Abstract
Bacterial nanocellulose (BNC) is a highly pure biopolymer with promising applications in the biomedical, food, and textile industries. However, the high production costs and low yields obtained in static conditions limit its scalability and industrial applications. This study addresses the sustainable production of BNC using a rotary disk bioreactor (RDB) and explores the use of grape pomace extract as an alternative carbon source for BNC production. Parameters such as the BNC production and biomass yield were evaluated using Komagataeibacter xylinus ATCC 53524 under different operational conditions (disk surface, rotation speed, and number of disks). The results showed that cellulose production increased using silicone-coated disks at 7–9 rpm (up to 2.72 g L−1), while higher yields (5.23 g L−1) were achieved when using grape pomace extract as the culture medium in comparison with conventional HS medium. FTIR and TGA characterizations confirmed that BNC obtained with grape pomace extract presents the same thermal and chemical characteristics than BNC produced with HS medium. This work provides insight into the feasibility of upscaling BNC production using a bioprocessing strategy, combining production in the RDB system and the use of an agro-industrial waste as a sustainable and cost-effective alternative.
1. Introduction
BNC is a linear biopolymer composed of β-1,4-glucopyranose units, which is produced in the form of laminar nanofibrils [1]. This material possesses outstanding properties, such as high purity (free of hemicellulose, lignin, and other impurities), high porosity, hydrophilic nature, mechanical strength, biocompatibility, crystallinity, high water absorption capacity, and flexibility to be molded into any shape with a nanofibrous morphology [2,3,4]. In addition, BNC has been classified as having Generally Recognized as Safe (GRAS) status by the FDA [5], generating increasing interest across a wide range of applications in the industrial, biomedical, food, cosmetic, and textile sectors.
However, the low yield and high production costs of BNC hinder its large-scale industrial implementation [2,6]. Most studies have focused on static culture methods, while the use of more sophisticated bioreactor designs remains underexplored [7,8]. Compared to conventional static culture, bioreactors offer the possibility to improve the mass transfer and mixing efficiency, enabling precise control of the physiological and nutritional parameters for microbial growth [1,4,9].
BNC synthesis can be improved by controlling parameters such as the pH and temperature and ensuring optimal oxygen transfer during fermentation [10]. Furthermore, oxygen mass transfer is often the limiting factor in aerobic processes in BNC production [2]. In recent years, rotary disk bioreactors (RDBs) have been suggested as a suitable strategy to enhance BNC productivity and improve the scalability of the process [11]. These systems consist of a series of disks mounted on a central horizontal axis, which are alternately exposed to the culture medium and the air phase, reducing the shear stress on bacterial cells and facilitating oxygen supply [11]. Variables such as the disk rotation speed, disk immersion depth, surface roughness, material type, inter-disk distance, biofilm characteristics, and dissolved oxygen (DO) levels influence BNC productivity [1,9,12]. Compared to other bioreactors, RDBs provide turbulence for better mixing and an increased surface area for improved air–biofilm contact. These benefits, along with their low energy consumption, maintenance cost, and compact design, make RDBs a promising and eco-friendly alternative for BNC production [4].
Herein, we designed and constructed a laboratory-scale RDB to investigate the effect of operational parameters on the BNC yield and obtain the necessary data for process scale-up (Figure 1 shows the research workflow of this study). The effects of the disk rotation speed and cultivation time were investigated in order to optimize the bioreactor performance. In addition, the BNC productivity was compared using a defined HS1717 medium and grape pomace extract, which is an agro-industrial residue from winemaking that is particularly abundant in Chile. Grape pomace is a nutrient-rich carbon source containing polyphenols and vitamins, offering a cost-effective alternative to pure glucose for reducing the scaling-up production costs through a sustainable bioprocess strategy. This study makes a substantial contribution to the advancement of knowledge at the intersection of bioprocess design and industrial waste valorization, providing insights into the feasibility of an integrated strategy for scaling-up BNC production.
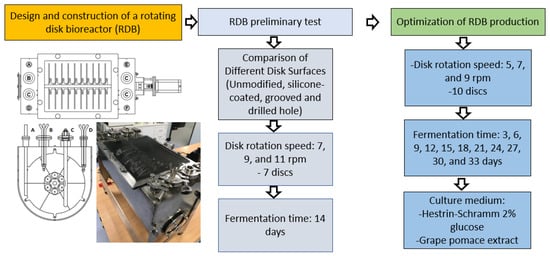
Figure 1.
Research work flow describing the design and optimization of the RDB-based bioprocess strategy.
2. Materials and Methods
2.1. Chemicals
D-glucose, sodium citrate 2-hydrate, citric acid 1-hydrate, NaOH, and HCl were supplied by Winkler Ltd., Santiago, Chile. Gibco Ltd., Miami, FL, USA, provided peptone and yeast extract. Di-sodium hydrogen phosphate (Na2HPO4) and sodium potassium tartrate were supplied by Merck KGaA., Darmstadt, Germany. Sigma-Aldrich Co., St. Louis, MO, USA provided cellulase, enzyme blend SAE0020 (≥1000 U g−1) and 3,5-dinitrosalicylic acid.
2.2. Strain and Hestrin–Schramm S1717 Medium Preparation
All the cultures were performed using the strain K. xylinus ATCC 53524. Sterile Hestrin–Schramm (HS) 1717 medium [13] was prepared with 2% glucose (added after autoclaving the medium using a 0.22 µm syringe filter), 0.5% peptone, 0.5% yeast extract, 0.27% Na2HPO4, and 0.115% citric acid, the pH was adjusted to 5 with NaOH and HCl 0.1 N, and finally, autoclaved at 121 °C for 20 min.
2.3. Preparation of the Inoculum
First, 5–10 mL of liquid culture was prepared from a colony streaked from an HS1717 agar plate, previously inoculated from a cryotube stored at −80 °C, and incubated for three days at 30 °C and 150 rpm. After incubation, the resulting cell suspension was used as inoculum or pre-inoculum. RDB inoculation was performed using 1 L of inoculum, which was prepared with 10 mL of pre-inoculum and 1 L of HS1717 medium and cultivated for three days at 150 rpm and 30 °C.
2.4. Fermentation in Static Culture
The fermentation kinetics of the BNC-producing strain in a static culture were determined in Petri dishes (Ø 90 mm) using 40 mL of HS1717 liquid medium and inoculated with 0.04 mL of the previously prepared cell suspension. The cultures were incubated for 10 days at 30 °C (optimal growth temperature) under static conditions and in triplicate. Every day, the cellulose was harvested and quantified from the Petri dish culture (in duplicates), and the pH and reducing sugars were measured from the liquid phase. In addition, the biomass was quantified from Petri dishes at the same time intervals.
2.5. Cellulose and Biomass Quantification
The cellulose pellicle was harvested and treated with 0.1 M NaOH for 3 h at 80 °C, then centrifuged, washed three times with distilled water and dried at 50 °C until a constant weight. For the biomass quantification, the pellicle-included culture broth was centrifuged to obtain a pellet. This pellet was subsequently treated with 0.5 mL of cellulase and 19.5 mL of 0.1 M citrate buffer (pH 5.5), and finally, incubated at 50 °C for 1 h. Following incubation, the sample was centrifuged (4500 rpm) and the resulting biomass was dried at 50 °C.
