguro, M.; Lin, Y.H.; Pan, S.F.; Ji, S.H.; Yu, C.R.; Liou, M.S.; Chang, Y.C.; Wu, H.C.; Yanagida, F. Lactococcus formosensis sp. nov., a lactic acid bacterium isolated from yan-tsai-chin (fermented broccoli stems). Int. J. Syst. Evol. Microbiol. 2014, 64, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Wang, L.T.; Liao, Y.J.; Lan, Y.S.; Chang, C.H.; Chang, Y.C.; Wu, H.C.; Lo, H.Y.; Otoguro, M.; Yanagida, F. Lactobacillus musae sp. nov., a novel lactic acid bacterium isolated from banana fruits. Int. J. Syst. Evol. Microbiol. 2017, 67, 5144–5149. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Zhang, D.; Lovitt, R.W. Performance assessment of malolactic fermenting bacteria Oenococcus oeni and Lactobacillus brevis in continuous culture. Appl. Microbiol. Biotechnol. 2006, 69, 658–664. [Google Scholar] [CrossRef]
- Bravo-Ferrada, B.M.; Tymczyszyn, E.E.; Gómez-Zavaglia, A.; Semorile, L. Effect of acclimation medium on cell viability, membrane integrity and ability to consume malic acid in synthetic wine by oenological Lactobacillus plantarum strains. J. Appl. Microbiol. 2013, 116, 360–367. [Google Scholar] [CrossRef]
- Torres-Guardado, R.; Rozès, N.; Esteve-Zarzoso, B.; Reguant, C.; Bordons, A. Influence of succinic acid on Oenococcus oeni and malolactic fermentation. Oeno One 2022, 56, 195–204. [Google Scholar] [CrossRef]
- Dokoozlian, N.K. Grape berry growth and development. In Raisin Production Manual; Christensen, L.P., Ed.; Agriculture and Natural Resources at University of California: Davis, CA, USA, 2000; pp. 30–37. [Google Scholar]
- Betteridge, A.L.; Sumby, K.M.; Sundstrom, J.F.; Grbin, P.R.; Jiranek, V. Application of directed evolution to develop ethanol tolerant Oenococcus oeni for more efficient malolactic fermentation. Appl. Microbiol. Biotechnol. 2018, 102, 921–932. [Google Scholar] [CrossRef]
- Shimazu, Y.; Uehara, M.; Watanabe, M. Transformation of citric acid to acetic acid, acetoin and diacetyl by wine making lactic acid bacteria. Agric. Biol. Chem. 1985, 49, 2147–2157. [Google Scholar] [CrossRef]
- Wibowo, D.; Eschenbruch, R.; Davis, C.R.; Fleet, G.H.; Lee, T.H. Occurrence and growth of lactic acid bacteria in wine. Am. J. Enol. Viticult. 1985, 36, 302–313. [Google Scholar] [CrossRef]
- Werner, M.; Rauhut, D.; Cottereau, P. Yeasts and natural production of sulphites. Internet J. Enol. Vitic. 2009, 12, 1–5. [Google Scholar]
- Arnink, K.; Henick-Klin, T. Influence of Saccharomyces cerevisiae and Oenococcus oeni strains on successful malolactic conversion in wine. Am. J. Enol. Vitic. 2005, 56, 228–237. [Google Scholar] [CrossRef]
- Jiang, J.; Sumby, K.M.; Sundstrom, J.F.; Grbin, P.R.; Jiranek, V. Directed evolution of Oenococcus oeni strains for more efficient malolactic fermentation in a multi-stressor wine environment. Food Microbiol. 2018, 73, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Franquès, J.; Araque, I.; Palahí, E.; Portillo, M.D.C.; Reguant, C.; Bordons, A. Presence of Oenococcus oeni and other lactic acid bacteria in grapes and wines from Priorat (Catalonia, Spain). LWT Food Sci. Technol. 2017, 81, 326–334. [Google Scholar] [CrossRef]
- Sumby, K.M.; Bartle, L.; Grbin, P.R.; Jiranek, V. Measures to improve wine malolactic fermentation. Appl. Microbiol. Biotechnol. 2019, 103, 2033–2051. [Google Scholar] [CrossRef]