2.6. Quantification of Reducing Sugars
The glucose concentration (g L−1) in the culture broth samples was determined using the 3,5-dinitrosalicylic acid (DNS) method to quantify the total reducing sugars as previously described [14]. Briefly, 500 µL of diluted culture broth sample (HS and grape pomace; dilution 1:10–1:20) was transferred into a 15 mL test tube, followed by the addition of 500 µL of DNS reagent. The mixture was then heated at 100 °C for 5 min using a heating block (DLAB, HB-120S). After heating, 5 mL of distilled water was added to each tube. A glucose calibration curve (0.2–1.0 g L−1) was prepared using the same procedure. The absorbance was measured at 540 nm in a quartz cuvette (2 mm path length) using a UV-vis spectrophotometer (Hanon, i3).
2.7. Optimization of BNC Production in Rotary Disk Bioreactor
2.7.1. Start-Up of the RDB
The bioreactor was assembled with a variable number of disks (7 or 10) and then autoclaved at 121 °C for 15 min. Both the media and the inoculum were added to the RDB using the top door, with the help of a Bunsen burner to prevent external contamination. Finally, the motor speed was configurated through the controller and the temperature was set to 20 °C.
2.7.2. Comparison of Different Disk Surfaces
Biofilm formation was studied on four different stainless steel disks (unmodified, silicone-coated, grooved, and drilled hole surfaces) to select the best adhesive conditions for a 7-day RDB fermentation. The bioreactor was filled with 9 L of sterile HS1717 medium and 1 L of the inoculum was added. The cellulose present on the disks was quantified by the dry weight after purification with 0.1 M NaOH, as previously described in Section 2.5.
2.7.3. Comparing RDB Operating Parameters
The cellulose pellicle growing over the RDB disks was compared between fermentations carried out during 14 days (using 7 disks) and 33 days (using 10 disks). Samples were collected every 2 (14-day fermentation) and 3 (33-day fermentation) days for cellulose quantification (in one disk), glucose quantification (reducing sugars) and pH measurements to assess the impact of the bioreactor rotation speed (rpm). The first set of fermentations (14 days and 7 disks) were performed at 7, 9, and 10 rpm, while the second set of fermentations (33 days and 10 disks) were performed at 5, 7, and 9 rpm (5 rpm was considered due to the results obtained in the first set of experiments). The bioreactor has an internal volume of 25 L with a variable number of disks (Ø 20 cm). Each experiment was performed with 9 L of HS1717 medium and inoculated with 1 L of the previously prepared cell suspension, submerging 46.5% of the disk. The RDB was equipped with a rotating motor (model 57HS112-3004A08-D21), which had a torque of 3 Nm and a current of 3.0 A, externally controlled via software.
2.8. Grape Pomace Extracts
The grape pomace used in this study consisted of the Pinot Noir and Moscatel varieties, obtained from a local winery located in the Guarilihue area, Itata Valley, Biobío Region, Chile. The pomace was oven-dried at 65 °C for 48 h and then ground using an ultracentrifuge mill ZM 300 equipped with a 1 mm sieve. The ground grape pomace was mixed with deionized water at a ratio of 1:10 (w/v) and incubated for 2 h at 40 °C with constant stirring at 150 rpm. A 1:1 mixture of dried Pinot Noir pomace and Moscatel pomace was used for the extractions intended for the RDB experiments. Individual varieties were used for the extraction used in the comparison experiments concerning BNC production using static culture conditions. After extraction, the mixtures were filtered through gauze to retain the coarse particles. The reducing sugars were quantified in the final extract and expressed in g L−1 of glucose.
2.9. Comparison Between Rotary Disk Bioreactor and Static Culture Using Grape Pomace
BNC production in the RDB was performed using grape pomace extracts as a substitute for glucose (carbon source). To make the bioreactor design comparable, 10 L of grape pomace extract was prepared as described in the previous section and supplemented with the same nutrient concentrations as the HS1717 medium, excluding glucose. The RDB was operated at 7 rpm and 20 °C (maximum capability of the system). Samples were taken every 2 days during 14 days to quantify the cellulose production. Similarly, the BNC production in static culture was evaluated using 40 mL of culture media prepared with grape pomace extract of each variety and supplemented with the nutrients of the HS1717 medium (without glucose). Fermentation in static culture was carried out during 7 days at 30 °C (optimal growth temperature). The BNC yields from the static and RDB fermentations using supplemented grape pomace extracts were compared with that obtained using HS1717 medium and glucose as a carbon source.
2.10. Determination of Productive Yields
The productive yields were calculated using the following equations, considering the total volume of the culture in the Petri dish (40 mL) or RDB (10 L):
where:
Yp/s = (△P)/(−△S)
Yp/x = (△P)/(△X)
YX/S = (△X)/(−△S)
Q = MP/(T × V)
- Yp/s: Product yield coefficient based on substrate
- Yp/x: Product yield coefficient based on biomass
- Yx/s: Cell yield coefficient based on substrate
- Q: Volumetric productivity (Q)
- ΔP: Mass of BNC produced (g)
- ΔS: Mass of substrate consumed (g)
- ΔX: Biomass generated (g)
- MP: Mass of BNC produced (mg)
- T: Cultivation time (days)
- V: Fermentation volume (L)
The specific growth rate was determined using the Monod equation [15], which describes the relationship between the specific growth rate and the limiting substrate concentration (Equation (5)).
where µMax is the maximum specific growth rate, S is the concentration of the limiting substrate, and Ks is the saturation constant at which µ = µMax/2 (h−1).
µ = (µMax S)/(Ks + S)
2.11. Characterization of the BNC Pellicles
The morphological characteristics of the BNC pellicles obtained with the HS and supplemented grape pomace extracts were examined using a scanning electron microscope (VEGA3 EasyProbe, Tescan, Brno, Czech Republic). Prior to analysis, samples were dried by critical point drying and sputter-coated with gold. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) spectroscopy (FT/IR-4600LE, Jasco, Tokyo, Japan) was used to identify the functional groups present in the BNC pellicles. The thermal behavior was evaluated by thermogravimetric analysis (TGA) using an STA 6000 instrument (Perkin Elmer, Shelton, CT, USA) over a temperature range of 25–600 °C, with a heating rate of 10 °C/min under a nitrogen atmosphere, as previously described in [16].
3. Results and Discussion
3.1. Fermentation in Static Culture
The BNC production and kinetic parameters were investigated using K. xylinus ATCC 53524 under static cultivation conditions. Slight change in the pH of the medium were measured, which decreased from 5.0 to 4.6 after 10 days of culture (Figure 2). Cellulose production reached 5.8 g L−1 at day 4, and it remained steady until day 10 without statistic differences in this period (p-value < 0.0001). Glucose, on the other hand, gradually decreased from 2% (in day 0) to 0.32% (in day 10). Biomass exhibited exponential growth until day 5 (0.8 g L−1) and then increased gradually to reach a maximum of 1.2 g L−1 on day 9, remaining stable until day 10 (1.15 g L−1). A maximum specific growth rate (µmax) of 0.6697 d−1 was calculated (equivalent to 0.0279 h−1) from the analysis of the cellular growth kinetics, with the following yield coefficients: Yp/s = 0.845 (g BNC g glucose −1), Yx/s = 0.118 (g biomass g glucose−1), and Yp/x = 7.147 (g BNC g biomass−1). The specific growth rate dropped to nearly a half of the value (0.3107 d−1) when the experiment was carried out at a lower temperature (20 °C instead of 30 °C), also achieving lower BNC production (1.3 g L−1, 7th day). Unlike other studies, BNC production under these experimental conditions proved to be favorable [17,18,19,20]. For example, Lestari et al. [17] reported significantly lower values of µ (0.0132 and 0.0082 h−1) and yields (Yp/x of 2.235 and 2.452 g BNC g biomass−1) after 12 days of culture than obtained in this study (7.147 g BNC g biomass−1) in fermentations with K. xylinus using coconut water and pineapple juice, respectively. Although the Yp/s values were higher with coconut water (3.612 g BNC g glucose−1), the efficiency per unit of biomass was more favorable under our conditions. Furthermore, Avirasdya et al. [18] evaluated the impact of different carbon sources using the strain Komagataeibacter xylinus FNCC 0001 and found that fructose resulted in the highest values of µ (0.1141 h−1) as well as the highest product yield values for Yp/s (0.317 g BNC g fructose−1) and Yp/x (0.5927 g BNC g biomass−1) [18]. In addition, glucose achieved the highest cell yield (Yx/s of 0.0983 g biomass g glucose−1) and the highest final cellulose production (5.83 g L−1), followed by fructose (4.91 g L−1).
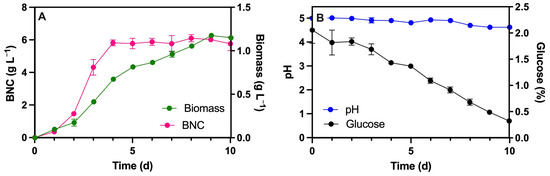
Figure 2.
Static culture of the cellulose-producing strain K. xylinus ATCC 53524 incubated at 30 °C using HS1717 medium at pH 5.0. BNC production and biomass were quantified as dry weight (A), along with pH variation and glucose consumption (B).
3.2. Rotary Disk Bioreactor Design
The bioreactor consists of a semi-cylindrical stainless steel vessel with dimensions L = 500 mm, H = 240 mm and W = 241 mm (Figure 3(1,5)). Additionally, a round side window with a diameter of 45 mm is incorporated for visualizing the liquid phase of the fermentation (Figure 3(2)). These dimensions allow for a maximum volume of 10 L of liquid phase. Internally, the system accommodates up to 10 removable stainless steel disks, which are mounted vertically on a rotating horizontal stainless steel axis measuring 458 mm in length and 13 mm in diameter. Each disk consists of six magnetized segments, each 3 mm thick, supported by magnetic rotating masses (Figure 3(3,4)). These dimensions were chosen to optimize the effective surface area of each disk. An immersion zone of 46.5% of the effective surface area in the culture medium was designed, based on results reported by Soleimani et al. [1]. Given this, the RDB system cyclically exposes the disks to both the aerobic phase and the nutrient medium. According to Serafica et al. [21], limiting the air exposure of the disk surface to less than 50% can enhance bacterial growth and prevent damage to the rotary stainless steel horizontal axis [21].
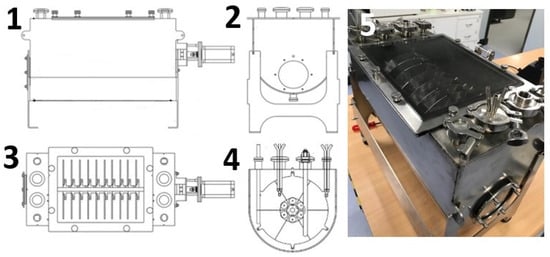
Figure 3.
(1): Front view of the RDB. A: Stainless steel tank; and B: stainless steel support. (2): Side view of the RDB. A: Left side window. (3): Top view of the RDB showing the locations of the measurement and inoculation components. (4): Internal left-side view highlighting the sensor and inoculation ports. (5): Photograph of the fully assembled RDB.
3.3. Optimization of BNC Production in Rotary Disk Bioreactor
3.3.1. Comparison of Different Disk Surfaces
The RDB system used in this study comprised circular disks with a diameter of 20 cm, each divided into six equal arrowhead-shaped sections. This study evaluated the influence of disk surface characteristics on BNC production, with a particular emphasis on biopolymer adhesion to the various disk surfaces. The results indicate that the silicone-coated disk significantly enhanced the BNC formation (0.479 g), likely due to its increased surface roughness, effective surface area, and hydrophobicity [22]. Figure 4 compares the BNC production across different disk surface modifications: unmodified (0.229 g), grooved (0.319 g), drilled hole (0.450 g), and silicone-coated. Images of the BNC pellicles adhered to each surface are also included (Figure 4). These findings are consistent with those reported by Jagtap and Dastager [2], who evaluated BNC production using disks made of wood, polypropylene, and smooth stainless steel. The authors observed that the rougher material (wood) promoted greater cell adhesion and, consequently, higher BNC yields compared to smooth stainless steel. Similarly, Sharma et al. [4] compared stainless steel mesh disks (mesh size 16) with drilled hole stainless steel disks (4 mm holes arranged in eight rows). They concluded that the metal mesh yielded the highest BNC production, whereas the drilled disks exhibited negligible adhesion. Pilafidis et al. [23] reported a significant increase in cellulose adhesion when using roughened (sanded) polycarbonate disks compared to smooth ones [23]. These findings confirm that the surface porosity and roughness are key parameters that favor the growth of K. xylinus and the attachment of the biopolymer within the system. The improved performance observed with etched disks suggests that surface roughness facilitates bacterial adhesion, which is a key initial step in biofilm formation and subsequent cellulose synthesis. Smooth surfaces may hinder this process due to the lower surface energy and limited anchoring points, leading to uneven BNC distribution or detachment during rotation. In contrast, a roughened surface increases the contact area and provides microtopographical features that promote stable bacterial colonization.
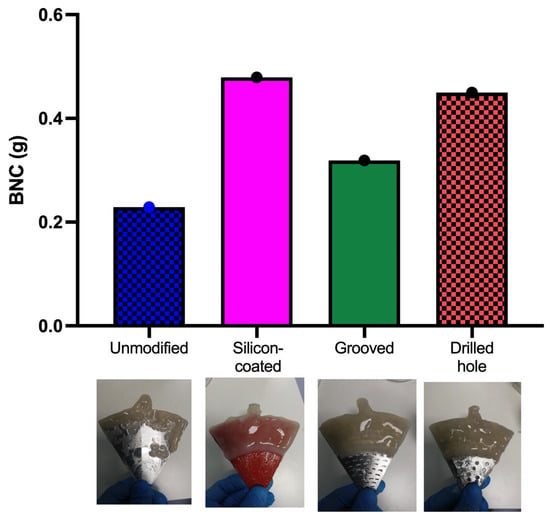
Figure 4.
Cellulose production obtained in the RDB after 7 days of cultivation using different disk surfaces (unmodified, silicone-coated, grooved, and drilled hole).