- Cibrario, A.; Peanne, C.; Lailheugue, M.; Campbell-Sills, H.; Dols-Lafargue, M. Carbohydrate metabolism in Oenococcus oeni: A genomic insight. BMC Genom. 2016, 17, 984. [Google Scholar] [CrossRef]
- Da Silveira, M.G.; Golovina, E.A.; Hoekstra, F.A.; Rombouts, F.M.; Abee, T. Membrane fluidity adjustments in ethanol-stressed Oenococcus oeni Cells. Appl. Environ. Microbiol. 2003, 69, 5826–5832. [Google Scholar] [CrossRef]
- Grandvalet, C.; Assad-García, J.S.; Chu-Ky, S.; Tollot, M.; Guzzo, J.; Gresti, J.; Tourdot-Marechal, R. Changes in membrane lipid composition in ethanol- and acid-adapted Oenococcus oeni cells: Characterization of the cfa gene by heterologous complementation. Microbiology 2008, 154, 2611–2619. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, J. Stress Responses of Oenococcus oeni. In Stress Responses of Lactic Acid Bacteria; Tsakalidou, E., Papadimitriou, K., Eds.; Food Microbiology and Food Safety; Springer: Boston, MA, USA, 2011. [Google Scholar] [CrossRef]
- Onda, T.; Kojima, M.; Nganuma, K. Technical Improvements of Malo-lactic Fermentation for the Production of Sparkling Wine from Wines with High Acidity. J Brew. Soc. Jpn. 2019, 114, 281–286. [Google Scholar] [CrossRef]
- Onda, T.; Kojima, M.; Nakayama, T. Changes in chemical and physical components during sparkling wine making using a traditional in-bottle secondary fermentation method. J. Brew. Soc. Jpn. 2017, 112, 836–848. [Google Scholar] [CrossRef]
- Yang, L.; Xia Zhu, X.; Mao, Y.; Zhang, X.; Xu, B.; Yang, X. Effect of different inoculation strategies of mixed culture Saccharomyces cerevisiae/Oenococcus oeni on the aroma quality of Chardonnay wine. Food Res. Int. 2024, 190, 114636. [Google Scholar] [CrossRef]
- Martineau, B.; Acree, T.E.; Henick-Kling, T. Effect of wine type on threshold for diacetyl. Food Res. Int. 1995, 28, 139–143. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [PubMed]

| Vintage Year | Sample Name | MLF * | Malic Acid | Lactic Acid | Citric Acid | Acetic Acid | Tartaric Acid | Succinic Acid |
|---|---|---|---|---|---|---|---|---|
| (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | |||
| 2017 | Koshu wine A (2017-A) | + | 1.62 | 0.60 | 0.16 | 0.38 | 2.56 | 0.78 |
| 2017 | Koshu wine B (2017-B) | + | 0.31 | 1.58 | 0.09 | 0.44 | 2.47 | 0.78 |
| 2017 | Koshu wine C (2017-C) | −− | 2.12 | 0.15 | 0.17 | 0.26 | 2.65 | 0.87 |
| 2018 | Koshu wine A (2018-A) | ++ | 0.22 | 1.27 | nd | 0.31 | 2.33 | 0.67 |
| 2018 | Koshu wine B (2018-B) | ++ | nd | 1.35 | nd | 0.23 | 2.45 | 0.71 |
| 2018 | Koshu wine C (2018-C) | −− | 1.72 | nd | 0.15 | 0.19 | 2.07 | 0.7 |
| Vintage Year | Sample Name * | No. of Isolates | Identification |
|---|---|---|---|
| 2017 | 2017-A | 20 | O. oeni 20 strains |
| 2017 | 2017-B | 0 | - |
| 2017 | 2017-C | 0 | - |
| 2018 | 2018-A | 22 | O. oeni 22 strains |
| 2018 | 2018-B | 18 | O. oeni 18 strains |
| 2018 | 2018-C | 0 | - |
| 2019 (Koshu grape) | 2019-GR | 5 | O. oeni 4 strains L. hilgardii 1 strain |
| Total | 65 | O. oeni 64 strains, | |
| L. hilgardii 1 strain |
| Strains | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sugars | ML3 | MLa20 | MLb3 | MLb5 | MLb6 | MLb11 | MLb14 | GR4 |
| Glycerol | - | - | - | - | - | - | - | - |
| Erythritol | - | - | - | - | - | - | - | - |
| D-arabinose | - | - | - | - | - | - | - | - |
| L-arabinose | - | + | + | + | + | + | + | + |
| D-ribose | - | - | - | - | - | - | - | + |