3.3.2. Comparison of Different Disk Rotation Speeds
The results obtained after 12 days of fermentation show that the rotation speed of the disks is a relevant factor influencing the surface production of BNC (Figure 5). In Experiment A, where the rotation speed was evaluated with seven disks, higher production was reached at 7 and 9 rpm (60.9 and 52.4 g m−2, respectively), but it drastically decreased at 11 rpm (23.5 g m−2). In Experiment B, conducted with 10 disks, a similar trend was observed. Production progressively decreased as the rotation speed increased, reaching a peak at 5 rpm (25.5 g m−2) and dropping to a minimum at 9 rpm (8.5 g m−2). Low to moderate speeds (5–9 rpm) favor the development of biofilms on the surface of the disks, while higher speeds (11 rpm) can generate excessive shear forces that hinder cellulose adhesion and/or remove already formed cellulose, as previously observed in moderate agitation systems [11]. Furthermore, a comparison between the two configurations (7 and 10 disks) reveals that increasing the number of disks does not necessarily improve the individual or total surface production. Thus, we can obtain 1.9 g L−1 (7 disks with spaced 18 mm apart) and 0.75 g L−1 (10 disks spaced 13 mm apart) at 12 days of fermentation by extrapolating the yields to the total number of disks. In fact, increasing the number of disks may lead to a decrease in the radial flow density, which is defined as the volumetric flow rate of culture medium or oxygen per unit of effective surface area in the radial direction (i.e., from the center of the reactor toward the periphery), as well as in oxygen availability per unit of effective surface area. This decline may negatively impact BNC synthesis. Decreasing the disk spacing from 18 mm to 13 mm likely led to the observed reduction in the radial flow density by restricting fluid circulation and thereby diminishing the oxygen transfer efficiency. Furthermore, upstream disks may partially obstruct the flow, limiting both nutrient and oxygen availability to downstream surfaces. Since Komagataeibacter spp. are obligate aerobes, insufficient oxygen supply per unit area can directly impair metabolic activity and BNC synthesis.
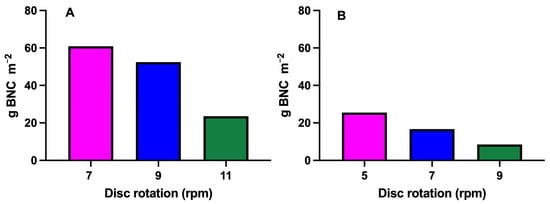
Figure 5.
Comparison of BNC production using different disk rotation speeds (rpm) and numbers of disks. The y-axis shows the density of cellulose production in grams per effective surface area of a disk (g m−2), while the x-axis indicates the rpm speed used in the RDB. These results were obtained after 12 days of fermentation, using 7 disks (A) and 10 disks (B).
This phenomenon has been well-described in studies of biofilm systems, where increased competition for nutrients and oxygen can constrain the yield per unit area when the total available surface area is excessively increased [24,25]. Previous studies have identified similar optimal rotation speeds. For example, Zahan et al. [26] found that a speed of 7 rpm resulted in the highest microbial cellulose production using liquid pineapple waste as a substrate [9]. Likewise, Sharma et al. [4] reported that speeds above 10 rpm decreased BNC production due to destabilization of the biofilm [27]. Our results confirm that the design of the RDB must consider a balance between maximizing the effective surface area and maintaining optimal hydrodynamic conditions [28]. In this regard, both excessive agitation and surface overload can adversely affect BNC biosynthesis. In addition to hydrodynamic limitations, the initial distribution of the bacterial inoculum across the available surface area also plays a critical role. Thus, when the same inoculum volume is distributed over an increasing number of disks (7 vs. 10), the number of bacterial cells available per unit area decreases (Figure 5). This dilution effect may delay biofilm formation, reduce local cell density, and ultimately, impair the production kinetics and yield of BNC. Consequently, increasing the number of disks without a proportional increase in inoculum likely resulted in a detrimental effect over colonization of the disks’ surfaces.
3.3.3. Kinetics of BNC Production in a Rotary Disk Bioreactor
Figure 6 illustrates the dry weight of BNC (in g L−1) produced in a 10 L RDB at different rotation speeds (7, 9, and 11 rpm). The graph shows that the highest production (2.7 g L−1) was achieved after 14 days at a rotation speed of 9 rpm. However, a production of 2.2 g L−1 was achieved at a lower speed of 7 rpm by day 14 of cultivation. The production decreased further (to 1.00 g L−1) at 11 rpm, with a concomitant decrease in the pH to 3.6 by day 14 of cultivation. Regarding glucose, the most significant decrease was observed at 11 rpm, with the levels dropping to 8.0 g L−1 by day 14. On the final day of fermentation, the glucose levels measured 12.6 g L−1 at 9 rpm and 18.8 g L−1 at 7 rpm.
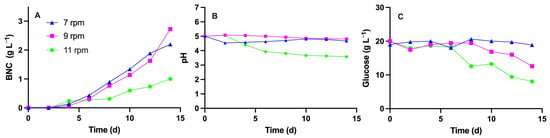
Figure 6.
BNC production (A), pH profile (B) and glucose consumption (C) of the BNC-producing strain K. xylinus ATCC 53524 after 14 days of cultivation in the RDB. All the tests were conducted at 7, 9 and 11 rpm.
Since the stationary phase was not reached during the 14-day fermentation, the cultivation period was extended to 33 days, with samples collected every 3 days. A lower rotation speed (5 rpm) was tested in this experiment, as previous experiments showed that speeds above 9 rpm are associated with reduced BNC production. The highest BNC production was achieved at 33 days with 7 rpm (5.9 g L−1), reaching 2.5 g L−1 at 5 rpm and 1.8 g L−1 at 9 rpm (Figure 7). Additionally, a decrease in pH was observed across all three conditions, dropping from an initial value of 5.0 to an average of 3.7 by day 33 of fermentation. Regarding glucose consumption, the greatest reduction was observed at 5 rpm, reaching 1.4 g L−1 on day 27. In contrast, the final glucose levels for the 7 and 9 rpm conditions were 6.8 g L−1 and 7.7 g L−1, respectively.
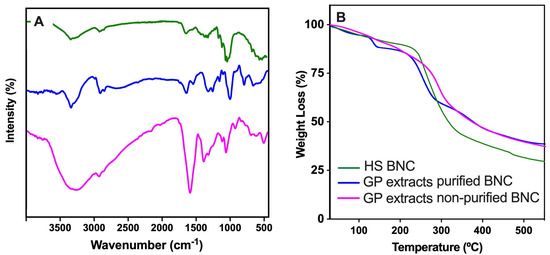
Figure 7.
BNC production in g L−1 (A), pH profile (B) and glucose consumption in g L−1 of the BNC-producing strain K. xylinus ATCC 53524 after 33 days of cultivation in the RDB at different speeds (5, 7, and 9 rpm).
The results demonstrate that a rotation speed of 7 rpm over 33 days of fermentation in the RDB produced the highest yield of BNC (5.9 g L−1), significantly surpassing the productivities obtained at 5 rpm (2.5 g L−1) and 9 rpm (1.8 g L−1). These findings are consistent with previous studies [29], which highlight the importance of moderate agitation to optimize BNC production. For example, Zahan et al. [9] found that a speed of 7 rpm during a 4-day fermentation resulted in optimal BNC production in a 10 L RDB [9]. The pH decrease observed on day 33 is consistent with the previous literature [29,30], which reports that organic acid production during fermentation can lower the medium’s pH and subsequently influence BNC synthesis. In this regard, the lower production observed at 9 rpm might be related to pH-dependent inhibition of enzymatic activities that are essential for cellulose biosynthesis. In particular, the greater level of glucose consumption observed at 5 rpm may be a consequence of reduced oxygen availability. Accordingly, a more anaerobic fermentation environment can lead to increased conversion of glucose into by-products rather than BNC [31,32].