| D-xylose | - | - | - | - | - | - | - | - |
| L-xylose | - | - | - | - | - | - | - | - |
| Adonitol | - | - | - | - | - | - | - | - |
| Methyl-β D-xylopyranoside | - | - | - | - | - | - | - | - |
| D-Galactose | - | - | - | - | - | - | - | - |
| D-Glucose | - | - | - | - | - | - | - | - |
| D-Fructose | + | + | + | + | + | + | + | + |
| D-mannose | - | - | - | - | - | - | - | - |
| L-sorbose | - | - | - | - | - | - | - | - |
| L-rhamnose | - | - | - | - | - | - | - | - |
| Dulcitol | - | - | - | - | - | - | - | - |
| Inositol | - | - | - | - | - | - | - | - |
| D-Mannitol | - | - | - | - | - | - | - | - |
| D-sorbitol | - | - | - | - | - | - | - | - |
| Methyl-α D-mannopyranoside | - | - | - | - | - | - | - | - |
| Methyl-α D-glucopyranoside | - | - | - | - | - | - | - | - |
| N-acetyl glucosamine | - | - | - | - | - | - | - | - |
| Amygdalin | - | - | - | - | - | - | - | - |
| Arbutin | - | - | - | - | - | - | - | - |
| Esculin | + | + | + | + | + | + | + | + |
| Salicin | - | - | - | - | - | - | - | - |
| D-cellobiose | - | - | - | - | - | - | - | - |
| D-maltose | - | - | - | - | - | - | - | - |
| D-lactose | - | - | - | - | - | - | - | - |
| D-melibiose | - | - | - | - | - | - | - | - |
| D-sucrose | - | - | - | - | - | - | - | - |
| D-trehalose | - | - | - | - | - | - | - | - |
| Inulin | - | - | - | - | - | - | - | - |
| D-melezitose | - | - | - | - | - | - | - | - |
| D-raffinose | - | - | - | - | - | - | - | - |
| Starch | - | - | - | - | - | - | - | - |
| Glycogen | - | - | - | - | - | - | - | - |
| Xylitol | - | - | - | - | - | - | - | - |
| Gentiobiose | - | - | - | - | - | - | - | - |
| D-turanose | - | - | - | - | - | - | - | - |
| D-lyxose | - | - | - | - | - | - | - | - |
| D-tagatose | - | - | - | - | - | - | - | - |
| D-fucose | - | - | - | - | - | - | - | - |
| L-fucose | - | - | - | - | - | - | - | - |
| D-arabitol | - | - | - | - | - | - | - | - |
| L-arabitol | - | - | - | - | - | - | - | - |
| Gluconate | - | - | - | - | - | - | - | - |
| 2-keto-gluconate | - | - | - | - | - | - | - | - |
| 5-keto-guluconate | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otoguro, M.; Inui, S.; Aoyanagi, T.; Misawa, A.; Nakano, H.; Shimazu, Y.; Misawa, S. Physiological, Genetic, and Fermentative Traits of Oenococcus oeni Isolates from Spontaneous Malolactic Fermentation in Koshu Wine. Fermentation 2025, 11, 440. https://doi.org/10.3390/fermentation11080440
Otoguro M, Inui S, Aoyanagi T, Misawa A, Nakano H, Shimazu Y, Misawa S. Physiological, Genetic, and Fermentative Traits of Oenococcus oeni Isolates from Spontaneous Malolactic Fermentation in Koshu Wine. Fermentation. 2025; 11(8):440. https://doi.org/10.3390/fermentation11080440
Chicago/Turabian StyleOtoguro, Misa, Sayaka Inui, Taichi Aoyanagi, Ayana Misawa, Hiromi Nakano, Yoshimi Shimazu, and Shigekazu Misawa. 2025. "Physiological, Genetic, and Fermentative Traits of Oenococcus oeni Isolates from Spontaneous Malolactic Fermentation in Koshu Wine" Fermentation 11, no. 8: 440. https://doi.org/10.3390/fermentation11080440
APA StyleOtoguro, M., Inui, S., Aoyanagi, T., Misawa, A., Nakano, H., Shimazu, Y., & Misawa, S. (2025). Physiological, Genetic, and Fermentative Traits of Oenococcus oeni Isolates from Spontaneous Malolactic Fermentation in Koshu Wine. Fermentation, 11(8), 440. https://doi.org/10.3390/fermentation11080440