Furthermore, studies have shown that higher agitation speeds can cause cell shearing and damage to the BNC structure [11], thereby compromising both its quality and the production yield. Therefore, a moderate rotation speed, such as 7 rpm, is expected to optimize BNC production by providing a balance between oxygen availability and the structural integrity of the material [9,29]. These results were compared with those reported in the literature for BNC production using different configurations and RDB conditions (Table 1). Although our maximum production of 5.9 g L−1 after 33 days at 7 rpm in an RDB falls within the range reported in previous studies, the productivity of 0.18 g L−1 d−1 (at 20 °C) was lower compared to other research that employed shorter cultivation periods and higher temperatures (typically 28–30 °C). However, we anticipate that operating the RDB at temperatures above 28–30 °C will significantly enhance the cellulose yield (in 4.5-fold) due to higher rates of cellulose biosynthesis and the well-described shortening of the lag phase and production time of the fermentation [33,34]. For instance, Sharma et al. [4] reported a 2.8-fold increase in cellulose production when cultivation was conducted at 30 °C instead of 20 °C.

Table 1.
BNC production and operational characteristics in RDB systems.
3.3.4. BNC Production in Rotary Disk Bioreactor Using Grape Pomace
The dry weight of BNC produced in static fermentation with different media is illustrated in Figure 8. BNC production of 5.8 g L−1 (day 7) was obtained using the HS1717 medium (control culture medium). Interestingly, the Pinot Noir grape pomace medium yielded 6.5 g L−1, while the Moscatel grape pomace medium resulted in the highest concentration, reaching 7.4 g L−1. This increase may be attributed to the presence of other nutrients in the supplemented grape pomace media [39,40], which promote greater bacterial biomass growth, and consequently, higher cellulose production. Grape pomace is composed of skins (~50%), seeds (~25%) and stalks (~25%), and its physicochemical composition varies among grape varieties [41]. In the case of Vitis vinifera L., reported data indicate the presence of proteins (8.5 g 100 g−1), soluble sugars (8.9 g 100 g−1 of fructose and 8.0 g 100 g−1 of glucose), lipids (8.2 g 100 g−1), fiber (46.2 g 100 g−1) and ash (4.7 g 100 g−1) [42]. The mineral profile is predominantly characterized by zinc, copper, iron, magnesium, sodium, calcium, potassium, and cobalt. Additionally, the bioactive composition includes vitamin E, vitamin C, anthocyanins, and a wide range of polyphenolic compounds [42].
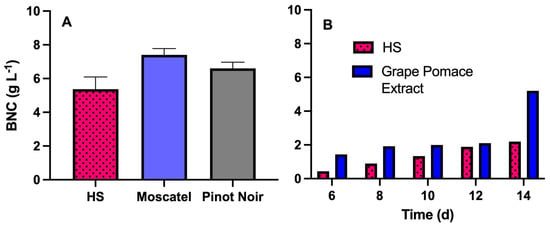
Figure 8.
Comparison of BNC production using HS and supplemented grape pomace extracts under static cultivation and RDB fermentation using K. xylinus ATCC 53524. For static cultivation (A), the production of BNC was compared between HS1717 medium and supplemented grape pomace extracts (Pinot Noir and Moscatel). For RDB fermentation (B), production of BNC was compared between HS1717 medium and supplemented grape pomace extract (1:1 Moscatel/Pinot Noir). Experiments were conducted for 14 days at 7 rpm and 20 °C, while BNC production in static culture was performed for 7 days at 30 °C. Standard deviation corresponds to three culture replicates.
Since the productivity levels in static fermentations using grape pomace extracts from the Moscatel and Pinot Noir varieties were similar, a mixture of these pomaces was used for the fermentation in the rotating disk bioreactor. The cellulose production turned out to be higher when using the grape pomace extract compared to the standard HS1717 medium during all of the fermentation days. On day 14, a BNC concentration of 2.2 g L−1 was obtained with HS medium, whereas the mixed grape pomace extract yielded a significantly higher concentration of 5.2 g L−1.
The interest in using of agro-industrial residues as cost-effective and sustainable substrates for BNC production is growing [43]. Grape pomace, a by-product of various winemaking processes, contains significant amounts of both simple sugars and polysaccharides. The simple sugar fraction can represent up to 40% of the dry weight, primarily composed of fructose and glucose [44], with glucose being an ideal precursor for BNC biosynthesis [45]. Although the sugar content can vary depending on the grape variety and vinification process, recent findings indicate that these variations do not significantly impact the concentration of reducing sugars in grape pomace hydrolysates [46]. The hydrolysates obtained in this study from grape pomace contained reducing sugar concentrations between 2.9 and 3.1 g L−1, which were adequate to sustain BNC production without requiring the addition of commercial sugars. It is worth noting that in static fermentation, grape pomace extracts have proven to be an effective culture medium, achieving BNC yields of up to 9 g L−1 after 9 days of culture [47].
The suitability of grape pomace extract as a substrate is not only attributed to its content of fermentable sugar but also to the presence of essential nutrients that support bacterial growth. For example, the presence of ions such as K+, Mg2+, and Ca2+ in grape pomace extract may enhance BNC production, as these minerals play central roles in bacterial metabolism and BNC biosynthesis [48,49]. Grape pomace is also a notable source of vitamin C (~0.2–0.3 mg g −1) [50], which has been linked to improved BNC yields [51]. For example, Andritsou et al. [51] reported 0.61 g L−1 d−1 after 11 days of fermentation using vitamin C-rich fruit waste. Furthermore, Keshk [52] demonstrated that supplementing HS medium with 0.5% vitamin C enhanced BNC production by reducing gluconic acid formation, thereby maintaining a favorable pH for bacterial activity. Pilafidis et al. [23] demonstrated improved production of BNC by 133.3% when using enzymatic hydrolysates of bread waste, additionally highlighting the potential of waste recovery in a sustainable model of BNC production [23]. Using agro-industrial waste streams not only offers an economical alternative for BNC production but also aligns with environmental sustainability goals by valorizing materials that would be otherwise discarded. Thus, a variety of waste products, such as citrus peels, sugarcane bagasse, and rice bran, have been successfully explored as substrates for microbial BNC synthesis [34].
3.4. BNC Characterization
The FTIR spectra in Figure 9A (left panel) show the functional group profiles of the BNC samples produced under different culture conditions and purification treatments. The green line corresponds to the spectrum of BNC synthesized in standard HS medium and purified with 0.1 N NaOH, which displays the characteristic peaks of cellulose. These include a broad O–H stretching vibration around 3300 cm−1, a C–H stretching band near 2900 cm−1, and prominent bands in the fingerprint region (1200–900 cm−1), particularly around 1050 cm−1, associated with C–O–C and C–O stretching vibrations of β-1,4-glycosidic linkages [16]. The blue line represents the spectrum of purified BNC produced with grape pomace extract as the culture medium. This profile is largely similar to the green spectrum, indicating that the chemical structure of cellulose is preserved despite the change in carbon source. Minor differences in the intensity and sharpness of certain peaks (notably in the region between 1600 and 1400 cm−1) may reflect slight variations in surface chemistry or residual metabolites derived from the extract. In addition, the magenta line corresponds to the spectrum of unpurified BNC produced with grape pomace extract that exhibits notable differences. While BNC-related peaks are still present, the spectrum shows additional and more intense bands in the region between 1700 and 1500 cm−1 [53]. This result is suggestive of residual proteins, lignin derivatives, and/or polyphenolic compounds commonly found in grape pomace [39,40,54]. The broader and less defined bands indicate a more heterogeneous chemical composition, consistent with the lack of purification.
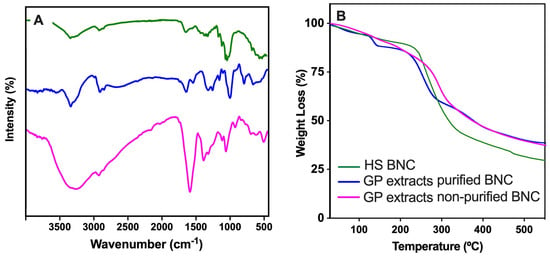
Figure 9.
ATR-FTIR spectra (A) and TGA decomposition profiles (B) of BNC produced using HS medium (green line) or grape pomace extracts: purified (blue line) and unpurified (magenta line).
The thermogravimetric analysis (TGA) profiles in Figure 9B (right panel) show the thermal decomposition behavior of the BNC samples produced under different culture media and purification conditions. All the samples display a typical two-step weight loss pattern: an initial weight loss (below 150 °C) and a main degradation stage (250–400 °C). The first stage is attributed to the evaporation of adsorbed and bound water. The three samples show a similar trend, though slight differences in the water content can be inferred from the onset temperature and the magnitude of the initial weight loss. The magenta curve (unpurified BNC) tends to show a slightly higher water loss, likely associated with its more hygroscopic and heterogeneous nature. The second stage corresponds to the thermal decomposition of cellulose and organic compounds [16]. The green (HS-derived) and blue (pomace-derived) purified samples exhibit comparable onset degradation temperatures, indicating similar thermal stability. However, the blue curve shows a slightly lower degradation onset, which may reflect compositional differences introduced due to residual particles of grape pomace extracts. In contrast, the magenta line (unpurified BNC) shows an earlier onset of degradation and a faster weight loss rate in this region, suggesting the presence of thermally less stable compounds such as proteins, polyphenols, and/or lignocellulosic residues from the pomace extract, which degrade at lower temperatures and affect the overall thermal behavior of the sample. The residual mass (above 450 °C) of the HS-derived BNC (green) is lower, likely due to the higher purity than the other BNCs.
Figure 10 presents SEM images of the nanofibrous networks corresponding to purified BNC produced using Moscatel (Figure 10A), Pinot Noir (Figure 10B) and a 1:1 mixture of both (C). All the samples exhibit an interconnected, three-dimensional network of randomly oriented nanofibers, characteristic of BNC matrices [16]. The presence of surface agglomerates scattered across the field is observed, even though all the samples were purified using same standard procedure [55]. These aggregates may correspond to residual grape pomace particles, suggesting that the conventional purification step might be insufficient when using agricultural residues and the BNC will need further purification steps depending on the intended application. These observations are consistent with the TGA and ATR-FTIR analyses. A comparison between the purified (Figure 10D–F) and unpurified (Figure 10G–I) BNC pellicles reveals that the standard purification process removes most of the coloration associated with the pomace extracts, while the remains are observed only at a nanometric scale.
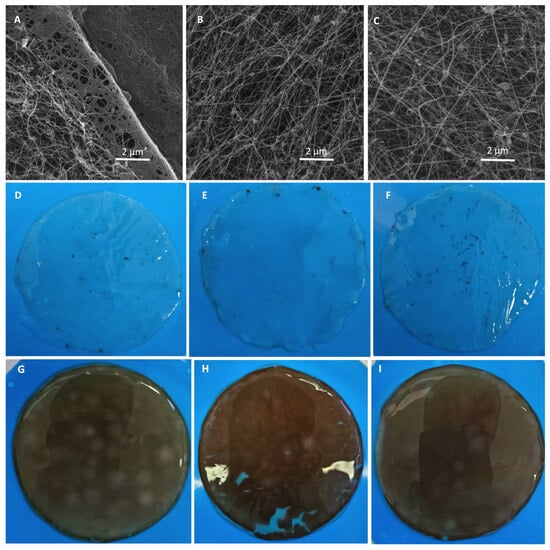
Figure 10.
SEM micrographs of BNC produced using Moscatel pomace extract (A), Pinot Noir pomace extract (B) and a 1:1 mixture of Moscatel and Pinot Noir extracts (C). Images of purified pellicles produced using Moscatel pomace extract (D), Pinot Noir pomace extract (E) and a 1:1 mixture of Moscatel and Pinot Noir extracts (F). Images of unpurified pellicles produced using Moscatel pomace extract (G), Pinot Noir pomace extract (H) and 1:1 mixture of Moscatel and Pinot Noir extracts (I).
4. Conclusions
This study demonstrates the optimized production of BNC using Komagataeibacter xylinus ATCC 53524 at a higher scale using an RDB (10 L of medium), obtaining a maximum production titer of 5.87 g L−1, similar to static fermentation but at a lower temperature. The design and optimization of the rotary disk bioreactor have been crucial for maximizing BNC production. A moderate rotation speed (7 rpm) appears to enhance biopolymer adhesion and minimize shear stress, while the number of disks also plays a key role in the overall performance. The comparative analysis of different disk surfaces revealed that rougher textures significantly improve biofilm adherence. The use of grape pomace extracts as an alternative carbon source has demonstrated promising potential for promoting a more sustainable process, enabling increased BNC production by replacing glucose in the HS medium. These findings are promising and offer valuable insights for advancing the scalability of BNC production through the integration of improved bioprocess design and industrial residue valorization. As a next step, implementing continuous or semi-continuous operation modes in the rotary disk system could enhance the process scalability and reduce the operational costs. Moreover, the integration of metabolic engineering strategies to improve the ability of bacterial strains to utilize complex substrates derived from agro-industrial residues may further boost yields. Additionally, combining grape pomace with other agricultural by-products could offer an effective alternative to conventional nitrogen sources.
Author Contributions
Conceptualization, P.O. and D.N.; formal analysis, E.E., I.M. and D.N.; funding acquisition, D.N.; investigation, R.C., J.P.V., F.C. and K.T.; methodology, R.C., J.P.V., F.C. and D.N.; project administration, D.N.; resources, D.N.; supervision, D.N.; validation, P.O., E.E. and I.M.; writing—original draft, R.C. and D.N.; writing—review and editing, P.O., E.E. and I.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Fondecyt regular, grant number 1241325, and the APC was partially funded by the Dirección de Investigación from the Universidad Católica de la Santísima Concepción, Concepción, Chile.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Research data is available upon request.
Acknowledgments
The authors gratefully acknowledge the Center for Advanced Microscopy, CMA BIO-BIO, for providing access to the scanning electron microscopy (SEM) facilities. The SEM analyses were conducted within the framework of the Proyecto PIA-ANID ECM-12, which supported the microscopy services.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| BNC | Bacterial nanocellulose |
| RDB | Rotary disk bioreactor |
| TGA | Thermogravimetric analysis |
| ATCC | American Type Culture Collection |
| SEM | Scanning electron microscope |
| HS | Hestrin–Schramm medium |
| GRAS | Generally Recognized as Safe |
| FDA | Food and Drug Administration |
| DO | Dissolved oxygen |
| CCD | Central composite design |
| GP | Grape pomace |
References
- Soleimani, A.; Hamedi, S.; Babaeipour, V.; Rouhi, M. Design, Construction and Optimization a Flexible Bench-Scale Rotating Biological Contactor (RBC) for Enhanced Production of Bacterial Cellulose by Acetobacter Xylinium. Bioprocess. Biosyst. Eng. 2021, 44, 1071–1080. [Google Scholar] [CrossRef]
- Jagtap, A.; Dastager, S.G. Bacterial Nanocellulose: A Versatile Biopolymer Production Using a Cost-Effective Wooden Disc Based Rotary Reactor. Biopolymers 2024, 115, e23577. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K.; Pathak, P.; Dey, P.; Gautam, S.; Kumar, S.; Dutt Purohit, S. Bacterial Nanocellulose by Static, Static Intermittent Fed-Batch and Rotary Disc Bioreactor-Based Fermentation Routes Using Economical Black Tea Broth Medium: A Comparative Account. Int. J. Biol. Macromol. 2024, 277, 134228. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K.; Pathak, P. Rotary Disc Bioreactor-Based Approach for Bacterial Nanocellulose Production Using Gluconacetobacter Xylinus NCIM 2526 Strain. Cellulose 2022, 29, 7177–7191. [Google Scholar] [CrossRef]
- Dourado, F.; Leal, M.; Martins, D.; Fontão, A.; Cristina Rodrigues, A.; Gama, M. Chapter 7—Celluloses as Food Ingredients/Additives: Is There a Room for BNC? In Bacterial Nanocellulose; Gama, M., Dourado, F., Bielecki, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 123–133. ISBN 978-0-444-63458-0. [Google Scholar]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial Cellulose: Molecular Regulation of Biosynthesis, Supramolecular Assembly, and Tailored Structural and Functional Properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of Bacterial Cellulose from Alternative Cheap and Waste Resources: A Step for Cost Reduction with Positive Environmental Aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Zahan, K.A.; Pa’e, N.; Muhamad, I.I. Monitoring the Effect of pH on Bacterial Cellulose Production and Acetobacter Xylinum 0416 Growth in a Rotary Discs Reactor. Arab. J. Sci. Eng. 2015, 40, 1881–1885. [Google Scholar] [CrossRef]
- Zahan, K.; Paé, N.; Muhamad, I. An Evaluation of Fermentation Period and Discs Rotation Speed of Rotary Discs Reactor for Bacterial Cellulose Production. Sains Malays. 2016, 45, 393–400. [Google Scholar]
- Zahan, K.A.; Pa’E, N.; Seng, K.F.; Muhamad, I.I. Monitoring Initial Glucose Concentration for Optimum pH Control during Fermentation of Microbial Cellulose in Rotary Discs Reactor. Key Eng. Mater. 2014, 594–595, 319–324. [Google Scholar] [CrossRef]
- Cruz, M.A.; Flor-Unda, O.; Avila, A.; Garcia, M.D.; Cerda-Mejía, L. Advances in Bacterial Cellulose Production: A Scoping Review. Coatings 2024, 14, 1401. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Chegeni, A.; Babaeipour, V. Mathematical Modeling and Simulation of Oxygen Mass Transfer in Rotating Biological Contactor (RBC) for Bacterial Cellulose Production. Biochem. Eng. J. 2023, 200, 109076. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of Cellulose by Acetobacter Xylinum. 2. Preparation of Freeze-Dried Cells Capable of Polymerizing Glucose to Cellulose *. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Núñez, D.; Oyarzún, P.; Cáceres, R.; Elgueta, E.; Gamboa, M. Citrate-Buffered Yamanaka Medium Allows to Produce High-Yield Bacterial Nanocellulose in Static Culture Using Komagataeibacter Strains Isolated from Apple Cider Vinegar. Front. Bioeng. Biotechnol. 2024, 12, 1375984. [Google Scholar] [CrossRef]
- Levenspiel, O. The Monod Equation: A Revisit and a Generalization to Product Inhibition Situations. Biotechnol. Bioeng. 1980, 22, 1671–1687. [Google Scholar] [CrossRef]
- Núñez, D.; Cáceres, R.; Ide, W.; Varaprasad, K.; Oyarzún, P. An Ecofriendly Nanocomposite of Bacterial Cellulose and Hydroxyapatite Efficiently Removes Lead from Water. Int. J. Biol. Macromol. 2020, 165, 2711–2720. [Google Scholar] [CrossRef] [PubMed]
- Lestari, P.; Elfrida, N.; Suryani, A.; Suryadi, Y. Study on the Production of Bacterial Cellulose from Acetobacter Xylinum Using Agro-Waste. Jordan. J. Biol. Sci. 2014, 7, 75–80. [Google Scholar] [CrossRef]
- Avirasdya, R.A.; Nursiwi, A.; Sari, A.M.; Zaman, M.Z.; Sanjaya, A.P. Kinetics Study of Bacterial Cellulose Production by Acetobacter Xylinum FNCC 0001 with Variation of Carbon Sources. E3S Web Conf. 2022, 344, 03002. [Google Scholar] [CrossRef]
- Guevara, K.M.; Martínez-Valenzuela, G.; Sánchez-Vásquez, V.; Guerrero-Ruiz, K.; Fiallos-Cárdenas, M. Trends and Perspectives on Bacterial Nanocellulose: A Comprehensive Analysis from the Three Helixes of Innovation. Mater. Today. Sustain. 2025, 30, 101090. [Google Scholar] [CrossRef]
- Kumaravel, A.; Lin, S.P.; Santoso, S.P.; Shanmugasundaram, S.; Hsu, H.Y.; Hsieh, C.W.; Chou, Y.C.; Lin, H.W.; Cheng, K.C. Unlocking the Potential of Bacterial Cellulose: Synthesis, Functionalization, and Industrial Impact. Int. J. Biol. Macromol. 2025, 311, 143951. [Google Scholar] [CrossRef]
- Serafica, G.; Mormino, R.; Bungay, H. Inclusion of Solid Particles in Bacterial Cellulose. Appl. Microbiol. Biotechnol. 2002, 58, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, S.F.; Li, Y.; Xu, H.; Qin, L.; Tay, J.H. The Influence of Cell and Substratum Surface Hydrophobicities on Microbial Attachment. J. Biotechnol. 2004, 110, 251–256. [Google Scholar] [CrossRef]
- Pilafidis, S.; Vardaxi, A.; Kourmentza, K.; Pispas, S.; Dimopoulou, M.; Tsouko, E. From Bread Waste to Bacterial Cellulose Nanostructures: Development of a Novel Rotating Disk Bioreactor. Int. J. Biol. Macromol. 2025, 314, 144374. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Tabuteau, H.; Gaffney, E.A.; Fostera, K.R.; Durham, W.M. Microbial Competition in Porous Environments Can Select against Rapid Biofilm Growth. Proc. Natl. Acad. Sci. USA 2017, 114, E161–E170. [Google Scholar] [CrossRef]
- Rendueles, O.; Ghigo, J.-M. Mechanisms of Competition in Biofilm Communities. Microbiol. Spectr. 2015, 3, 3. [Google Scholar] [CrossRef]
- Zahan, K.A.; Pa’, N.; Muhamad, I.I. Process Parameters for Fermentation in a Rotary Disc for Optimum Microbial Cellulose Production Response Surface Methodology. Bioresources 2014, 9, 1858–1872. [Google Scholar] [CrossRef]
- Choi, C.N.; Song, H.J.; Kim, M.J.; Chang, M.H.; Kim, S.J. Properties of Bacterial Cellulose Produced in a Pilot-Scale Spherical Type Bubble Column Bioreactor. Korean J. Chem. Eng. 2009, 26, 136–140. [Google Scholar] [CrossRef]
- Krsmanovic, M.; Biswas, D.; Ali, H.; Kumar, A.; Ghosh, R.; Dickerson, A.K. Hydrodynamics and Surface Properties Influence Biofilm Proliferation. Adv. Colloid. Interface Sci. 2021, 288, 102336. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Kari, Z.A.; Noor, N.H.M.; Ray, R.R. Bacterial Cellulose: Production, Characterization and Application as Antimicrobial Agent. Int. J. Mol. Sci. 2021, 22, 12984. [Google Scholar] [CrossRef]
- Beluhan, S.; Herceg, F.; Leboš Pavunc, A.; Djaković, S. Preparation and Structural Properties of Bacterial Nanocellulose Obtained from Beetroot Peel Medium. Energies 2022, 15, 9374. [Google Scholar] [CrossRef]
- Chen, G.; Chen, L.; Wang, W.; Chen, S.; Wang, H.; Wei, Y.; Hong, F.F. Improved Bacterial Nanocellulose Production from Glucose without the Loss of Quality by Evaluating Thirteen Agitator Configurations at Low Speed. Microb. Biotechnol. 2019, 12, 1387–1402. [Google Scholar] [CrossRef]
- Páez, M.A.; Casa-Villegas, M.; Aldas, M.; Luna, M.; Cabrera-Valle, D.; López, O.; Fernández, D.; Cruz, M.A.; Flor-Unda, O.; García, M.D.; et al. Insights into Agitated Bacterial Cellulose Production with Microbial Consortia and Agro-Industrial Wastes. Fermentation 2024, 10, 425. [Google Scholar] [CrossRef]
- Aswini, K.; Gopal, N.O.; Uthandi, S. Optimized Culture Conditions for Bacterial Cellulose Production by Acetobacter Senegalensis MA1. BMC Biotechnol. 2020, 20, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ruka, D.R.; Simon, G.P.; Dean, K.M. Altering the Growth Conditions of Gluconacetobacter Xylinus to Maximize the Yield of Bacterial Cellulose. Carbohydr. Polym. 2012, 89, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Kim, J.-N.; Wee, Y.-J.; Park, D.-H.; Ryu, H.-W. Bacterial Cellulose Production by Gluconacetobacter Sp. RKY5 in a Rotary Biofilm Contactor. Appl. Biochem. Biotechnol. 2007, 137–140, 529–537. [Google Scholar] [CrossRef]
- Krystynowicz, A.; Czaja, W.; Wiktorowska-Jezierska, A.; Gonçalves-Miśkiewicz, M.; Turkiewicz, M.; Bielecki, S. Factors Affecting the Yield and Properties of Bacterial Cellulose. J. Ind. Microbiol. Biotechnol. 2002, 29, 189–195. [Google Scholar] [CrossRef]
- Lin, S.P.; Liu, C.T.; Hsu, K.D.; Hung, Y.T.; Shih, T.Y.; Cheng, K.C. Production of Bacterial Cellulose with Various Additives in a PCS Rotating Disk Bioreactor and Its Material Property Analysis. Cellulose 2016, 23, 367–377. [Google Scholar] [CrossRef]
- Lin, S.P.; Hsieh, S.C.; Chen, K.I.; Demirci, A.; Cheng, K.C. Semi-Continuous Bacterial Cellulose Production in a Rotating Disk Bioreactor and Its Materials Properties Analysis. Cellulose 2014, 21, 835–844. [Google Scholar] [CrossRef]
- Samarakoon, K.; Rupasinghe, H.P.V. Valorization of Grape Pomace by Microbial Fermentation: Composition, Biological Activities and Potential Applications for the Food Industry. J. Food Compos. Anal. 2025, 144, 107656. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef]
- Karastergiou, A.; Gancel, A.L.; Jourdes, M.; Teissedre, P.L. Valorization of Grape Pomace: A Review of Phenolic Composition, Bioactivity, and Therapeutic Potential. Antioxidants 2024, 13, 1131. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 14. [Google Scholar] [CrossRef]
- Taokaew, S. Bacterial Nanocellulose Produced by Cost-Effective and Sustainable Methods and Its Applications: A Review. Fermentation 2024, 10, 316. [Google Scholar] [CrossRef]
- Katia Cury, R.; Yelitza Aguas, M.; Ana Martinez, M.; Rafael Olivero, V.; Linda Chams, C. Residuos Agroindustriales Su Impacto, Manejo y Aprovechamiento. RECIA 2017, 9, 122–132. [Google Scholar] [CrossRef]
- Keshk, S.; Sameshima, K. Evaluation of Different Carbon Sources for Bacterial Cellulose Production. Afr. J. Biotechnol. 2005, 4, 478–482. [Google Scholar]
- Gorgieva, S.; Jančič, U.; Cepec, E.; Trček, J. Production Efficiency and Properties of Bacterial Cellulose Membranes in a Novel Grape Pomace Hydrolysate by Komagataeibacter Melomenusus AV436T and Komagataeibacter Xylinus LMG 1518. Int. J. Biol. Macromol. 2023, 244, 125368. [Google Scholar] [CrossRef]
- Tsouko, E.; Pilafidis, S.; Kourmentza, K.; Gomes, H.I.; Sarris, G.; Koralli, P.; Papagiannopoulos, A.; Pispas, S.; Sarris, D. A Sustainable Bioprocess to Produce Bacterial Cellulose (BC) Using Waste Streams from Wine Distilleries and the Biodiesel Industry: Evaluation of BC for Adsorption of Phenolic Compounds, Dyes and Metals. Biotechnol. Biofuels Bioprod. 2024, 17, 1–17. [Google Scholar] [CrossRef]
- Fan, X.; Gao, Y.; He, W.; Hu, H.; Tian, M.; Wang, K.; Pan, S. Production of Nano Bacterial Cellulose from Beverage Industrial Waste of Citrus Peel and Pomace Using Komagataeibacter Xylinus. Carbohydr. Polym. 2016, 151, 1068–1072. [Google Scholar] [CrossRef]
- Coelho, C.C.S.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.C.; Teixeira, J.A. Cellulose Nanocrystals from Grape Pomace: Production, Properties and Cytotoxicity Assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef]
- Cortina, J.L.; Nayak, A.; Bhushan, B.; Rosales, A.; Rodriguez-Turiel, L. Valorisation Potential of Cabernet Grape Pomace for the Recovery of Polyphenols: Process Intensification, Optimisation and Study of Kinetics. Food Bioprod. Process. 2018, 109, 74–85. [Google Scholar] [CrossRef]
- Andritsou, V.; Melo, E.; Tsouko, E.; Ladakis, D.; Maragkoudaki, S.; Koutinas, A.; Matharu, A. Synthesis and Characterization of Bacterial Cellulose from Citrus-Based Sustainable Resources. ACS Omega 2018, 3, 10365–10373. [Google Scholar] [CrossRef]
- Keshk, S. Vitamin C Enhances Bacterial Cellulose Production in Gluconacetobacter Xylinus. Carbohydr. Polym. 2014, 99, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Fuller, M.E.; Andaya, C.; McClay, K. Evaluation of ATR-FTIR for Analysis of Bacterial Cellulose Impurities. J. Microbiol. Methods 2018, 144, 145–151. [Google Scholar] [CrossRef]
- Lopes, J.d.C.; Madureira, J.; Margaça, F.M.A.; Cabo Verde, S. Grape Pomace: A Review of Its Bioactive Phenolic Compounds, Health Benefits, and Applications. Molecules 2025, 30, 362. [Google Scholar] [CrossRef] [PubMed]
- Chawla, P.R.; Bajaj, I.B.; Survase, S.; Singhal, R.S. Microbial Cellulose: Fermentative Production and Applications (Review). Food Technol. Biotechnol. 2009, 47, 107–124. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).